A Technique for the Prolonged Incubation of Neuronal Tissue of the Retina
Abstract
Source: Cameron, M. A., et al. Prolonged Incubation of Acute Neuronal Tissue for Electrophysiology and Calcium-imaging. J. Vis. Exp. (2017).
This video demonstrates a method for prolonged incubation of neuronal tissue of the retina. Eyecups containing retina are obtained from a mouse eye; the inner limiting membrane (ILM) is enzymatically digested to expose the neuronal cells of the retina, and either the treated eyecup or the isolated retina is transferred to a specialized incubator for prolonged incubation at a low temperature.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Retinal Wholemount Preparation and Inner Limiting Membrane Removal
- Prepare retinal wholemounts under either normal laboratory lighting conditions or dim red/infra-red light.
- Euthanize animal by cervical dislocation and immediately enucleate eyes. Make a small cut along the ora serrata, and place in either Ames media, or artificial cerebrospinal fluid (aCSF) containing (mM): 125 NaCl, 25 NaHCO3, 3 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 0.5 L-glutamine, and saturate with carbogen (95% O2/5% CO2 mixture; ~300 mOsm; pH 7.4), at room temperature (RT).
- Immediately remove the cornea, lens and vitreous by cutting along the ora serrata with small scissors and removing the lens and vitreous with forceps. Place tissue in the incubation system at RT.
NOTE: Retinal tissue can be maintained in the eyecup, after slow temperature reduction to 15 – 16 °C, for >24 h in the incubation system until needed. - To remove the ILM, transfer the eyecup containing the retina to a small glass jar containing 30 U/mL papain, 1 mM L-cystine, 0.5 mM ethylenediaminetetraacetic acid (EDTA) and 0.005% deoxyribonuclease (DNase) in Earl's Balanced Salt Solution (BSS) at 37 °C for 20 min. Apply 95% O2/5% CO2 to the solution through the lid but do not bubble. If using tissue from young animals (<6 weeks), dilute the solution to half-strength.
- Stop enzymatic digestion by placing the tissue in an ovomucoid (10 mg/mL) and bovine serum albumin (BSA, 10 mg/mL) solution for 10 min in Earl's BSS. Wash tissue thoroughly with aCSF before transferring to the incubation system and reduce temperature to 15 – 16 °C.
- Maintain retinal tissue in the eyecup until needed. For transfer to the microscope, isolate the retina from the eyecup and cut into 4 pieces with a razorblade. If the entire retina is required, make four small cuts in the periphery of the retina to allow it to lie flat.
NOTE: For imaging experiments, load with calcium-dyes before transfer to the incubation system, see below.
2. Maintaining Tissue in the Incubator
- Place tissue in the main chamber containing probes for pH and temperature measurements (Figure 1A, B).
- Circulate aCSF through a second chamber (UVC chamber, built as previously described) isolated from the main chamber and exposed to 1.1 W ultraviolet C (UVC) light (254 nm, 5W/2P sterilizer UV lamp) in order to eradicate bacteria floating in the solution (Figure 1A). Control UVC light timing via a programmable timer using a random feature that turns ON at times varying between 15 and 26 min every 15 – 30 min to avoid excessive heating of the aCSF.
- Set the flow rate in the UVC chamber to 12 mL/min as previously reported. Cover the UVC chamber with aluminum foil to prevent UVC illumination outside the chamber, which can damage neuronal tissue.
NOTE: For dark-adapted retinal tissue, apply a custom-made cover to the main chamber to exclude light. - Use a peristaltic pump to circulate the solution (aCSF) through the two chambers and a Peltier thermoelectric cold plate cooler to either cool or heat the main chamber to the desired temperatures in the range of 0 – 50 °C. For optimal tissue incubation, use 15 – 16 °C for long-term viability.
Representative Results
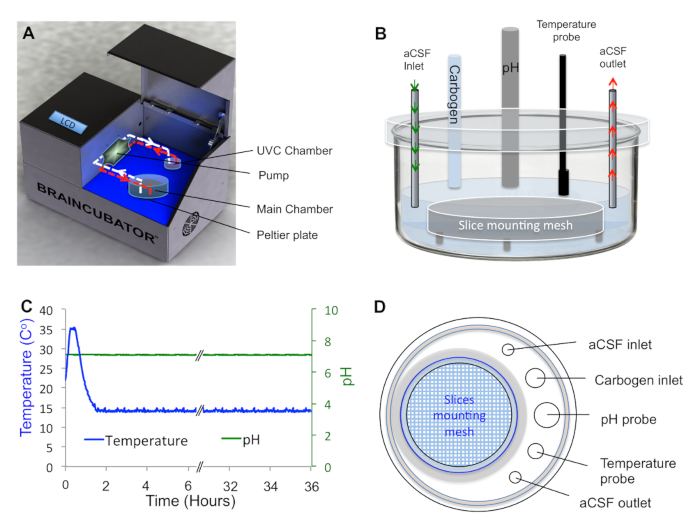
Figure 1. An Incubation System That Enables Tight Regulation of Tissue Environment. A, Schematic diagram of the incubation system composed of a cooling Peltier plate, main chamber, peristaltic pump and UVC chamber. B, Side view of the main chamber showing the inlet and outlet points of the aCSF, as well as the carbogen inlet, temperature and pH probes. Tissue is placed on the mesh as indicated. All measuring probes are attached to the lid to allow structural stability. C, aCSF Parameters Stamp describing the temperature and pH of the aCSF during incubation. D, Top view of the main chamber showing the mounting holes for the measuring probes.
Disclosures
The authors have nothing to disclose.
Materials
| Sodium Chloride | Sigma Aldrich VETEC | V800372 | |
| Potassium chloride | Sigma Aldrich | P9333 | |
| N-Methyl-D-glucamine | Sigma Aldrich | M2004 | |
| D-glucose | Sigma Aldrich | G5767 | |
| Sodium bicarbonate | Sigma Aldrich | S6014 | |
| Magnesium chloride | Sigma Aldrich | M9272 | |
| Calcium chloride | Sigma Aldrich | 223506 | |
| Papain Dissociation System | Worthington | LK003150 | |
| Fixed Stage Upright Microscope | Olympus | BX51WI | |
| Microscope Objective | Olympus | XLUMPlanFLN 20x/1.00w | 20X |
| Microscope Objective | Olympus | LUMPlanFLN 60x/1.00w | 60X |
| Braincubator | Payo Scientific | BR26021976 | www.braincubator.com.au |
| Peltier-Thermoelectric Cold Plate Cooler | TE Technology | CP-121 | |
| Temperature Controller | TE Technology | TC-36-25 RS232 |

