Direct Neuronal Reprogramming of Mouse Astrocytes into Neurons
Abstract
Source: Hersbach, B. A. et al., Isolation and Direct Neuronal Reprogramming of Mouse Astrocytes. J. Vis. Exp. (2022)
This video showcases the direct reprogramming of astrocytes expressing transforming factors into neurons. Transformation factors trigger a cascade of molecular events facilitating astrocyte-to-neuron conversion. The process is further supported by supplying specific agents and supplements through a neuronal differentiation medium.
Protocol
1. Seeding of astrocytes for reprogramming
NOTE: The following steps have to be performed under a biological safety cabinet with a safety level 1 (SL1).
- Prepare 24-well plates with poly-D-lysine coated glass coverslips in the same way culture flasks were prepared previously. To determine the number of plates needed, consider that astrocytes are plated at a density of 5-5.5 x 104 cells per well in a 24-well plate. Usually, isolating six spinal cords from postnatal day 2 (P2) mice yields around 1 x 106 cells.
- Aspirate media from the T25 culture flasks containing the cultured astrocytes and wash once with 1x Phosphate-buffered saline (PBS). Detach the astrocytes from the culture flask by adding 0.5 mL of 0.05% Trypsin/ Ethylenediaminetetraacetic acid (EDTA) and incubate at 37 °C for 5 min. Gently tap the side of the flask to release cells from the culture flask surface and check detachment under a brightfield microscope using a 10X magnification.
- Stop trypsinization with 2.5 mL of astrocyte culture medium and collect cell suspension in 15 mL tubes. Centrifuge at 300 x g for 5 min, aspirate supernatant, and resuspend cells in 1 mL of astrocyte culture medium. Calculate cell concentration using a haemocytometer or an automated cell counting system.
- Based on the number of cells, dilute the cell suspension with fresh astrocyte medium to obtain a solution of 1-1.1 x 105 cells per mL. Supplement the medium with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) at 10 ng/mL per factor. Add 500 µL of cell suspension, equivalent to 5-5.5 x 104 cells, to each well of the previously prepared 24-well plates and culture cells at 37 °C and 5% CO2.
2. Forced expression of transcription factors
NOTE: Before proceeding with the protocol, it is essential to properly design the experiment. In particular, it is important to always include a negative control for the reprogramming, namely a condition where no reprogramming factor is expressed. For instance, when using vectors carrying the cDNA for the reprogramming factor and a reporter (e.g., green fluorescent protein (GFP), DsRed), the negative control is represented by the same vector carrying only the reporter. When expressing multiple factors carrying different reporters, the negative control should be accordingly adjusted.
- The day after plating, inspect the 24-well plates to make sure cells have adhered to the coverslips.
- Based on the experimental aim and available resources, the forced expression of reprogramming factors can be achieved by viral transduction (see step 2.3) or DNA transfection (see step 2.4).
NOTE: The use of retrovirus or lentivirus requires the approval of government authorities and must be performed under a biological safety cabinet within a laboratory with safety level 2 (SL2). - Reprogram the astrocytes by transducing the cells with the retrovirus or lentivirus carrying the genetic information to express the reprogramming factor(s) of interest as described below.
- Transduce cells with a high virus titer of 1 x 1010-1 x 1012 particles/mL by adding 1 µL of the cell suspension directly to the astrocyte medium. This ensures a high infection rate. Culture the cells with the astrocyte medium containing viral particles at 37 °C for 24-36 h before proceeding with section 3, depending on the purpose of the experiment.
NOTE: Different promoters can be used to drive the expression of the transgenes (see also Representative Results). Constitutive promoters {e.g., cytomegalovirus (CMV), chicken β‑actin (CAG)} induce the expression of the transgene earlier than inducible promoters; however, both types of promoter types have been successfully used to reprogram cells into neurons.
- Transduce cells with a high virus titer of 1 x 1010-1 x 1012 particles/mL by adding 1 µL of the cell suspension directly to the astrocyte medium. This ensures a high infection rate. Culture the cells with the astrocyte medium containing viral particles at 37 °C for 24-36 h before proceeding with section 3, depending on the purpose of the experiment.
- DNA plasmids can also be introduced into astrocytes via DNA transfection as described below.
NOTE: This can be done under a biological safety cabinet approved for SL1.- Before transfection, obtain the transfection reagent, a plasmid DNA of the desired constructs, fresh astrocyte medium, and serum-reduced medium (see Table of Materials). Calculate the required amount of serum-reduced medium (see Table of Materials) by considering that each well of a 24-well plate requires 300 µL of serum-reduced medium. Add the appropriate amount of serum-reduced medium to a 50 mL tube and warm it to 37 °C.
- When the serum-reduced medium is warm, aspirate astrocyte medium from all wells and collect it in a 50 mL tube. Filter the collected astrocyte medium with a 0.45 µM syringe filter to remove detached cells.
- Add an equal volume of fresh astrocyte medium to the filtered medium to obtain a solution sufficient to add 1 mL of astrocyte medium per well. Maintain the astrocyte conditioned medium in the incubator at 37 °C until use (step 2.4.9).
NOTE: The culture medium is re-used as it contains several secreted factors that support the viability of the culture. - Add 300 µL of pre-warmed serum-reduced medium to each well and place the 24-well plate back to the incubator.
- Prepare solution A, consisting of DNA and serum-reduced medium. For each well, use a total of 0.6 µg of DNA and add it to 50 µL of serum-reduced medium. Store solution A at room temperature until use.
NOTE: Typically, a technical triplicate per condition is considered. Therefore, a mix sufficient for 3.5 reaction is prepared (e.g., 2.1 µg of total DNA diluted in 175 µL of serum-reduced medium) to ensure enough material to transfect three wells of a 24-well plate. - Prepare solution B, composed of the transfection reagent and serum-reduced medium. For each well, add 0.75 µL of transfection reagent to 50 µL of serum-reduced medium. As this solution is common to all transfection conditions, prepare it in bulk to reduce the variability across transfections.
- Incubate solution B at room temperature for 5 min. Add solution B to solution A drop by drop at a 1:1 ratio and gently mix. Do not vortex. Incubate solution A+B for 20-30 min at room temperature under the hood.
- Repeat step 2.4.5-2.4.7 for all transfection conditions. After 20-30 min, add solution A+B to each well drop by drop for a final volume of 100 µL. Gently shake the plate and place the cells back in the incubator at 37 °C for 4 h.
- After 4 h, remove the transfection medium and add 1 mL of the pre-warmed astrocyte conditioned medium prepared in step 2.4.3. Maintain cells for 36-48 h before proceeding with section 3, depending on the purpose of the experiment.
3. Reprogramming of astrocytes (7 days analysis)
- Prepare neuronal differentiation medium by adding 1 mL of B27-supplement to 49 mL of basic culture medium. After 24-48 h, depending on whether cells have been transduced or transfected (see steps 2.3 and 2.4, respectively), replace astrocyte medium with 1 mL of neuronal differentiation medium per well and culture cells at 37 °C and 9% CO2.
NOTE: Cells can also be kept at 5% CO2 if no 9% CO2 incubator is available. However, neuronal reprogramming is more efficient under these conditions. - Optional: To increase the reprogramming efficiency, supplement neuronal differentiation medium with Forskolin (final concentration of 30 µM) and Dorsomorphin (final concentration of 1 µM) when replacing the astrocyte medium to differentiation medium. When opting to treat cells with Forskolin and Dorsomorphin, provide a second dose of Dorsomorphin 2 days after the initial treatment. Add this second treatment directly to the culture medium.
- Direct reprogramming of murine astrocytes into neuronal cells normally occurs within 7 days after changing the medium (Figure 1). Hence, 7 days after the initiation of neuronal reprogramming, fix or collect cells for downstream analysis.
Representative Results
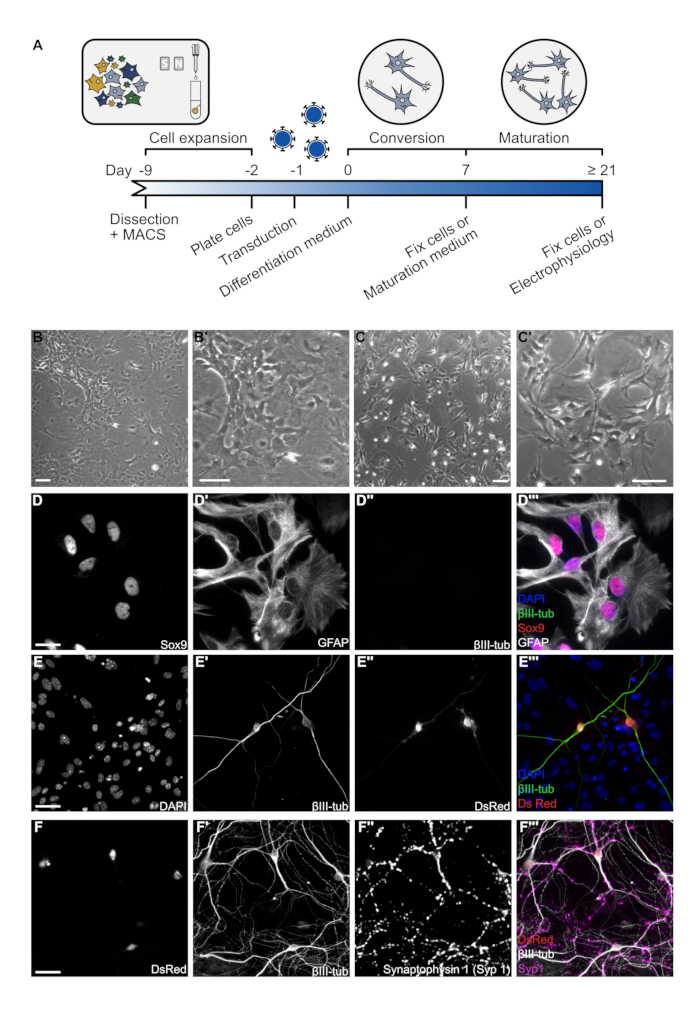
Figure 1: Overview of astrocyte culture and reprogramming. (A)Timeline of astrocyte-to-neuron direct conversion. Each black line represents an important step in the protocol. (B) Representative brightfield images of cultured spinal cord-derived astrocytes after 7 days in culture. Pictures were taken using a brightfield microscope and 10x objective. Scale bar represents 100 µm. (C) Representative brightfield images of spinal cord astrocytes 1 day after re-plating at a density of 5.5 x 104 cells per well in a 24-well plate. Images were taken using a brightfield microscope and a 10x objective. Scale bar represents 100 µm. (D) Immunofluorescence image of a βIII-tub, Sox9, GFAP triple staining on astrocytes fixed 1 day after plating to demonstrate culture purity. Cells were fixed in 4% paraformaldehyde for 10 min and washed twice with 1x PBS. Cells were blocked using a 3% BSA, 0.5% Triton-X 100 in 1x PBS solution. Primary antibodies were diluted at the proper concentration (e.g., anti-GFAP 1:250; anti-βIII-tub 1:250; anti-Syp1 1:500) in blocking solution and incubated for 2 h at room temperature. Cells were washed three times with 1x PBS and incubated with fluorophore-conjugated secondary antibodies for 1 h at room temperature. Coverslips were washed three times with 1x PBS before mounting with Aqua Poly/Mount. Images were acquired using an epifluorescence microscope and a 40x objective. Scale bar represents 20 µm. (E) Immunofluorescence image of a βIII-tub, DsRed double staining to demonstrate astrocyte to neuron conversion with Ascl1 after 7 DPT. Protocol of immunofluorescence and image acquisition was as described above. Scale bar represents 20 µm. (F) Immunofluorescence images of a βIII-tub, DsRed, Synaptophysin 1 (Syp1) triple staining to demonstrate neuronal maturity after 21 DPT of reprogramming with Ascl1. Protocol of immunofluorescence and image acquisition was as described above. Scale bar represents 20 µm.
Disclosures
The authors have nothing to disclose.
Materials
| 0.05% Trypsin/EDTA | Life Technologies | 25300054 | |
| B27 Supplement | Life Technologies | 17504044 | |
| bFGF | Life Technologies | 13256029 | |
| DMEM/F12 | Life Technologies | 21331020 | |
| Dorsomorphin | Sigma Aldrich | P5499 | |
| EGF | Life Technologies | PHG0311 | |
| Fetal Bovine Serum | PAN Biotech | P30-3302 | |
| Forskolin | Sigma Aldrich | F6886 | |
| Lipofectamine 2000 (Transfection reagent) | Thermo Fisher | Cat# 11668019 | |
| OptiMEM – GlutaMAX (serum-reduced medium) | Thermo Fisher | Cat# 51985-026 | |
| Penicillin/Streptomycin | Life Technologies | 15140122 | |
| Poly-D-Lysine | Sigma Aldrich | P1149 |

