Fluorescent Reporter-Based Paralysis Assay: A Technique to Assess Age-Associated Progressive Formation of Polyglutamine Fluorescent Reporter and Associated Paralysis in Caenorhabditis elegans
Abstract
Source: Lazaro-Pena, M. I. et al. Quantifying Tissue-Specific Proteostatic Decline in Caenorhabditis elegans. J. Vis. Exp. (2021)
This video demonstrates an in vivo imaging-based assay to determine proteostasis decline due to aging. The assay uses Caenorhabditis elegans expressing polyglutamine YFP-fusion protein in body wall muscles to measure the age-associated aggregation of polyglutamine and induction of proteotoxicity, leading to paralysis.
Protocol
1. Measuring the decline in proteostasis in muscle tissue by using polyglutamine-expressing animals
NOTE: Two methods can be used to identify proteostasis decline in muscle cells: imaging the formation of protein aggregates during aging (1.1) and measuring the proteotoxicity these aggregates cause with age through the onset of paralysis (1.2).
- Imaging polyglutamine aggregate formation in the muscle during aging
NOTE: The age-dependent progression of protein aggregation in the muscle cells is imaged using polyglutamine (polyQ) repeats fused to a yellow fluorescent protein (YFP). This protocol outlines the use of strain AM140 rmIs132[unc-54p::Q35::YFP], but other polyglutamine variant strains can also be used. The polyQ::YFP transgene is expressed in the muscle using the unc-54 muscle-specific promoter. Synchronized unc-54p::polyQ::YFP expressing animals are visualized at Day 1, 2, 3, and 4 of adulthood. To visualize unc-54p::polyQ::YFP aggregates, use a compound microscope equipped for fluorescence or a fluorescent dissecting microscope. AM140 is available from the Caenorhabditis Genetics Center (CGC) at: https://cgc.umn.edu/strain/AM140- On imaging days (Day 1, 2, 3, and 4 of adulthood), pick 20 animals and mount them on a microscope slide setup with a 3% agarose pad and a 5 μL drop of 10 mM sodium azide (diluted in M9 buffer).
- After all the animals are immobilized by sodium azide (~ 5 minutes), image the whole bodies of the animals using a 10x magnification lens (Figure 1A). For imaging, use a FITC filter and the same exposure for every animal. Discard slides after imaging.
NOTE: Alternatively, YFP foci can also be quantified directly on plates, but the movement must be inhibited by applying a stream of carbon dioxide onto the plate while scoring. This alternative method is most appropriate when screening a large number of conditions. - After the acquisition of images, count the number of foci in the body wall muscles of the whole animal. Foci are brighter punctuated signals that can be differentiated from the dimmer soluble signal in the background.
- Plot the progression of YFP foci accumulation from days 1, 2, 3, and 4. Plot an XY graph where X represents days of adulthood and Y represents the number of unc-54p::polyQ::YFP foci (Figure 1B). The number of foci in the experimental condition (e.g., gene downregulation or overexpression) is compared to control animals. Perform statistical analysis between the groups at each time point observed for each trial.
- When comparing foci counts for only two conditions, perform an independent sample t-test at each time point.
- When comparing foci counts for more than two conditions, perform an omnibus one-way ANOVA analysis for each time point, including the data for all the conditions at that time point. If the omnibus ANOVA yields a significant p-value (i.e., p < 0.005), perform a post-hoc analysis with pairwise independent sample t-tests to determine the specific conditions between which a significant difference in foci was detected (e.g., for groups A, B, and C compare A & B, A & C, and B & C).
2. Measuring animal paralysis rates as a surrogate for polyglutamine toxicity in muscle cells
NOTE: The age-dependent increase of polyQ aggregates in muscle causes the decline of muscular function that drives locomotion. This defect in locomotion can be determined by measuring the progressive rate of paralysis in polyQ-expressing animals. For the paralysis assay, synchronized unc-54p::polyQ::YFP expressing animals are scored at Day 3, 5, 7, and 9 of adulthood. Add additional time points as needed, to extend the scoring range such that the effect of genetic perturbation can be assessed.
- On scoring days (Day 3, 5, 7, and 9 of adulthood), look at the 6 cm plates containing 50 animals and record the number of paralyzed animals. Remove paralyzed animals from the plate. If animals have crawled off the plate or have died, they should be censored; record the event so it can be accounted for in the analysis.
NOTE: An animal is considered to be paralyzed when no movement is observed after exposing them to light or gentle touch stimuli. Pharyngeal pumping activity is used to determine whether nonmoving animals are alive or dead. - At the completion of the experiment, calculate the paralysis rate for each condition. Do this at each time point by dividing the fraction of paralyzed animals by the total number of animals observed for that condition. If observing multiple plates per condition per time point (i.e., replicate plates), calculate the ratio separately for each replicate.
- Plot the progression of the paralysis rate in an XY graph (scatterplot). X represents days of adulthood, and Y represents paralysis rates. The paralysis rate of unc-54p::polyQ::YFP animals is compared to control animals (Figure 1C). Perform statistical analysis between the groups; for multiple trials, treat trials independently. Use the Cox Proportional-Hazards Regression and the Wald test. Most data analysis tools that support survival analysis also support Cox modeling.
- Transform the data: each condition should have two columns, one for time and another for "event." At each time point observed, add a row with "0" for each paralyzed animal and a "1" for each censored animal. The total number of rows should be equal to the number of starting animals on the plate for that condition.
- Perform Cox Proportional-Hazards modeling, followed by the Wald test, according to instructions for the statistical software. A univariate model will be appropriate for typical experiment designs. For more than two conditions, if a test with all conditions included indicates a significant result (i.e., p < 0.005), pairwise tests between conditions can be performed to determine the specific significant pair(s) of conditions.
NOTE: The Cox model relies on the assumption that the test condition(s) modulate the probability of an event proportionally over time. This means, for example, that the Cox model would not be appropriate for comparing a condition under which most animals are paralyzed at day 1 to a condition under which most animals are paralyzed at day 9. Extensions of the Cox model, and other approaches for comparing proportions paralyzed at each time point, are available for cases where the proportional hazards assumption does not hold.
Representative Results
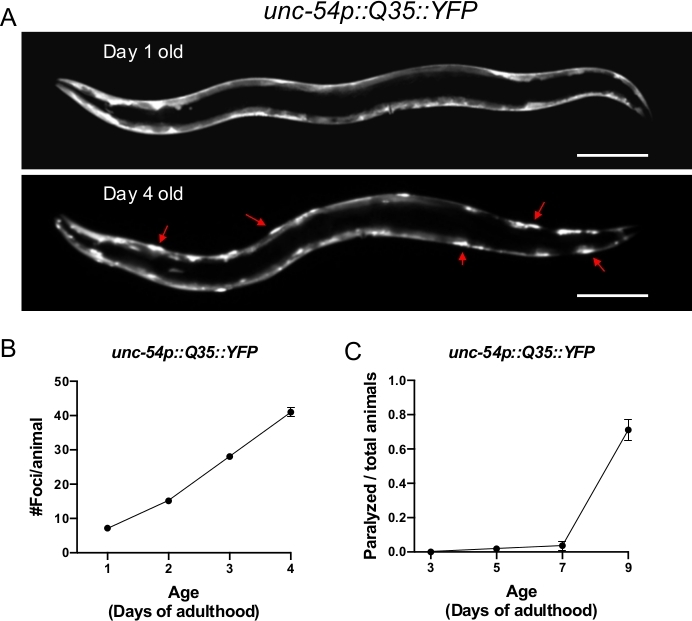
Figure 1: Expression of polyQ::YFP within C. elegans muscle results in progressive foci accumulation and paralysis during aging. (A) C. elegans unc-54p::Q35::YFP expression at days 1 and 4 of adulthood (upper and lower panel, respectively). Arrows indicate representative foci. (B) Quantification of fluorescent foci over the first 4 days of adulthood. Foci are resistant to FRAP, consistent with an insoluble protein aggregate. Error bars represent the standard error of the mean (SEM) (C) unc-54p::Q35::YFP animals become paralyzed during aging. Error bars represent standard error of proportion. The scale bar represents 100 μm in all panels.
Disclosures
The authors have nothing to disclose.
Materials
| Glass microscope slides | VWR | 160004-422 | |
| Sodium Azide, CAS #26628-22-8 | Sigma-Aldrich | S2002 | |
| Zeiss Axio Imager M2m microscope with AxioVision v4.8.2.0 software | Zeiss | ||
| Zeiss StemiSV11 M2 Bio Quad microscope | Zeiss |

