6.11:
Standard Enthalpy of Formation
34,542 Views
•
•
Enthalpy changes are typically tabulated for reactions in which both the reactants and products are at the same conditions. A standard state is a commonly accepted set of conditions used as a reference point for the determination of properties under other different conditions. For chemists, the IUPAC standard state refers to materials under a pressure of 1 bar and solutions at 1 M and does not specify a temperature. Many thermochemical tables list values with a standard state of 1 atm. Because the ΔH of a reaction changes very little with such small changes in pressure (1 bar = 0.987 atm), ΔH values (except for the most precisely measured values) are essentially the same under both sets of standard conditions. A superscripted “o” in the enthalpy change symbol designates standard state. Since the usual (but not technically standard) temperature is 298.15 K, this temperature will be assumed unless some other temperature is specified. Thus, the symbol (ΔH°) is used to indicate an enthalpy change for a process occurring under these conditions. (The symbol ΔH is used to indicate an enthalpy change for a reaction occurring under nonstandard conditions.)
The enthalpy changes for many types of chemical and physical processes are available in the reference literature, including those for combustion reactions, phase transitions, and formation reactions. Since the enthalpy change for a given reaction is proportional to the amounts of substances involved, it may be reported on that basis (i.e., as the ΔH for specific amounts of reactants). However, we often find it more useful to divide one extensive property (ΔH) by another (amount of substance), and report a per-amount intensive value of ΔH, often “normalized” to a per-mole basis.
Standard Enthalpy of Formation
The standard enthalpy of formation ΔHf° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free elements in their most stable states under standard state conditions. These values are especially useful for computing or predicting enthalpy changes for chemical reactions that are impractical or dangerous to carry out, or for processes for which it is difficult to make measurements. Using known values of standard enthalpies of formation, the enthalpy change for any reaction can be determined.
The standard enthalpy of formation of CO2 (g) is −393.5 kJ/mol. This is the enthalpy change for the exothermic reaction:
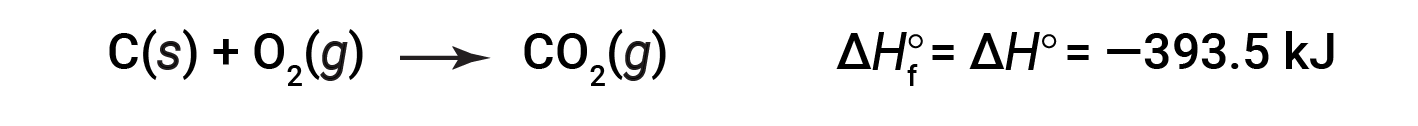
starting with the reactants at a pressure of 1 atm and 25 °C (with the carbon present as graphite, the most stable form of carbon under these conditions) and ending with one mole of CO2, also at 1 atm and 25 °C. For nitrogen dioxide, NO2 (g), ΔHf° is 33.2 kJ/mol. This is the enthalpy change for the endothermic reaction:

A reaction equation with 1/2 mole of N2 and 1 mole of O2 is correct in this case because the standard enthalpy of formation always refers to 1 mole of product: NO2 (g).
By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions. For example, the standard enthalpies of formation of carbon (graphene), diatomic oxygen gas, diatomic nitrogen gas, sodium metal, and liquid mercury are zero under standard conditions.
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
