Analysis of Physiologic E-Selectin-Mediated Leukocyte Rolling on Microvascular Endothelium
Summary
This report provides a visual depiction of parallel-plate flow chamber analysis for studying leukocyte endothelial interactions under physiologic shear stress. This method is particularly useful for investigating the role of endothelial (E)-selectin and leukocyte E-selectin ligands that trigger leukocyte rolling on endothelial cell surfaces.
Abstract
Protocol
1. Preparation of hBMEC (HBMEC-60 Cell) Monolayer on Petri Dishes for Flow Chamber
- Coat 35 x 10mm tissue-culture dishes with 2ml of 20µg/ml fibronectin (in PBS) overnight at 4°C or for 3 hours at 37°C.
- Aspirate fibronectin solution from dishes and add 1.5 x105 HBMEC-60 cells [Medium 199 with HEPES & Glutamine,10% FBS,10% Human Serum, 5 units/ml heparin, 1ng/ml recombinant human fibroblast growth factor,1%Penicillin-Streptomycin] to the dish and allow them to grow to >90% confluence. (This takes 2 days)
- Aspirate growth media and, to up-regulate E-selectin expression, add fresh medium with 50ng/mL IL-1β for 4-6 hrs. Cell monolayers are now ready for rolling assay. To block E-selectin function, neutralizing anti-human E-selectin moAb (clone BBIG-E4(5D11)) can be added at 20µg/ml for ~1hr at 37°C.
2. Preparation of Human Hematopoietic Progenitor KG1a Cells for Flow Chamber
- Human hematopoietic progenitor KG1a cells are grown to confluency (1×106 cells/ml) in RPMI-1640 with glutamine and 10% FBS/1% penicillin-streptomycin and are pipetted directly from the flask for use in flow chamber assay.
- Cell number is calculated using a hemacytometer and then cells are re- suspended at 1 x 106 cells/mL in HBSS with 10mM HEPES (H/H Buffer) and 2mM CaCl2
- Cells are stored on ice until use in assay.
3. Preparation of Microscope, Parallel-Plate Flow Chamber and Syringe Pump (Figure 1)
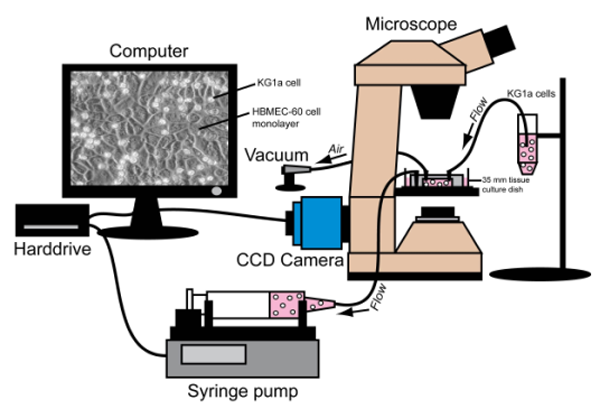
Figure 1.Illustration of Parallel-Plate Flow Chamber Analysis. Inverted microscope, Harvard Syringe Pump, computer/camera and parallel-plate flow chamber are placed in an optimal arrangement for efficient and reproducible cell analysis.
- Turn on power and light source to inverted microscope and power to Syringe Pump and select the 10X Objective on the microscope.
- Place 50mL conical tube in holder and fill with H/H and 2mM CaCl2.
- Place 5ml plastic test tubes in test tube holder below the 50mL conical tube
- Place 60mL syringe in Syringe Pump (Be sure that both the plunger and body of syringe are both secured).
- Attach 3-way stop-cock to end of 60mL syringe and attach 5mL syringe to 3-way stop-cock.
- Place Parallel Flow Chamber apparatus (GlycoTech, Inc.) on microscope stage with input tube to the right, output tube to the left and vacuum tube to the back.
- Attach vacuum tubing to vacuum and open air valve to allow parallel plate apparatus to adhere to the stage. (This tests if the vacuum skirt on the flow chamber is air-tight.) Turn off air valve.
- Attach output tubing line (on left of microscope) to 3-way stop-cock. Apparatus is now prepared for placing on HBMEC-60 monolayer plate.
- Place HBMEC-60 monolayer plate and place on center of microscope.
- Turn on vacuum and position the flow chamber apparatus above the plate such that the monolayer plate is in the center.
- Gently lower the flow chamber apparatus onto the HBMEC-60 monolayer plate and allow flow chamber apparatus to suction down. (There should be no sounds of air escaping and at this point you may need to apply pressure to chamber to compress the rubber gasket.)
- Once vacuum has been established, place input tube (on the right) into the 50mL conical tube containing H/H with 2mM CaCl2.
- Open 3-way stop-cock to allow flow into the 60mL syringe and place pump in “pump mode.”
- To remove air from input/output lines without lifting the cells on the plate, use the Syringe Pump set at 2mL/minute to fill the intake tube, then quickly set to 0.5 mL/minute just before the fluid reaches the apparatus. It is imperative to prime this set up carefully and avoid air bubbles which will lift the monolayer from the plate.
- Once fluid is seen to move into the 60ml syringe, the flow chamber and Syringe Pump have been primed and the Flow chamber is ready for use. This set up will be repeated for each cell input run and for each new plate needed.
4. Capturing Leukocyte Rolling Events using NIS Elements
- Open NIS Elements on desktop.
- Press “play” icon for live image. Auto exposure will allow you to see the plate easier for now and is important for focusing.
- Be sure that microscope is focused properly on the cell monolayer.
- Once focused, re-set exposure to 1 frame/ second and adjust light on the microscope. The image should appear on the computer and no longer be visible in the microscope viewfinder due to the low light level. Light histogram can then be adjusted to remove background exposure or improve cell clarity.
- Click on 2X2 bin or Live Bin to obtain picture that is ideal for movie capture
- Click on “Macro” on menu bar and select “write to port” ; select port “com3,” and “open”. (This opens the port to coordinate with the Syringe Pump.
- Pipette 1mL of KG1a cell suspension into the 5ml test tube near the input tubing line and then place input tubing line to the bottom of the test tube.
- Go to menu bar, click on “Capture” ; “time-lapse acquisition.”
- A new menu will pop up. Select the protocol lasting 210 seconds, this is the length of the program for rolling on HBMEC-60 Cells. (Each phase is programmed to take a picture every second for the duration of the pump protocol. It is also set up to send a command through the com3 port to start the pump.) If time-lapse is interrupted, the pump will not stop on its own and must be reset. Press Cancel to return to live image.
- Set the Syringe Pump to “Program Mode” according to instruction manual. (Program mode allows for manual programming of specific flow rates (i.e. shear stress levels) to be imparted within the chamber and dictate KG1a cell interactions over the HBMEC-60 cell monolayer.) Settings for KG1a cell rolling on hBMEC Cells were as follows 4.2 dynes/cm2 for 45 s, 0.3 dynes/cm2 for 60 s, and stepwise increases every 15 s to a maximum of 4.2 dynes/cm2
- Wall shear stress (Wst) in the chamber was calculated according to the following equation: Wst = 6µQ/a2b, where μ is the estimated viscosity of the media (0.0076 P), Q is the volumetric flow rate in ml/s, a is the channel height (gasket thickness) in cm (0.03 cm), and b is the channel width in cm (0.5 cm). Flow rates were regulated using the program mode of the Syringe Pump.
- Microscope and computer are now ready to capture time-lapse photography. Press “start run” on the computer to begin acquiring data. While running the program, be sure to add media to the test tube containing KG1a cells in order to keep the input/output tubing full of medium (Make sure there are no air bubbles).
5. Dependence of Terminal Sialylation and Surface Glycoprotein for E-selectin-Mediated KG1a Cell 5. Rolling (Figure 2; Videos Clips of 5.1-5.5)
- Assaying of KG1a cell rolling on non-stimulated HBMEC-60 cells. (Negative control) KG1a cells were prepared and infused into the flow chamber over live non-IL-1β-stimulated HBMEC-60 as described above.
- Assaying of KG1a cell rolling on HBMEC-60 cells pre-treated with IL-1β. (E-selectin-dependence control) KG1a cells were prepared and infused into the flow chamber over live IL-1β-stimulated HBMEC-60 as described above.
- Assaying of KG1a cell rolling on HBMEC-60 cells pre-treated with IL-1β and then neutralizing anti-human E-selectin moAb. (E-selectin-dependence control) KG1a cells were prepared and infused into the flow chamber over live IL-1β- stimulated HBMEC-60 pretreated with neutralizing anti-human E-selectin moAb as described above.
- Assaying of sialidase-digested KG1a cell rolling on IL-1β-stimulated HBMEC-60. KG1a cells pretreated with 0.1U/ml Vibrio Cholerae neuraminidase for 1hr at 37°C were prepared and infused into the flow chamber over live IL-1β-stimulated HBMEC- 60 as described above.
- Assaying of protease-digested KG1a cell rolling on IL-1β-stimulated HBMEC-60. KG1a cells pretreated with protease, 0.2U/ml Bromelain for 1hr at 37°C, were prepared and infused into the flow chamber over live IL-1β-stimulated HBMEC-60 as described above.
6. Analysis of NIS-Elements-Captured KG1a Cells and Graphing of KG1a Cell Rolling Data
- After time sequence is acquired, save the data.
- Go to “measure” tab on menu bar and select “define threshold.” Move bars so that desired cells are colored in red. Select “all images” then “Okay”. The entire time-lapse sequence should now be modified with visible red rolling cells.
- Go to “Measure” and select “restrictions.” (Restrictions allow the user to exclude thresholded objects that do not represent rolling cells. This is primarily background created by the HBMEC-60 monolayer. ) Select “area” and input min and max values for the area for rolling cells. Repeat this step for “elongation” and “circularity.”
- Select “measure” and select “create binary using restrictions”, and set the restriction values to optimize NIS-Elements software recognition of rolling cells on monolayer of HBMEC-60 cells.
- Go back to “measure”, select “Field data”, and then select “reset data”. Close this menu.
- Return to “Measure” and select “scan field”.
- Open “Measure”; “Field data”, select “number or Objects” and then select “all fields” and export data to Microsoft Excel.
- Select “clear data” to prepare NIS-Elements cell acquisition for next data collection.
Results:
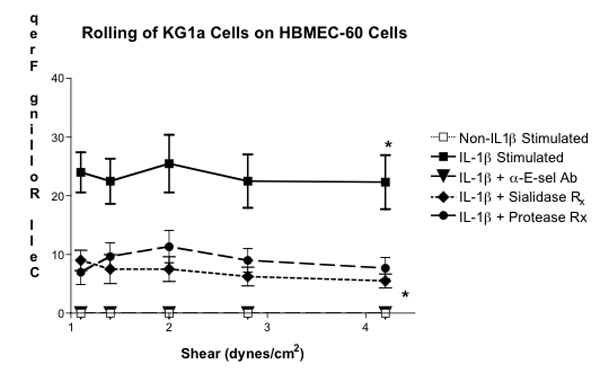
Figure 3. Parallel-Plate Flow Chamber Analysis of E-selectin-Binding Activity on KG1a Cells. KG1a cells treated with sialidase or protease were analyzed for rolling efficiency on monolayers of HBMEC-60 cells pre-treated with IL-1β. Negative control rolling experiments were also conducted by analyzing KG1a cell rolling on non-IL-1β-stimulated HBMEC -60 cells or on IL-1β-stimulated HBMEC-60 cells treated with neutralizing anti-human E-selectin moAb (5D11 ). Cell rolling frequencies were performed in triplicate and analyzed with NIS-Elements software. (* Statistical significance; p<0.001)
In this video-based report, we provided a visual depiction of how to analyze leukocyte – endothelial cell interactions under physiologic shear stress conditions. In particular, we analyzed the role of E-selectin – E-selectin ligands in mediating leukocyte tethering and rolling, an adhesive behavior necessary for commitment of secondary more stable binding activities through receptors, such as integrins and hyaluronan receptors. Using the parallel-plate flow chamber adapted for use with an inverted microscope, Harvard Syringe Pump, video camera and computer/cell acquisition hardware, we conducted experiments wherein E-selectin ligand+ KG1a cells were used as our leukocyte model and hBMEC cells (HBMEC-60) stimulated with pro-inflammatory cytokine, IL-1β, were used as our E-selectin+ human endothelial cell model (1-6). As previously reported, we observed robust KG1a cell rolling on IL-1β-stimulated HBMEC-60 cells over a range of shear stress levels in an E-selectin-dependent manner (Figure 3)(Statistically significant difference when compared with KG1a cell rolling on non-IL-1β stimulated HBMEC-60 cells). Negative controls, which consisted of KG1a cell rolling on non-IL-1β-stimulated HBMEC-60 cells or on IL-1β-stimulated HBEMC-60 cells pretreated with anti-human E-selectin mAb, were performed in parallel to demonstrate the dependence of cytokine stimulation and E-selectin expression for rolling activity (Figure 3). Furthermore, rolling analysis of KG1a cells pretreated with Vibrio cholerae neuraminidase (sialidase) or with bromelain, a non-specific protease known for digesting surface E-selectin glycoprotein ligand, highlighted the importance of terminal sialic acid residues and surface glycoprotein for E-selectin ligand activity (2,3).
Discussion
Parallel-plate flow chamber analysis is a specialized in vitro assay system used for studying leukocyte – endothelial adhesive interactions under shear stress conditions similar those imparted on the surface of a post-capillary venule. As depicted here in our visual presentation, we demonstrate the value of this system for investigating the role of E-selectin – E-selectin ligands in mediating leukocyte tethering and rolling on the surface of activated microvascular endothelium. We also reveal the application of protease, glycosidase and blocking antibody treatments for elucidating the role of E-selectin and its ligands in this system. These analyses are highly reproducible and help form an experimental basis for studying E-selectin – E-selectin ligand function in vivo.
In addition to studying cell adhesion with monolayers of cytokine-activated microvascular endothelial cells (hBMEC or human umbilical vein or dermal microvascular endothelial cells), assays can be performed using purified recombinant human E-selectin or E-selectin-Ig chimeric molecules as a substrate for cell adhesion. Other adhesive interactions mediated through leukocyte (L-)-selectin and platelet (P)-selectin can also be assayed using parallel-plate flow chamber technology. By varying the type of cell monolayer/protein substrate or of input leukocyte/selectin-transfectant cell models, investigators can study the role of these interactions under physiologic shear stress conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding for this project was provided by an American Cancer Society Research Scholar Award, (06-024-01-CSM to Charles J. Dimitroff); a National Institutes of Health/National Cancer Institute grant (RO1CA118124 to Charles J. Dimitroff); and a National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant (P30 AR042689 to Dr. Thomas S. Kupper; Brigham and Women’s Hospital, Boston, MA).
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| FBS | SIGMA | F6178-500ML | ||
| DPBS | GIBCO | 14190 | ||
| 0.5 M EDTA | GIBCO | 15575-038 | ||
| 1M HEPES | GIBCO | 15630 | ||
| Parallel-Plate Flow Chamber Kit | GlycoTech | 31-001 | ||
| PHD2000 Programmable Syringe Pump | Harvard Apparatus | PHD 2000 | ||
| 35x10mm Tissue Culture Dish | BD FALCON | 353001 | ||
| Fibronectin , from human plasma | SIGMA | 86088-83-7 F2006-2MG | ||
| Interleukin-1_, Human; Recombinant | SIGMA | I9401-5UG | ||
| HBSS (Hank s Buffered Saline Solution) | GIBCO | 14170 | ||
| Medium 199 w/ HEPES & Glutamine | Invitrogen | 12320-039 | ||
| Human Serum | Innovative Research, Inc. | IPLA-SER1 | ||
| Heparin | Abraxis Pharmaceutical Products | 504011 | ||
| Recombinant human fibroblast growth factor | R&D Systems | 233-FB | ||
| Penicillin Streptomycin | GIBCO | 15140 | ||
| Plastic Test tubes | BD Bioscience | 352008 | ||
| Anti-human E-selectin clone BBIG-E4(5D11) | R&D Systems | BBA16 | ||
| Bromelain | SIGMA | B-4882 |
References
- Dimitroff, C. J., Kupper, T. S., Sackstein, R. Prevention of leukocytic migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J. Clin. Invest. 112, 1008-1018 .
- Dimitroff, C. J., Lechpammer, M., Long-Woodward, D., Kutok, J. L. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 64, 5261-5269 (2004).
- Dimitroff, C. J., Descheny, L., Trujillo, N., Kim, R., Nguyen, V., Huang, W., Pienta, K. J., Kutok, J. L., Rubin, M. A. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 65, 5750-5760 (2005).
- Descheny, L., Gainers, M. E., Walcheck, B., Dimitroff, C. J. Ameliorating Skin-Homing Receptors on Malignant T cells with a Fluorosugar Analog of N-acetylglucosamine: P-selectin Ligand is a More Sensitive Target than E-selectin Ligand. J Invest Dermatol. 126 (9), 2065-2073 (2006).
- Gainers, M. E., Descheny, L., Barthel, S. B., Liu, L., Wurbel, M., Dimitroff, C. J. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J. Immunology. 179 (12), 8509-8518 (2007).
- Rood, P. M., Calafat, J., von dem Borne, A. E., Gerritsen, W. R., van der Schoot, C. E. Immortalization of human bone marrow endothelial cells: characterisation of new cell lines. Eur J Clin Invest. 30 (7), 618-629 (2000).

