Evaluation of the Effect of Growth Factors on Chlorophylls a and b Production from Microalgae
Summary
The present study highlights the advantages of employing the method developed by Jeffrey and Humphrey for extracting and quantifying fat-soluble pigments from microalgae. This method serves as a valuable tool for assessing the influence of growth factors on chlorophyll production and cellular content in these organisms.
Abstract
Microalgae contain two main groups of pigments: chlorophylls and carotenoids. Chlorophyll is a green pigment that absorbs light energy and transforms it into chemical energy to facilitate the synthesis of organic compounds. This pigment serves as a valuable primary source for biotechnological input products in the food, pharmaceutical, and cosmetic industries due to its high antioxidant properties and coloring capabilities. The objective of this research was to evaluate the effect of growth factors (CO2 concentration, light color, and light intensity) through a Taguchi L4 experimental design on cell growth and the cellular content of chlorophyll a and b in Chlorella sorokiniana, followed by validation of the method using Haematococcus pluvialis microalgae as an additional study model. Cell growth was quantified using the optical density spectrophotometric technique at a wavelength of 550 nm. For the quantification of chlorophylls, a cell extract was obtained using a 90% pure acetone solution, and subsequently, the concentrations of chlorophylls a and b were quantified using spectrophotometric techniques at wavelengths of 647 nm and 664 nm, according to the method described by Jeffrey and Humphrey. The experimental results indicated that controlling conditions of low CO2 addition, purple light, and low light intensity increases both cell growth and the concentration of chlorophylls a and b within the cells. The implementation of this chlorophyll quantification method allows for quick, simple, and precise determination of chlorophyll content, as the wavelengths used are at the absorbance peaks of both types of chlorophylls, making this technique easily reproducible for any microalgae under study.
Introduction
In recent years, the growing environmental problems caused by anthropogenic activities and their adverse effects on the health and balance of ecosystems have driven the search for more efficient and environmentally friendly production systems. This has accelerated processes in industries and fostered the implementation of bioremediation treatments and the development of bio compounds to mitigate these harmful effects1.
This context has led to a significant growth in the study of microalgae, driven by the need to find innovative solutions to current environmental and economic challenges. Microalgae thrive in aquatic environments, using sunlight and carbon dioxide as sources of energy and carbon, respectively. This characteristic makes them a sustainable and promising alternative for producing various valuable products. Research in this field has focused on understanding the physiology and metabolism of these cells, as well as developing efficient technologies for their cultivation and processing2.
There is a clear need to develop accessible and reliable tools for studying microalgae to accelerate research processes and deepen the understanding of their physiology, metabolism, and potential applications. These tools should enable rapid analysis of the effect of environmental and production factors on key parameters, such as chlorophyll concentration, which is a fundamental indicator of the health and development of these organisms. A decrease in chlorophyll content may indicate environmental stress, nutritional deficiencies, or diseases.
These green pigments play a crucial role in photosynthesis, capturing sunlight energy to convert carbon dioxide and water into glucose and oxygen and constitute between 0.5% and 5% of the biomass of microalgae3. Beyond their essential role in sustaining life processes, chlorophylls have found applications in various industries. Chlorophyll extracts are utilized in food and beverage processing as natural colorants, imparting vibrant green hues to products while also providing antioxidant properties. Moreover, chlorophyll-based supplements are gaining popularity in the health and wellness sector due to their purported detoxifying and anti-inflammatory effects. By harnessing the multifaceted properties of chlorophyll, industries can develop innovative products that contribute to both visual appeal and consumer well-being1.
In this regard, one microalgae of great biotechnological relevance is C. sorokiniana. This microorganism stands out for its rapid growth rate, making it highly efficient in biomass production. Additionally, C. sorokiniana exhibits a diverse content of highly nutritional compounds, including proteins, lipids, and vitamins, which renders it valuable for various applications in food, feed, and biofuel production2. Moreover, this microalgae species has been found to produce extracellular enzymes with diverse functions, opening up possibilities for biotechnological applications such as wastewater treatment, bioremediation, and pharmaceuticals4. In addition to its rapid growth and versatile applications, C. sorokiniana also demonstrates significant potential for chlorophyll production. As a photosynthetic microorganism, C. sorokiniana possesses the machinery necessary for the synthesis of chlorophyll, which constitutes between 0.5% and 5% of the biomass of microalgae3. This capability makes C. sorokiniana an attractive candidate for commercial-scale chlorophyll production and promises to be a sustainable solution to urgent environmental and nutritional challenges5.
On the other hand, another microalgae species of significant interest is H. pluvialis. This microalga is renowned for its production of astaxanthin, a potent antioxidant pigment with numerous industrial applications. Astaxanthin serves as a protective mechanism for H. pluvialis photosystem, shielding it from oxidative stress induced by environmental factors. This pigment is highly sought after in cosmetics, nutraceuticals, and aquaculture industries, owing to its antioxidant properties and potential health benefits. With its abundant ability to produce astaxanthin, H. pluvialis presents a promising avenue for developing innovative products catering to various industrial and consumer needs6.
Recent studies have also highlighted H. pluvialis1 potential for chlorophyll production, further enhancing its industrial significance. For instance, a study investigated the chlorophyll content of H. pluvialis under varying growth conditions and found that under optimal cultivation parameters, H. pluvialis exhibited remarkable chlorophyll production rates, surpassing those of other microalgae species7. This finding underscores the potential of H. pluvialis as a sustainable source of chlorophyll, offering novel opportunities for its utilization in various industrial sectors.
Protocol
1. Culture media preparation and inoculum preparation
- Prepare 1 L of 3N-BBM+ V(CCAP) growth medium (Macronutrients: NaNO3 [0.75 g L-1], CaCl2 [0.019 g L-1], MgSO4 [0.019 g L-1], K2HPO4 [0.057 g L-1], NaCl [0.025 g L-1], KH2PO4 [0.175 g L-1]; micronutrients: Na2 EDTA [0.0186 g L-1], FeCl3 [0.0024 g L-1], MnCl2 [0.001 g L-1], ZnCl2 [1.25 x 10-4 g L-1], CoCl2 [5 x 10-5 g L-1], Na2MoO4 [9.98 x 10-5 g L-1])8 and sterilize it for 15 min at 121 °C and 1.5 psi.
- Inoculate a flask with 1 L of growth medium at an approximate concentration of 3 x 106 cells mL-1 for the microalga C. sorokiniana; or add 5% to 10% (v/v) of inoculum to the growth medium.
NOTE: To maintain sterile conditions, it is recommended to use a laminar flow hood, gloves, lab coat, and face mask. - Incubate the inoculated flask under red light sources with an approximate intensity of 3,600 to 4,200 lux, at a temperature between 22-26 °C, with a photoperiod of 12 h light and 12 h darkness for C. sorokiniana and photoperiod of 16 h light and 8 h darkness for H. pluvialis, and manually agitate every 24 h, approximately for 7 days for both strains.
NOTE: Maintain under growth conditions for at least 7-10 days (until obtaining an intense green color) for C. sorokiniana and 10-12 days for H. pluvialis. To obtain the microalgae concentrate that will serve as the inoculum in the kinetics, it is necessary to remove the supernatant medium from the produced algae. - In a sterile environment, fill sterile 15 mL conical centrifugation tubes with approximately 10 mL of inoculated medium and centrifuge at 3,000 x g for 20 min under 25-30 °C.
- Under sterile conditions, remove the supernatant using a micropipette or by pouring and concentrating the biomass in a sterile 50 mL conical centrifugation tube.
- Repeat this process as necessary until the required volume of inoculum for the kinetics is obtained.
- Measure the optical density (OD550 nm) of the inoculum to determine the cell concentration of the microalgae and store it at a temperature of 3-4 °C until ready for use.
NOTE: Direct cellular counting was initially performed to correlate cell concentration with absorbance. Once counted, the inoculum can be stored under refrigeration for no more than 4 days.
2. Study of the growth kinetics
- Prepare the required volume of 3N-BBM+ V(CCAP) suitable for each experiment (8 L for photobioreactor level tests for C. sorokiniana and 0.5 L for the flask-level tests for H. pluvialis) according to standard laboratory procedures, ensuring proper sterilization.
- For both photobioreactor and flasks, configure the light source to the desired color spectrum (Blue at 460 nm, purple, mix of 460 nm and 630 nm for C. sorokiniana and red at 630 nm for H. pluvialis); cover both systems to avoid external light interference.
NOTE: Due to the variation in wavelength emitted from each light-emitting diode, spectroradiometry was performed using a spectroradiometer on the light sources to determine the wavelength and emission power of each diode9. Purple light was achieved by combining red and blue LEDs, resulting in wavelengths of 460 nm with an emission power of 1.65 W nm-1 for the blue LEDs and 630 nm with an emission power of 0.6 W nm-1 for the red LEDs. These measurements were used for the tests conducted solely under blue and red light. - Program the timer to alternate between 12 h light and 12 h dark cycles for C. sorokiniana and 16 h light and 8 h dark cycles for H. pluvialis.
- Connect the CO2 aeration system to the photobioreactor culture vessel to provide carbon dioxide supply in intervals of 12 h for C. sorokiniana.
- Inoculate the culture vessel with a sufficient volume of pre-inoculum to achieve a starting cell density of 3 x 105 cells mL-1 or its equivalent in optical density (OD550 nm) = 0.180.
NOTE: Ensure aseptic technique during inoculation to prevent contamination. - Sample the culture at regular intervals of 12 h for C. sorokiniana and 8 h for H. pluvialis to monitor cell growth and physiological parameters. Collect samples using sterile techniques to maintain the integrity of the culture.
3. Quantification of chlorophylls
NOTE: Data for cellular quantification is provided in Supplementary File 1.
- Place 40 mL of the sample into plastic conical centrifugation tubes.
- Centrifuge at 3,000 x g for 20 min at 15 °C and discard the supernatant by decantation or using a Pasteur pipette.
- Transfer the cell pellet into a glass tube covered with aluminum foil and a screw cap to prevent oxidation.
- Resuspend the cell pellet with 3 mL of 90% pure acetone solution using a vortex.
- Sonicate the suspended sample in an ice bath for two cycles of 5 min each and let it rest at 4 °C for 16 h10.
- After 16 h, sonicate again under the same conditions mentioned in the previous step (step 3.5) and centrifuge again under the previously described conditions (step 3.2).
- Separate the pigment extract using a Pasteur pipette and transfer it to another clean tube protected from light.
- Place the pigment extract in a quartz cell to read in a spectrophotometer at 630 nm, 647 nm, and 664 nm wavelengths.
NOTE: Calibrate the spectrophotometer beforehand with 90% acetone. - To calculate chlorophyll concentrations, use the following equations11:
Chlorophyll a = 11.93 A664 – 1.93 A647
Chlorophyll b = 20.36 A647 – 5.50 A664
NOTE: Results are expressed in µg mL-1 of extract, which are multiplied by the extract volume and divided by the number of mL of sample to obtain chlorophyll concentrations in µg mL-1 of culture. - To obtain values in µg chl cell-1, use the following formula:
μg chl cell-1 = µg chl in 1 mL of culture / [cells mL-1] cells concentration in the culture.
Representative Results
To observe the efficiency of the technique detecting variations in chlorophyll cellular concentration and evaluate the effect of growth factors in C. sorokiniana, a Taguchi L4 experimental design was established, evaluating CO2 volume addition, light color, and light intensity. Each factor was assessed at low and high levels, as shown in Table 1, under the conditions defined by the experimental design in Table 2.
Once the experimental process was concluded, the results showed that the combination of low addition of CO2, purple light, and high light intensity (C1, P, I2) corresponding to treatment 2, increased microbial growth to 7.83 x 105 cells mL-1. On the other hand, chlorophyll content was higher in treatment 4 under conditions C2, P, I2, reaching a concentration of 5.92 x 10-7 µg cel-1 at 132 h (Figure 1) coinciding with purple light and high light intensity as the optimal levels of the factors in both experiences.
At the same time, a similar behavior was observed across the four evaluated lines. However, a clear favorability was observed in the tests conducted under purple light. This pattern suggests that purple light may positively impact the outcomes, potentially promoting optimal conditions for cellular growth or activity. These findings underscore the importance of carefully considering lighting conditions in biological experiments.
From the statistical analysis, the main effects plot was obtained. In Figure 2, it can be observed how each level of the evaluated factors influences chlorophyll production in C. sorokiniana. The best conditions for chlorophyll production were high addition of CO2, purple light, and high light intensity (C2, P, I2), where color and carbon dioxide concentration show very close significance values, meanwhile light intensity shows a lower significant effect on the evaluated response variables.
In addition, a contrast diagram was generated (Figure 3), showing the behavior of cellular chlorophyll concentration according to the effect of CO2 volume addition and the light color to which they were subjected.
It was observed that purple light (a mixture of blue and red light) and a high light intensity significantly promote microbial growth and chlorophyll production. Meanwhile, a high addition of CO2 influences both parameters positively and negatively, respectively, as it increases chlorophyll cellular concentration to a maximum during the experimentation but hinders microbial growth. However, it is noticeable that under purple light, a CO2 volume addition of 20 s pulses of CO2 every 12 h at a flow rate of 8.5 ft³ h-1 allows a favorable response for both parameters.
To validate the method and results obtained from C. sorokiniana, a confirmatory flask-level test was conducted on H. pluvialis under red light due to its natural characteristic of degrading chlorophyll to synthesize astaxanthin under stress conditions, which were induced at 168 h by increasing the salinity of the medium. A concentration graph was generated (Figure 4) where the maximum chlorophyll concentration was obtained at 0 h with 1.41 x 10-7 µg cel-1. In this case, due to the natural microalgae behavior, a decrease in chlorophyll concentration was observed, suggesting potential physiological changes in the biological model under investigation. This is further illustrated in Figure 5, which shows a micrograph of C. sorokiniana (Figure 5A) and two micrographs of H. pluvialis: one in the green phase (Figure 5B) and one in the phase of change and astaxanthin production (Figure 5C). Interestingly, despite this decline, no interference occurred from the presence of carotenoid compounds, which are, in turn, fat-soluble pigments present in them; this is strong evidence of the selectivity and reproducibility of the technique in microalgae with diverse capacities and behaviors.
The graph depicts a decrease in total chlorophyll concentration throughout the experiment. Notably, there is an increase in chlorophyll a compared to chlorophyll b at 50 h, where the concentration of chlorophyll b decreases significantly. The most substantial decline in chlorophyll concentration occurs at the end of the exponential growth phase. This decrease is attributed to the production of carotenoids in H. pluvialis.
Similarly, the analysis of chlorophyll a and b composition was conducted for the treatment with the highest production of C. sorokiniana (C1, P, I2) in Figure 6. Contrary to H. pluvialis, the production of chlorophylls displayed a consistent increase throughout the entire experimentation period, which was solely analyzed during the exponential phase. It is observed that similar to H. pluvialis, chlorophyll a is initially present in lower proportions than chlorophyll b at the beginning of the experiment; however, these proportions undergo a notable shift at the 50 h mark, with chlorophyll a showing a higher production rate than chlorophyll b thereafter.
The analytical technique employed in this study has proven to be efficient in detecting changes within a biological model and across different biological models. These findings underscore the sensitivity and precision of the analytical method, allowing for a nuanced understanding of chlorophyll dynamics within and between biological systems. Such insights are invaluable for unraveling the intricate mechanisms governing pigment synthesis and metabolism, paving the way for further advancements in biotechnological and environmental research.
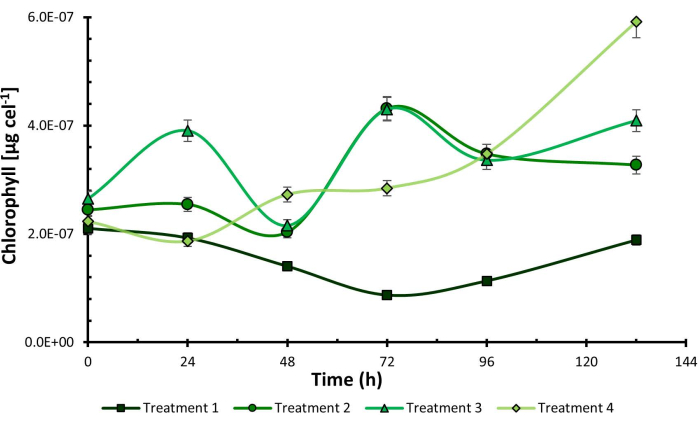
Figure 1: Chlorophyll cellular content under experimental design treatments. Chlorophyll cellular content measured in Chlorella sorokiniana across different experimental treatments. Data presented as standard deviation for n = 3. Please click here to view a larger version of this figure.
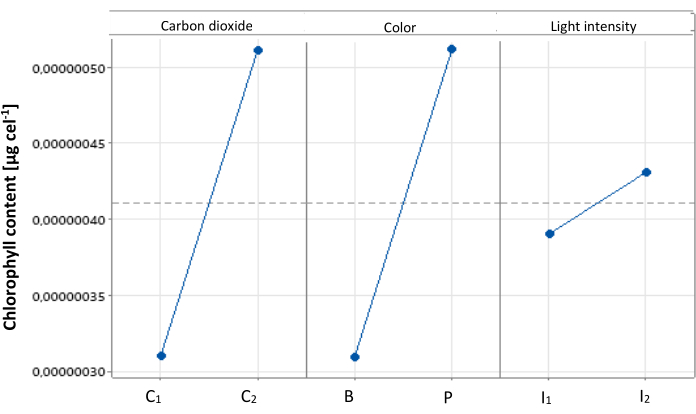
Figure 2: Main effects plot for cellular chlorophyll content. Main effects plot illustrating the impact of different treatments on cellular chlorophyll content in Chlorella sorokiniana. Data presented as standard deviation for n = 3. Please click here to view a larger version of this figure.
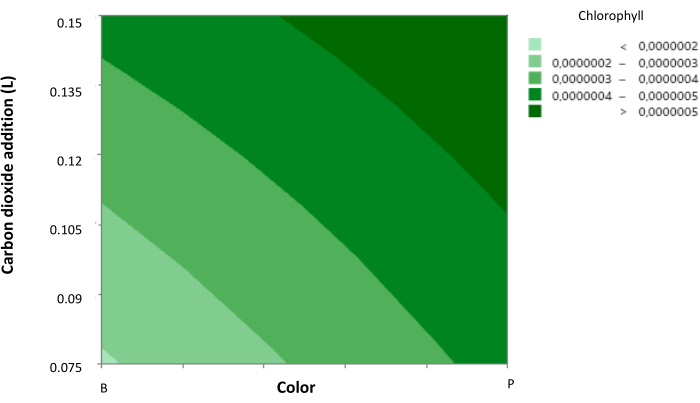
Figure 3: Contour plot of cellular chlorophyll concentration. Contour plot showing the relationship between cellular chlorophyll concentration, CO2 concentration, and light color. Light color is represented with axis 1 as blue and axis 2 as purple, with intermediate levels indicating the addition of red light. Data presented as standard deviation for n = 3. Please click here to view a larger version of this figure.
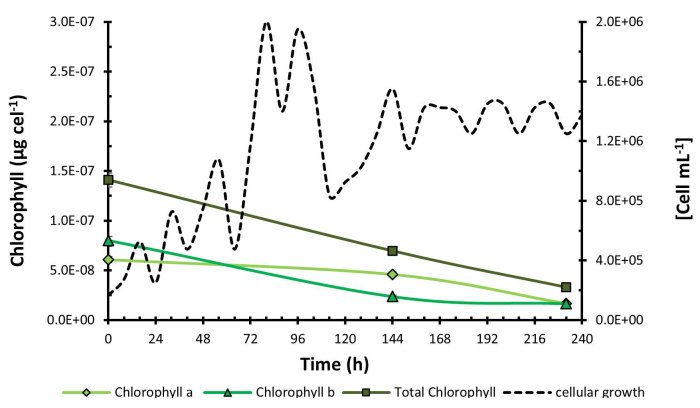
Figure 4: Chlorophyll concentration during H. pluvialis growth kinetics. Chlorophyll concentration measured over the growth kinetics of Haematococcus pluvialis. Data presented as standard deviation for n = 3. Please click here to view a larger version of this figure.
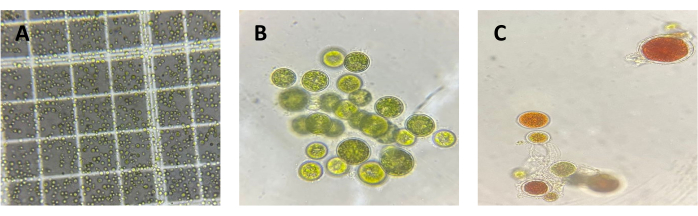
Figure 5: Micrographs of C. sorokiniana and H. pluvialis. Micrograph images of Chlorella sorokiniana (A) and Haematococcus pluvialis, illustrating one specimen in the green phase (B) and another in the early red phase (C). Please click here to view a larger version of this figure.
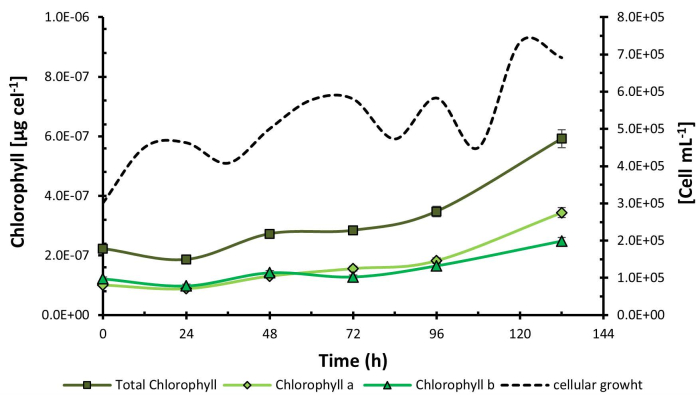
Figure 6: Chlorophyll concentration during C. sorokiniana growth kinetics. Chlorophyll concentration measured over the growth kinetics of Chlorella sorokiniana. Data presented as standard deviation for n = 3. Please click here to view a larger version of this figure.
| Factor | Low level | High level |
| CO2 addition (L) | C1 | C2 |
| Light color | Blue (B) | Purple (P) |
| Light intensity (Lux) | I1 | I2 |
Table 1: Kinetics factors and levels. Overview of kinetics factors and their respective levels: C1 = 0.078 L; C2 = 0.15 L; B = Blue light; P = Purple light; I1 = 550 lux; I2 = 1200 lux.
| Treatment | CO2 addition | Light color | Light intensity (Lux) |
| (L) | |||
| 1 | C1 | B | I1 |
| 2 | C1 | P | I2 |
| 3 | C2 | B | I1 |
| 4 | C2 | P | I2 |
Table 2: Experimental design and treatments. Summary of the experimental design and treatments applied: C1 = 0.078 L; C2 = 0.15 L; B = Blue light; P = Purple light; I1 = 550 lux; I2 = 1200 lux.
Supplementary File 1: Cellular quantification data. Please click here to download this File.
Discussion
The comparative study between H. pluvialis and C. sorokiniana revealed significant differences in chlorophyll production dynamics. While H. pluvialis exhibited a decrease in chlorophyll concentration throughout the experiment, C. sorokiniana showed a steady increase. Additionally, there was initially a lower proportion of chlorophyll a in both species, but this ratio reversed in particular growth conditions, which may give indications of an induction of the production of said chlorophylls through the selectivity or exposure of specific wavelengths. These findings suggest a unique response of each species to the experimental conditions, highlighting the sensitivity of the analytical technique used to detect changes within and between biological models. The ability to discern subtle changes in the proportions of chlorophyll a and b provides new insights into the regulation of pigment synthesis in microalgae, which may have significant implications in fields such as biotechnology and environmental research.
Detecting periods of increased chlorophyll production is crucial due to its role as the primary pigment in the photosynthesis process. Chlorophyll a is essential for capturing light energy and converting it into chemical energy, which is then used to drive cellular growth and metabolism. Therefore, an increase in chlorophyll a production indicates an enhancement in the photosynthetic capacity of organisms, suggesting optimal conditions for microbial growth12; this supports the observation in C. sorokiniana that the tests that generated a higher concentration of chlorophyll were those in which greater cell growth was obtained, providing valuable insights into when environmental conditions are most favorable for the development and proliferation of photosynthetic microorganisms.
In particular, understanding the proportions of chlorophyll in H. pluvialis is especially intriguing as they can provide important signals regarding the microalgae stress status. When conditions become unfavorable, such as under high light or nutrient deprivation, the microalgae may reduce chlorophyll production and instead synthesize carotenoid pigments like astaxanthin11. This transition from green growth phases, characterized by high chlorophyll concentrations, to astaxanthin-rich stress-induced phases is beneficial for maximizing astaxanthin yield. It underscores the importance of monitoring chlorophyll levels as a proxy for stress response and pigment production dynamics in H. pluvialis cultivation.
Furthermore, while the technique’s limitations are few due to its high selectivity, precautions such as keeping extracts away from light to prevent oxidation and handling centrifuged samples carefully to eliminate turbidity from biomass residues are essential. Additionally, a drawback of the method is the requirement for relatively large sample volumes, especially for diluted media or long-term kinetic studies at the flask level, which can pose logistical challenges. These considerations highlight the importance of careful experimental design and sample handling to maximize the effectiveness of chlorophyll analysis techniques in microalgae research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge the partial funding from the TecNM under the Call for Scientific Research, Technological Development, and Innovation (16898.23-P) for the Institutos Tecnologicos Federales. They also appreciate the support from the Instituto de Ciencia, Tecnología e Innovación del Estado de Michoacán de Ocampo (FCCHTI23_ME-4.1.-0001).
Materials
| C3H6O | Meyer | 67-64-1 | Acetone 90% |
| 15 mL tube | Biologix | 10-9502 | Test tube |
| 2510-DTH | Branson | D-73595 | Sonicator |
| 5 mL screw cap test tube | Kimax | 45066-13100 | Test tube |
| 50 mL centrifuge tube | Biologix | 10-9151 | Test tube |
| Aluminum foil | Reynolds | 611 standard, 12" x 1000 feet | Test tube cover |
| CaCl2 | Meyer | 0925-250 | Calcium Chloride |
| Centrifuge | Dynamica | 14 R | Centrifuge Refrigerated |
| CoCl2 | Merck | 1057-100 | Cobalt dichloride |
| FeCl3 | Merck | 157740 | Iron(III) Chloride |
| K2HPO4 | Meyer | 2051-250 | Dipotassium Phosphate |
| KH2PO4 | Meyer | 2055-250 | Monopotassium Phosphate |
| MgSO4 | Meyer | 1605-250 | Magnesium Sulphate |
| Micropipette | LabNet | Model Beta-Pette | Micropipette |
| MnCl2 | Merck | 429449 | Manganese(II) Chloride |
| Na2 EDTA | Merck | 200-449-4 | Edatamil, Edetato Disodium Salt Dihydrate |
| Na2MoO4 | Merck | 243655 | Sodium Molybdate |
| NaCl | Meyer | 2365-500 | Sodium Chloride |
| NaNO3 | Meyer | 2465-250 | Sodium Nitrate |
| RGB LED stripe | Steren | GAD-LED2 | Light source |
| Spectrophotometer | PerkinElmer | Model Lambda35 | Spectrophotometer |
| spectroradiometer | Gigahertz-Optik | model BTS256 | |
| Vortex | Scientific Industries | Vortex-Genie® 2 | Vortex |
| ZnCl2 | Merck | 208086 | Zinc Chloride |
References
- Khan, M. I., Shin, J. H., Kim, J. D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 17 (1), 36 (2018).
- Otero-Paternina, A. S. M., Cruz-Casallas, P. Effect of the hydrocarbon phenanthrene on Chlorella vulgaris (Chlorellaceae) growth. Acta Biol Col. 18 (1), 87-98 (2013).
- Pancha, I., et al. Salinity-induced oxidative stress enhanced the biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Techno. 189, 341-348 (2015).
- Ortiz, M. L., Cortés, C. E., Sánchez, J., Padilla, J., Otero, A. M. Evaluating microalgae Chlorella sorokiniana growth in different culture mediums in autotrophic and mixotrophic conditions. Orinoquia. 16 (1), 11-20 (2012).
- Camacho Kurmen, J. E., González, G., Klotz, B. Astaxanthin production in Haematococcus pluvialis under different stress conditions. Nova. 11 (19), 94-104 (2013).
- . Production of chlorophyll and astaxanthin from Haematococcus pluvialis under nitrogen deficiency stress in a 5-liter Biostat Aplus bioreactor Available from: https://repositorio.unicolmayor.edu.co/handle/unicolmayor/2735 (2019)
- Bischoff, H. W., Bold, H. C. Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. University of Texas. 6318, 95 (1963).
- Cerezo, R. LED characterization through the use and programming of a spectrometer. Universidad Politécnica de. 22323, (2013).
- Couso, I., Vila, M., Vigara, J., Cordero, B. F., Vargas, M. &. #. 1. 9. 3. ;. Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur J Phycol. 47 (3), 223-232 (2012).
- Vera-López, F., Martínez, A. Pigments in microalgae: Functions, applications, and overproduction techniques. BioTechnology. 25 (5), 35-51 (2021).

.
