A Protocol for Constructing a Rat Wound Model of Type 1 Diabetes
Özet
The streptozotocin-induced diabetic wound model in male SD rats is currently the most widely used model for studying wound healing in type I diabetes mellitus. This protocol describes the methods used to construct this model. It also presents and addresses potential challenges and examines the progression and angiogenic characteristics of diabetic wounds.
Abstract
A single high dose of streptozotocin injection followed by full-thickness skin excision on the dorsum of rats is a common method for constructing animal models of type 1 diabetic wounds. However, improper manipulation can lead to model instability and high mortality in rats. Unfortunately, there are few existing guidelines on type 1 diabetic wound modeling, and they lack detail and do not present specific reference strategies. Therefore, this protocol details the complete procedure for constructing a type 1 diabetic wound model and analyzes the progression and angiogenic characteristics of the diabetic wounds. Type 1 diabetic wound modeling involves the following steps: preparation of the streptozotocin injection, induction of type 1 diabetes mellitus, and construction of the wound model. The wound area was measured on day 7 and day 14 after wounding, and the skin tissues of the rats were extracted for histopathological and immunofluorescence analysis. The results revealed that type 1 diabetes mellitus induced by 55 mg/kg streptozotocin was associated with lower mortality and a high success rate. The blood glucose levels were relatively stable after 5 weeks of induction. The diabetic wound healing rate was significantly lower than that of normal wounds on day 7 and day 14 (p < 0.05), but both could reach more than 90% on day 14. Compared with the normal group, the epidermal layer closure of diabetic wounds on day 14 was incomplete and had delayed re-epithelialization and significantly lower angiogenesis (p < 0.01). The type 1 diabetic wound model constructed based on this protocol has the characteristics of chronic wound healing, including poor closure, delayed re-epithelialization, and decreased angiogenesis compared to normal rat wounds.
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic metabolic disease characterized by hyperglycemia and the destruction of pancreatic β-cells1. A T1DM wound is a chronic non-healing wound and the most common and devastating complication of diabetes in humans2,3. Animal models are the most appropriate prototypes for studying the pathological changes during wound healing and the safety and efficacy of potential therapeutic agents4. Compared to other types, male Sprague-Dawley (SD) rats are more sensitive to streptozotocin (STZ) and show a lower related mortality rate, making them popular in diabetic wound research5,6.
Numerous methods for constructing T1DM wound models have been described. Regarding the T1DM model, studies have primarily focused on the effect of the STZ injection method on the success rate of diabetes induction7,8. However, the modeling process suffers from the inconsistent operation of this same step. In one study, rats fasted for 18 h before the STZ injection; rats with blood glucose levels higher than 16.67 mmol/L 1 week after the STZ injection were deemed diabetic, and the diabetic wound was introduced after 3 weeks9. Conversely, in a related study, Zhu et al. fasted rats for 12 h before the STZ injection; rats with blood glucose levels higher than 16.7 mmol/L at 72 h after the injection were considered diabetic, and the diabetic wound was introduced after 4 weeks10. Overall, there are inconsistencies in the STZ injection protocols, diabetes diagnosis criteria, and wound introduction times.
In terms of wound modeling, in most studies, the full thickness of the dorsal skin is excised to construct T1DM wounds after successful diabetes induction11,12,13. Although this model is susceptible to skin contracture in rats, it is the most commonly used model in wound healing research because it is less labor-intensive and is cheap14,15. Nevertheless, method-guided research on this full-thickness excision technique is lacking. Furthermore, there are no uniform standards in existing studies regarding wound size and location12,16. The size and location of the wound can indirectly affect the consistency of the experimental design and the scientific validity of the results. Therefore, there is an urgent need for a standard protocol for T1DM induction and wound modeling as a reference for researchers. The goal of this study is to visualize a specific protocol for T1DM wound modeling that can be used as a reference for T1DM wound studies.
Protocol
The protocol was conducted following the Declaration of Helsinki, and all animal experiments were approved by the Management Committee from Chengdu University of Traditional Chinese Medicine (Record No. 2021-13).
1. Preparation of the streptozotocin injection
- Select 15 SD male specific pathogen-free (SPF) rats aged 8 weeks and weighing 220 g ± 20 g. Using the simple randomization method, divide the rats into a diabetic group (n = 10) and a normal group (n = 5).
- Measure the initial weight of the rats, and determine the dosage of STZ through the administration of 55 mg/kg.
NOTE: Based on pre-experiments, 55 mg/kg is the optimal STZ dose. - Weigh the STZ powder accurately, and place it in a light-proof container.
- Add an appropriate amount of 0.1 mol/L sodium citrate buffer (pH 4.5) to dissolve the STZ to a concentration of 1 %.
NOTE: The sodium citrate buffer must be pre-cooled for 2 h in a 4 °C refrigerator before use. The preparation of the STZ solution must assure sterility. - Shake for 30 s using a drug oscillator. Place in an ice box, and set aside.
NOTE: The injection should be used within 15 min.
2. Induction of the T1DM model
- Before the STZ injection, fast the rats for 18 h, and allow free access to water.
- Perform an intraperitoneal injection of 1% STZ solution.
- Grasp the rat, and expose the abdominal skin and the injection site (the intersection of the line connecting the roots of the two thighs and the midline of the abdomen).
- Disinfect the injection site twice using a cotton ball soaked in 75% alcohol (once clockwise and once counterclockwise). Place the rat's head below the abdomen.
- Insert the needle parallel to the abdominal midline at an angle of 45°, and after piercing the skin, reduce the needle angle to 30°, and then insert the needle 2-3 mm. Gently pull the needle plug, ensuring no blood or fluid is sucked into the syringe. Inject the STZ solution, pull out the needle, and stop the bleeding with a cotton swab.
NOTE: If a yellow fluid is drawn back into the syringe, the needle may have penetrated the bladder, and if a dark green fluid is drawn, the needle could have penetrated the large intestine or caecum. In either case, the needle should be removed immediately. The animal should be evaluated by veterinary personnel.
- Measure the casual (non-fasting or fasting) blood glucose levels at 09:00 am on day 3 and day 7 after STZ induction.
NOTE: The time for random the blood glucose measurement is fixed. In this protocol, it is fixed at 09:00 am. However, it is not the only time used. Blood collected from the caudal vein by a needle puncture is less susceptible to tissue fluid than blood taken from a severed tail, so blood glucose values are more accurate.- Immobilize the rat using a rat fixator (Figure 1).
- Find the location of the caudal vein. Disinfect the tail of the rat twice using a cotton ball soaked in 75% alcohol.
- Puncture the caudal vein to induce bleeding, and measure the blood glucose using a glucometer. Stop the bleeding with a cotton swab.
NOTE: A glucose level higher than 16.7 mmol/L on day 7 after the STZ injection is considered T1DM.
- Weigh the rats weekly, and measure the blood glucose levels and other parameters, including diet, water intake, and urine output.
- Feed the animals normally for 8 weeks after STZ induction.
3. Construction of the wound model
- Shave the rats with an electric razor 1 day before wound modeling. A shaved area of 5 cm x 5 cm on the dorsum side of the rat is generally ideal.
- Wipe the shaved area with a warm normal saline cotton ball, allow it to dry, and then apply depilatory cream for 5 min. Clean the area with gauze, and wash any residual depilatory cream with warm normal saline.
- Weigh the rats, and calculate the required dose of Nembutal based on the 35 mg/kg standard. Dissolve the Nembutal using normal saline to a concentration of 3%. Other general anesthetics such as ketamine/xylazine or isoflurane can be used for this procedure. Please work with Institutional Animal Care and Use Committees to ensure what is best.
NOTE: To ensure efficacy, the solution should be freshly prepared, and the Nembutal powder and solution should be protected from light. - Fast the rats for 12 h before anesthetization. Inject the anesthesia intraperitoneally. Use tetracycline eye ointment or a general eye lubricant to prevent eye dryness following the anesthesia administration.
NOTE: Anesthesia was considered moderate when the rat's muscles were relatively relaxed, the eye movements disappeared, the breathing was regular, and the response to painful stimuli was small. - Disinfect the dorsal skin twice using cotton balls soaked in iodine (once clockwise and once counterclockwise) and 75% alcohol (alternative rounds).
- After drying, cut the skin with a 20 mm diameter circular biopsy punch.
- Tent the skin with sterile forceps, and then use sterile surgical scissors to remove the full-thickness skin along the punch cut marks. Stop the bleeding with a normal saline cotton ball.
NOTE: The wound's superior border should be 5-10 mm below the lower scapular border and 5-10 mm to the right/left of the spine of the rat (Figure 2). The wounds are symmetrical along the spine when two wounds are constructed. - Use Vaseline gauze to cover the wounds, and wrap them with a gauze and breathable bandage held in place with rubber tape. Inject carprofen subcutaneously (5 mg/kg) once daily to relieve postoperative pain. Change the wound bandage once a day (use of carprofen for pain relief).
NOTE: Observe the rat's movement and respiration for any abnormalities after the bandage is completed, and ensure that the breathable bandage is appropriately tight. - Place a ruler under the wound, and photograph the wound with a digital camera until day 14. Euthanize the rats on day 14 according to the institutional animal care and use guidelines. Cut the wound skin tissue 5 mm from the wound edge. Divide the tissue sample into two parts, wash them with PBS to remove visible blood stains, and then fix them with 4% paraformaldehyde solution.
4. Calculation of the wound area with ImageJ software
- Click on the File button after opening the software, and then drop down and click on Open to open the wound pictures.
- Select the Straight tool, and draw a straight line of 1 cm along the ruler in the wound pictures.
- Click on the Set Scale command in the Analyze menu, and set the Known distance to 1.
- Select the Freehand selections tool, and sketch the outline of the wound on the picture.
- Click on the Measure command in the Analyze menu, and read the Area value after the result pops up.
5. Hematoxylin and eosin (H&E) staining
- Remove the skin tissue from the fixative, cut it into thin sections with a scalpel in a fume hood, and place it in a dehydration cassette.
- Put the dehydration cassette in a dehydration machine, and dehydrate the tissues in the following steps: 75% alcohol for 4 h; 85% alcohol for 2 h; 90% alcohol for 2 h; 95% alcohol for 1 h; anhydrous ethanol I and II for 30 min each; alcohol benzene for 5-10 min; xylene I and II for 5-10 min each; and wax I, II, and III for 1 h each.
- Embed the tissues in wax. Cool at −20° on a freezing table, and correct the wax block neatly.
- Cut the wax blocks longitudinally using a paraffin sectioning machine into 3 µm thick sections.
- Sequentially soak the section in xylene I and II for 20 min each, anhydrous ethanol I and II for 5 min each, and tap water for 5 min.
- Flood the tissues with hematoxylin stain for 3-5 min, 0.5% aqueous hydrochloric acid solution differentiation, 0.5% aqueous ammonia solution back to blue, and rinse with water.
- Dehydrate the tissue sections with 85% and 95% alcohol. Flood the tissues with eosin staining solution for 5 min.
NOTE: Normally, eosin staining takes 30 s to 2 min, and the time can be adjusted according to the staining results and requirements. - Dehydrate the sections sequentially with the following solutions: anhydrous ethanol I, anhydrous ethanol II, anhydrous ethanol III, xylene I, and xylene II, each for 5 min. Finally, cover the glass slides with neutral balsam.
- Examine the H&E-stained tissues under the microscope at 40x, 20x, and 10x, and take pictures to retain representative images of each slide.
6. CD31 immunofluorescence staining
- Soak the tissue sections in xylene I and II for 15 min each, anhydrous ethanol I and II for 5 min each, 85% alcohol for 5 min, and 75% alcohol for 5 min, and rinse with distilled water.
- Antigen repair
- Add a suitable 10 mM citric acid buffer of pH 6.0 to a microwave oven container, heat it to boiling on high, and then place the glass slide into it.
- Boil for 8 min on a medium heat, stop for 8 min, and then boil again at a medium-low heat for 7 min.
- Allow the slides to cool, place them in PBS (pH 7.4), and wash them three times for 5 min each using a decolorization shaker.
- Add 5% goat serum dropwise in the circle, and incubate for 30 min.
- Gently shake off the closure solution (5% goat serum), and add rabbit anti-CD31 antibody (diluted using PBS at a ratio of 1:200) dropwise onto the sections. Place the sections in a wet box, and incubate overnight at 4°C.
- Wash the slides three times with PBS (pH 7.4) on a decolorization shaker for 5 min each. Lightly shake the sections to dry them, and then cover them with a circular drop of FITC-labeled goat anti-rabbit IgG. Incubate at room temperature for 50 min in darkness.
- Wash the slides three times with PBS (pH 7.4) on a decolorization shaker for 5 min each. Air-dry the sections with light shaking, and add DAPI staining solution. Incubate the sections in the dark for 10 min at room temperature.
- After drying the sections, draw circles around the tissue with the PepPen (to prevent the loss of antibodies), add an autofluorescence quenching agent (0.3% Sudan Black B) to the circles for 5 min, and thereafter rinse them under running water for 10 min.
- Wash the slides three times with PBS (pH 7.4) on a decolorization shaker for 5 min each. Shake the sections slightly, and seal them with an antifade mounting medium.
- Observe and photograph the sections under a fluorescence microscope at 40x, 20x, and 10x.
NOTE: The DAPI UV excitation wavelength is 330-380 nm, and the emission wavelength is 420 nm (blue light). The FITC excitation wavelength is 465-495 nm, and the emission wavelength is 515-555 nm (green light).
7. Statistical analysis
- Collect and analyze the data using SPSS.
- Report the data as mean ± standard deviation.
- Use an independent samples t-test to analyze the differences between the diabetic and normal groups.
- Set the statistical significance at **p < 0.01 and *p < 0.05.
Representative Results
A total of 10 SD rats received a single STZ intraperitoneal injection to induce the T1DM model. One rat prematurely died (10%), but diabetes was induced in all the rats (100%). After 3 days of STZ injection, the blood glucose levels of all the rats were higher than 16.7 mmol/L, and the blood glucose levels stabilized 5 weeks after induction (Figure 3A). The weight of the diabetic group increased gradually after the STZ injection but decreased in week 3 and then slowly increased again from week 4 (Figure 3B). In contrast, the weight of rats in the normal group increased steadily, and their mean weight 3 days after diabetes induction was higher than that of the diabetic group (Figure 3B). The diabetic rats all exhibited typical symptoms of thirst, polyuria, and weight loss, similar to the findings of Hao et al.17.
On day 7 and day 14 after wounding, the macroscopic analysis revealed that re-epithelialization was more pronounced in rats in the normal group than in the diabetic group (Figure 4A). The quantitative results revealed that the wound healing rate was significantly lower in the diabetic group than in the normal group on day 7 and day 14 (p < 0.01). However, on day 14, the wound healing rates could also be above 90% in the diabetic group (p < 0.05, Figure 4B). This suggests that the T1DM wound model is characterized by poor closure but not to the extent of the chronic non-healing seen in human diabetic wounds.
H&E staining on day 14 of wound healing revealed an incomplete wound epidermis, slow proliferation of keratinocytes, and delayed re-epithelialization in the diabetic group compared to the normal group. The diabetic wounds showed partial loss of the hair follicles and sebaceous glands. There were also fewer visible capillaries (Figure 5).
Diabetes causes endothelial cell dysfunction, glycosylation of the extracellular matrix proteins, and vascular denervation18. These complications result in lower-than-normal wound angiogenesis in diabetic wounds18. Angiogenesis is necessary for wound healing, and wound angiogenesis is frequently analyzed by CD31 immunostaining (Figure 6A)19,20. Based on the average optical density (AOD) of CD31 expression, angiogenesis at the wound site was significantly higher in the normal than in the diabetic group (p < 0.01, Figure 6B).
Figure 1: Picture of rats immobilized by fixators. Please click here to view a larger version of this figure.
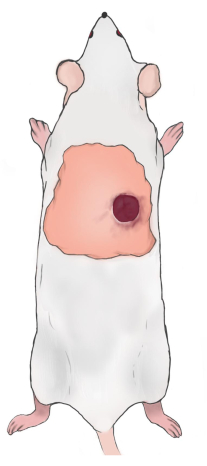
Figure 2: Diagram of the rat wound location. Please click here to view a larger version of this figure.
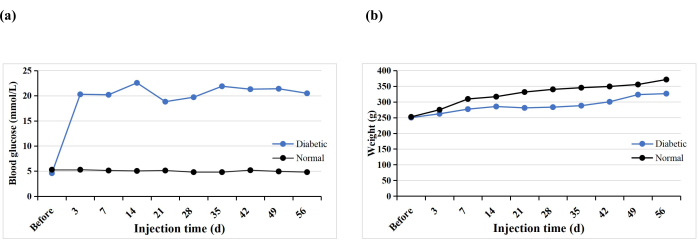
Figure 3: Blood glucose levels and weights of the experimental rats. Please click here to view a larger version of this figure.
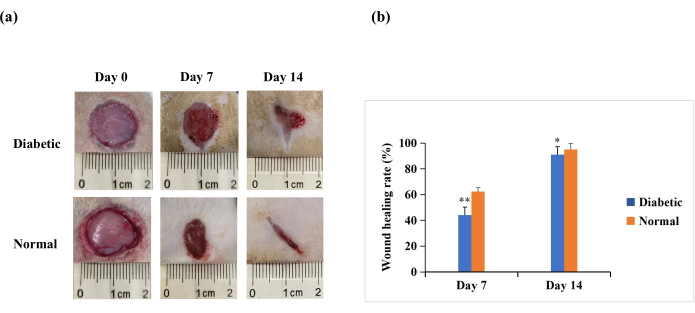
Figure 4: Full-thickness skin wounds (20 mm in diameter) on the backs of the experimental rats. (A) The macroscopic appearance of the wounds on day 0, day 7, and day 14. The wound morphology images on day 0, day 7, and day 14 were captured with a digital camera. (B) The wound area was measured using ImageJ software and was used to calculate the wound healing rate. The wound healing rate (%) was calculated as follows: (initial wound area − wound area at the indicated time point)/initial wound area × 100. The values are presented as mean ± SD (n = 14). Statistical significance was set at ** p < 0.01 and * p < 0.05. Please click here to view a larger version of this figure.
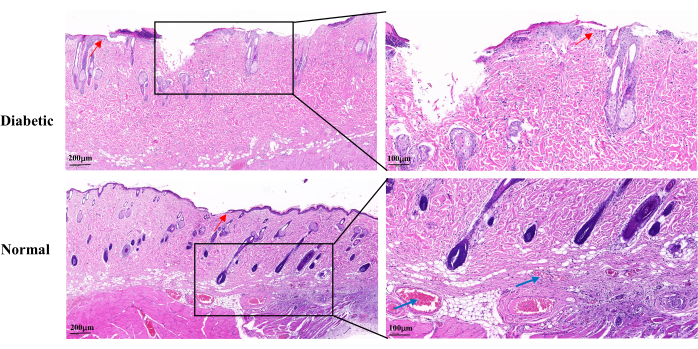
Figure 5: Representative histopathological H&E images on day 14 after wound establishment. The blue arrows indicate capillaries. The red arrows show the proliferation of keratinocytes. Left scale: one bar = 200 µm; right scale: one bar = 100 µm. Please click here to view a larger version of this figure.
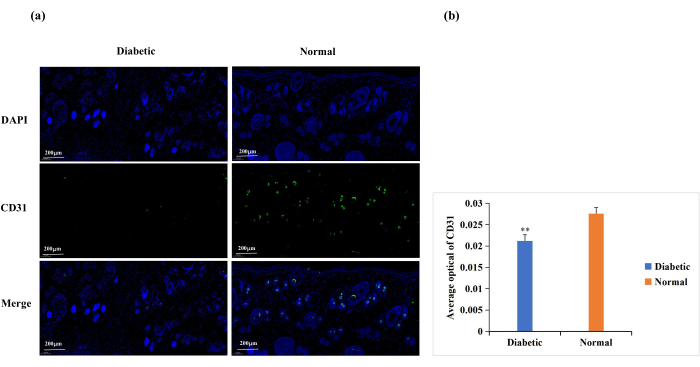
Figure 6: Immunofluorescence staining analysis for the expression of CD31. CD31 levels were used to determine the state of angiogenesis. (A) Representative images of CD31 immunofluorescence staining in the diabetic and normal groups. The integrated optical density (IOD) value and the pixel area (AREA) for each skin sample were calculated with Image-Pro Plus 6.0 software. The average optical density (AOD) value (AOD = IOD/AREA) was also derived. The AOD value was directly proportional to the positive expression of CD31. (B) Quantitative comparison of CD31 positive expression in the diabetic and normal groups. Data are presented as mean ± SD. ** p < 0.01. Scale: one bar = 200 µm. Please click here to view a larger version of this figure.
Discussion
This protocol clarifies the disputed operations in T1DM wound modeling. Concerns on the STZ injection protocols, T1DM induction success criteria, blood glucose stabilization time, and wound location and size have been addressed in this work. Furthermore, the pathological characteristics and measurable parameters for T1DM wound healing assessment have been clarified.
The rats fasted for 18 h before the STZ injection to avoid the competitive binding of glucose or its analogs to β-cells, which could affect the efficacy of STZ. The most commonly used method to induce T1DM is a single high dose of STZ, which increases blood glucose by damaging the islets and decreasing insulin secretion21. Pre-experimental trials revealed that the optimal STZ dose for a high success rate and a low mortality rate was 55 mg/kg, which is lower than the optimal doses reported in previous studies22,23,24. In this protocol, T1DM was induced using a single intraperitoneal injection of 55 mg/kg STZ.
The blood glucose levels were all higher than 16.7 mmol/L 3 days after the STZ injection. However, a blood glucose level higher than 16.7 mmol/L on day 7 after STZ injection is the recommended criterion for successful T1DM modeling, because the extent of islet damage varies among rats, and an appropriate extension of the diagnostic time can reduce the false-negative rate. In addition, the blood glucose fluctuations stabilized 5 weeks after the STZ injection, and the rats gradually gained weight during this period, consistent with previous findings25,26. This indicates that the blood glucose level in the T1DM model should be stabilized for at least 6 weeks, and an increase in rat weight after 6 weeks reduces the mortality rates during the wound modeling. Hence, this protocol conducted wound modeling 8 weeks after the STZ injection.
The wound closure rate on day 7 and day 14 after wounding was significantly lower in the diabetic than in the normal wound group, indicating slow healing. Moreover, wound re-epithelialization and angiogenesis were significantly lower in the diabetic than in the normal group. This demonstrates that the T1DM wound model shows slower wound healing and delayed re-epithelialization than in normal rats, which may be related to the pathological changes of reduced wound angiogenesis. However, on day 14, the T1DM wound healing rate was also above 90%, which is different from the chronic non-healing characteristic of human diabetic wounds. This could be because rodents' physiological mechanisms for wound healing differ from those of humans27. Consequently, the best wound diameter is at least 20 mm, which is large enough to allow time to assess an intervention's efficacy in a diabetic wound study. The wound location should avoid the scapula and spine, as continuous motion in these two sites could disrupt wound healing.
In conclusion, the construction of the T1DM wound model using the method of this protocol is effective. The protocol replicates some of the characteristics of chronic diabetic wounds, such as slower wound healing, delayed re-epithelialization, and reduced angiogenesis compared to normal rat wounds. However, it is unknown whether the model can replicate other chronic phenotypes of diabetic wounds. Furthermore, this protocol describes the most fundamental and widely used method, which does not account for the issue of skin contraction in rats. Future research can incorporate the use of wound splints into this protocol or explore additional models of chronic diabetic wounds, which will be a significant challenge for researchers in the future.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (82104877).
Materials
| Antifade mounting medium | Southern Biotechnology Associates, Inc. | 0100-01 | |
| AutoFluo Quencher | Servicebio Technology co., Ltd. | G1221 | |
| Automatic slide stainer | Thermo Fisher Scientific Inc. | Varistain™ Gemini ES | |
| CD31 | Servicebio Technology co., Ltd. | GB11063-2 | |
| Citrate antigen retrieval solution | Servicebio Technology co., Ltd. | G1201 | |
| Cover glass | Citotest Labware Manufacturing Co., Ltd. | 10212432C | |
| DAPI | Servicebio Technology co., Ltd. | G1012 | |
| Decolorization shaker | Scilogex | S1010E | |
| Depilatory cream | Guangzhou Ruixin Biotechnology Co., Ltd. | — | |
| Dimethyl benzene | Chengdu Kelong Chemical Co., Ltd. | 64-17-5 | |
| Drug oscillator | Shenzhen Jiashi Technology Co., Ltd. | VM-370 | |
| Electric razor | Shanghai Flyco Electrical Appliance Co., Ltd. | FC5908 | |
| Embedding machine | Wuhan Junjie Electronics Co., Ltd. | JB-P5 | |
| Ethanol absolute | Chengdu Kelong Chemical Co., Ltd. | 1330-20-7 | |
| Fitc-labeled goat anti-rabbit IgG | Servicebio Technology co., Ltd. | GB22303 | |
| Goat serum | Thermo Fisher Scientific Inc. | 16210064 | |
| Hematoxylin and eosin staining solution | Beijing Regan Biotechnology Co., Ltd. | DH0020 | |
| Image J software | National Institutes of Health | — | |
| Microwave oven | Midea Group Co., Ltd. | M1-L213B | |
| Mini centrifuge | Scilogex | D1008 | |
| Neutral balsam | Sinopharm Chemical Reagent Co., Ltd | 10004160 | |
| PBS buffer | Biosharp | G4202 | |
| Portable blood glucose meter | Sinocare Inc. | GA-3 | |
| Rapid tissue processor | Thermo Fisher Scientific Inc. | STP420 ES | |
| Rat fixator | Globalebio (Beijing) Technology co., Ltd | GEGD-Q10G1 | |
| Slicing machine | Thermo Fisher Scientific Inc. | HM325 | |
| Slides glass | Citotest Labware Manufacturing Co., Ltd. | 80312-3181 | |
| sodium citrate buffer | Beijing Solarbio Science & Technology Co., Ltd. | c1013 | |
| Streptozotocin | Sigma | 57654595 |
Referanslar
- Zimmet, P., Alberti, K. G., Shaw, J. Global and societal implications of the diabetes epidemic. Nature. 414 (6865), 782-787 (2001).
- Grennan, D. Diabetic foot ulcers. Journal of the American Medical Association. 321 (1), 114 (2019).
- Eming, S. A., Martin, P., Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Science Translational Medicine. 6 (265), 265sr6 (2014).
- Patel, S., Srivastava, S., Singh, M. R., Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomedicine & Pharmacotherapy. 112, 108615 (2019).
- Deeds, M. C., et al. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Laboratory Animals. 45 (3), 131-140 (2011).
- Chao, P. C., et al. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomedicine & Pharmacotherapy. 101, 155-161 (2018).
- Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Current Protocols. 1 (4), e78 (2021).
- Wu, J., Yan, L. J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 8, 181-188 (2015).
- Yang, J., Chen, Z., Pan, D., Li, H., Shen, J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. International Journal of Nanomedicine. 15, 5911-5926 (2020).
- Zhu, Y., Wang, Y., Jia, Y., Xu, J., Chai, Y. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair and Regeneration. 27 (4), 324-334 (2019).
- Shao, Z., et al. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioactive Materials. 20, 561-573 (2022).
- Asfour, H. Z., et al. Enhanced healing efficacy of an optimized gabapentin-melittin nanoconjugate gel-loaded formulation in excised wounds of diabetic rats. Drug Delivery. 29 (1), 1892-1902 (2022).
- Wei, L., et al. Mesenchymal stem cells promote wound healing and effects on expression of matrix metalloproteinases-8 and 9 in the wound tissue of diabetic rats. Stem Cells and Development. 32 (1-2), 25-31 (2022).
- Pastar, I., et al. . Preclinical models for wound-healing studies. In Skin Tissue Models., edited by. , 223-253 (2018).
- Yang, R. H., et al. Epidermal stem cells (ESCs) accelerate diabetic wound healing via the Notch signalling pathway. Bioscience Reports. 36 (4), e00364 (2016).
- Suliman Maashi, M., Felemban, S. G., Almasmoum, H. A., Jarahian, M. Nicaraven-loaded electrospun wound dressings promote diabetic wound healing via proangiogenic and immunomodulatory functions: A preclinical investigation. Drug Delivery and Translational Research. 13 (1), 222-236 (2023).
- Hao, M., Ding, C., Sun, S., Peng, X., Liu, W. Chitosan/sodium alginate/velvet antler blood peptides hydrogel promotes diabetic wound healing via regulating angiogenesis, inflammatory response and skin flora. Journal of Inflammation Research. 15, 4921-4938 (2022).
- Kolluru, G. K., Bir, S. C., Kevil, C. G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. International Journal of Vascular Medicine. 2012, 918267 (2012).
- Okonkwo, U. A., DiPietro, L. A. Diabetes and wound angiogenesis. International Journal of Molecular Sciences. 18 (7), 1419 (2017).
- Yi, C., et al. Targeted inhibition of endothelial calpain delays wound healing by reducing inflammation and angiogenesis. Cell Death & Disease. 11 (7), 533 (2020).
- Goodson 3rd, W. H., Hung, T. K. Studies of wound healing in experimental diabetes mellitus. Journal of Surgical Research. 22 (3), 221-227 (1977).
- Luippold, G., Klein, T., Mark, M., Empagliflozin Grempler, R. a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus. Diabetes, Obesity & Metabolism. 14 (7), 601-607 (2012).
- Sayed, N., et al. Effect of dapagliflozin alone and in combination with insulin in a rat model of type 1 diabetes. The Journal of Veterinary Medical Science. 82 (4), 467-474 (2020).
- Han, Y., et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. European Journal of Medical Research. 24 (1), 10 (2019).
- Ansell, D. M., Marsh, C., Walker, L., Hardman, M. J., Holden, K. Evaluating STZ-induced impaired wound healing in rats. Journal of Investigative Dermatology. 138 (4), 994-997 (2018).
- Liu, Y., et al. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Research & Therapy. 13 (1), 258 (2022).
- Zindle, J. K., Wolinsky, E., Bogie, K. M. A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health. Journal of Tissue Viability. 30 (3), 291-300 (2021).

