Two-step Approach to Explore Early- and Late-stages of Organ Formation in the Avian Model: The Thymus and Parathyroid Glands Organogenesis Paradigm
Özet
This article provides an updated approach to the classical quail-chicken chimera system to study organ formation, by combining novel in vitro and in ovo experimental procedures.
Abstract
The avian embryo, as an experimental model, has been of utmost importance for seminal discoveries in developmental biology. Among several approaches, the formation of quail-chicken chimeras and the use of the chorioallantoic membrane (CAM) to sustain the development of ectopic tissues date back to the last century. Nowadays, the combination of these classical techniques with recent in vitro methodologies offers novel prospects to further explore organ formation.
Here we describe a two-step approach to study early- and late-stages of organogenesis. Briefly, the embryonic region containing the presumptive territory of the organ is isolated from quail embryos and grown in vitro in an organotypic system (up to 48 h). Cultured tissues are subsequently grafted onto the CAM of a chicken embryo. After 10 days of in ovo development, fully formed organs are obtained from grafted tissues. This method also allows the modulation of signaling pathways by the regular administration of pharmacological agents and tissue genetic manipulation throughout in vitro and in ovo developmental steps. Additionally, developing tissues can be collected at any time-window to analyze their gene-expression profile (using quantitative PCR (qPCR), microarrays, etc.) and morphology (assessed with conventional histology and immunochemistry).
The described experimental procedure can be used as a tool to follow organ formation outside the avian embryo, from the early stages of organogenesis to fully formed and functional organs.
Introduction
Avian embryos have been widely used in seminal developmental biology studies. The main advantages of the avian model include the possibility to open the egg, the relatively easy access to the embryo, and the ability to perform micromanipulation. Some examples comprise the classic quail-chicken chimera system for studying cell fate1, application of specific growth factors to the embryo2, and the growth of ectopic cellular structures in the CAM1,3,4.
To get new insights into distinct stages of organ formation, we have recently developed a method which combines grafting techniques with in vitro manipulation of embryonic tissues5. The two-step approach enables the discrimination and exploration of both early- and late-stages of organogenesis, which are often limited due to highly dynamic and complex tissue interactions2. Moreover, the lack of suitable tissue-specific markers frequently limits the use of genetically modified animal models6. This novel method of the two-step approach largely overcomes such limitations.
To study early-stages of organ formation, in the first step, the quail embryonic territory comprising the prospective organ rudiment is isolated and grown in an in vitro organotypic system for 48 h. During this period, pharmacological modulation of specific signaling pathways can be performed by adding drugs to the culture medium5,7. Additionally, cultured tissues can be collected at any stage of in vitro growth and probed for gene-expression (using methods such as qPCR, microarrays, etc.).
In the second step, 48 h-cultured tissues are then grafted onto the CAM of a chicken (c) embryo at embryonic day (E) 8 (cE8) (Hamburger and Hamilton (HH)-stages 33-35)8. The CAM behaves as a vascular supplier of nutrients and allows gas exchanges1,3,4 to grafted tissues enabling its development in ovo for longer periods of time. This experimental step is especially well suited to study late-stages of organogenesis, as fully formed organs can be obtained after 10 days of in ovo development5,9,10,11. Morphological analysis is easily performed by conventional histology to confirm proper organ formation and donor origin of cells can be identified by immunohistochemistry using species-specific antibodies (i.e., MAb Quail PeriNuclear (QCPN)). During the CAM incubation period, grafts can also be grown in the presence of pharmacological agents and collected at any stage of development to evaluate the progression of organogenesis.
The two-step approach, described here in depth, has already been employed in Figueiredo et al.5 to explore the avian parathyroid/thymus common primordium development. Accordingly, the inherent particularities of the embryonic territories and stages of development involved in the organogenesis of the thymus and parathyroid glands will be presented below.
The thymus and parathyroid glands epithelia, though functionally distinct, derive from the endoderm of the pharyngeal pouches (PP)12. In avian, the epithelia of these organs originate from the third and fourth PP endoderm (3/4PP)12, while in mammals the thymic epithelium derives from the 3PP and the epithelium of parathyroid glands derives from the 3PP and 3/4PP in mouse and human, respectively13,14.
One of the earliest stages in the formation of these organs is the emergence of discrete thymus and parathyroid domains in the common primordium. In chicken, these domains can be identified by in situ hybridization, with specific molecular markers, at E4.515. As development proceeds, these organ rudiments individualize and separate from the pharynx, while a thin mesenchymal capsule, formed by neural crest-derived cells, surrounds them (at E5; HH-stage 27). Later on, the thymic epithelium is colonized by hematopoietic progenitor cells (at E6.5; HH-stage 30)12.
As in classical quail-chicken studies1,12, the two-step approach is particularly useful to study the formation of hematopoietic/lymphoid organs, namely the thymus5. As the quail explant, with the organ rudiment, is grafted in the chicken embryo prior to hematopoietic progenitor cell colonization, a chimeric thymus is formed with chicken blood-borne progenitor cells infiltrating a quail thymic epithelial counterpart. This method is, therefore, a useful tool to explore the contribution of hematopoietic cells in the development of the avian hemato/lymphoid system.
Protocol
All these experiments follow the animal care and ethical guidelines of the Centro Académico de Medicina de Lisboa.
1. Incubation of Fertilized Quail and Chicken Eggs
- Incubate Japanese quail (Coturnix coturnix japonica) and chicken (Gallus gallus) fertilized eggs for 3 and 8 days, respectively.
- Place the eggs with the air chamber (egg blunt end) facing up in a humidified incubator at 38 °C.
- Use around 20 quail eggs and 40 chicken eggs to perform this experiment.
NOTE: These numbers should be doubled when establishing this procedure for the first time.
2. Isolation of Quail Embryonic Region Containing the Presumptive Territory of Thymic and Parathyroid Rudiments
Note: Perform egg manipulation procedures in sterile conditions using a horizontal laminar flow hood and sterilized instruments and materials.
- Prepare a large borosilicate glass bowl about 3/4 filled with cold phosphate-buffered saline (PBS) solution.
- Open a quail egg after 3 days of incubation by tapping the shell and cutting a circular opening on the opposite side of its blunt end with curved scissors. Carefully remove pieces of shell and transfer the embryo to the glass bowl filled with cold PBS.
- Hold the quail (q) embryo at E3 (qE3) (the quail stage corresponding to the HH-stage 21 of the chicken) with the help of thin forceps. Make a cut into the vitelline membrane enveloping the yolk using curved scissors. Continue to cut around and externally to the circumference of extra-embryonic vessels.
- Transfer the embryo to a small bowl about 3/4 filled with cold PBS with the help of thin forceps. Thoroughly wash the embryo from the remaining attached yolk.
- Use a skimmer to transfer the embryo to a 100 mm glass Petri dish with black base (see Table of Materials) containing 10 mL of cold PBS.
- Place the Petri dish under a stereomicroscope.
Note: From this point forward, perform the microsurgery procedures under a stereomicroscope for progressive magnification. As an illumination source, it is advised to use LED lights incorporated in the stereomicroscope or in the optic fibers, considering the limited heat load. - Hold the embryo to the bottom of the plate with thin insect pins. Place four pins forming a square shape in the extra-embryonic region.
- Remove the extra-embryonic membranes of the cephalic region with the help of thin forceps and place a fifth pin there.
Note: If the embryo is correctly positioned, then the otic vesicle, the heart tube, and the 1st, 2nd, and 3rd pharyngeal arches (PAs) should be visible. - Dissect the embryonic region of interest, i.e., the 3rd and 4th pharyngeal arch region (3/4PAR), using Wecker eye scissors.
- Start cutting longitudinally and parallel to the embryo axis, between the somite/neural tube area and the PAs.
- Remove the ventrally positioned heart tube by cutting it. Keeping the scissors in the same position, rotate the Petri dish to reposition the embryo according to the direction of the cut.
- Cut between the 2nd and 3rd PAs and below the 4th PA.
- Detach the remaining membranes from the 3/4PAR with the help of thin forceps.
- Aspirate the isolated tissues (the 3/4PAR) and transfer them to a glass dish 3/4 filled with cold PBS using a 2 mL sterile plastic pipette.
Note: Hereafter, tissues can be grown in vitro up to 48 h or be immediately grafted onto the CAM of a chicken embryo at E8. - Keep the glass dish containing the isolated 3/4PAR on ice during the preparation of the in vitro assay.
3. In Vitro Organotypic Assay: Culture of the Embryonic Region Containing the Presumptive Territory of Thymic and Parathyroid Rudiments
- Prepare the culture medium with RPMI-1640 Medium supplemented with 10% FBS and 1% penicillin/streptomycin.
NOTE: Soluble pharmacological reagents can be added to the medium (for example, LY-411.575 (Ly) and di-benzazepine or cyclopamine and vismodegib, to inhibit Notch and Hedgehog (Hh) signaling pathways, respectively. For this assay, use 50-200 nM of Ly or 5-15 µM of Di-benzazepine to inhibit Notch signaling in the 3/4PAR. Use 20 µM of cyclopamine or 10 µM of vismodegib to inhibit Hh signaling in the 3/4PAR5. - Prepare the in vitro culture of the 3/4PP explant in a 6-well plate.
- Fill one well from the 6-well plate with 5 mL of culture medium. Place a 24 mm Polycarbonate Membrane Insert (see Table of Materials) on the well with the help of thin forceps.
- Under the stereomicroscope, transfer the 3/4PAR explant from the glass dish to the membrane surface by gently sliding with the help of a transplantation spoon (or spatula) and thin forceps. Place the explants with the ventral side up and the dorsal side in contact with the membrane. Add up to seven explants per membrane insert. Proceed to step 3.4.
- Alternatively, culture explants in floating membrane filters.
- Prepare a 35 mm Petri dish with 5 mL of culture medium. With the help of thin forceps, float a membrane filter (see Table of Materials) and keep a dry surface in contact with air.
- Under the stereomicroscope, transfer the 3/4PAR explant from the glass dish to the membrane filter by gentle sliding with the help of a transplantation spoon (or spatula) and thin forceps. Place the explants with the ventral side up and the dorsal side in contact with the membrane. Add up to 8 explants per membrane filter.
- Carefully place the explants prepared in steps 3.2 and 3.3 in a humidified incubator at 37 °C with 5% CO2.
- After a 48 h incubation period, remove the 6-well plate and the 35 mm Petri dish from the incubator.
- Collect the cultured explants from the membrane insert of the 6-well plate.
- Add PBS at room temperature (RT) to the membrane insert.
- Detach the explants from the membrane by vigorous flushing using a 2 mL sterile plastic pipette.
- With the help of the spatula and thin forceps, transfer the cultured explants to a glass dish 3/4 filled with PBS at RT.
- Similarly, collect the cultured explants from the floating membrane filter in the 35 mm Petri dish.
- Transfer the membrane filter with thin forceps to a new 35 mm Petri dish filled with PBS at RT.
- Detach the explants from the membrane filter by vigorous pipetting using a 2 mL sterile plastic pipette.
- With the help of thin forceps, discharge the explant-free membrane filter after confirming that no explants remained attached to it.
- With a spatula and thin forceps, transfer the explants to a glass dish filled with PBS at RT.
- Collect the cultured explants from the membrane insert of the 6-well plate.
- Transfer the cultured explants with a spatula to 1 mL of a reagent for total RNA isolation and use for gene-expression studies.
CAUTION: Exposure to this reagent (see Table of Materials) can be a serious health hazard. Wear appropriate protective eyewear, clothing, and gloves. Follow the handling instructions and read the safety data sheets provided by the manufacturer. - Alternatively, graft the cultured tissues onto CAM of chicken embryos at E8. Follow to step 4.
4. Preparation of the CAM
- Remove the chicken eggs with 8 days of embryonic development from the incubator.
NOTE: Eggs were incubated with air chamber facing upwards at 38 °C in a humidified incubator. - Cover the blunt end of the egg with clear plastic tape to prevent pieces of the shell from falling into the air chamber. Tap the shell and cut a circular opening in the egg with curved scissors. The air chamber should be visible.
- Remove with caution the white membrane of the air chamber with thin forceps. CAM is then visible and accessible for ectopic tissue transplantation.
Note: Do not use PBS to hydrate the CAM, before or after transplantation, since PBS promotes sliding and misplacement of the explants. If the membrane dries out, discard the egg.
5. Grafting of Cultured Explants onto the CAM
- Create small vascular lesions/wounds in the smaller vessels of the CAM with a microscalpel in a holder.
Note: Use the tip of a Pasteur pipette to remove blood by capillarity in the case of excess bleeding. - Use a spatula and thin forceps to transfer the cultured explant to the wounded area of the CAM.
- Cut a piece of a filter paper slightly larger than the explant and place it on the top of the explant.
Note: The filter paper helps tracking the explant location after its development in the CAM. Also, it allows daily drug delivery to the explant during in ovo development, if necessary (described in step 5.6). - Seal the egg window with clear plastic tape and identify it using a charcoal pencil.
Note: The plastic tape protects the embryo from dehydration during the incubation period. - Incubate the manipulated egg for 10 days in a humidified incubator at 38 °C. Follow to step 6.
- Optional Step: Daily drug administration during incubation period.
- To access the filter, partially lift the plastic tape. Add 100 µL of drug solution, drop by drop, on top of the paper. Re-seal the window and place the egg back in the humidified incubator at 38 °C.
Note: For this assay, the dose of 20 µM of cyclopamine will inhibit Hh signaling during in ovo development5.
- To access the filter, partially lift the plastic tape. Add 100 µL of drug solution, drop by drop, on top of the paper. Re-seal the window and place the egg back in the humidified incubator at 38 °C.
6. Ectopic Organ Formation in the CAM After 10 Days of In Ovo Development
- After 10 days of incubation, remove the egg from the incubator and carefully withdraw the plastic tape.
- Cut the CAM around the filter region using curved scissors and transfer the CAM-derived explant with filter paper to a small glass bowl about 3/4 filled with cold PBS.
- With the help of thin forceps transfer the CAM-derived explant to a 100 mm Petri dish with black base containing 10 mL of PBS. Gently remove the filter paper and the excess of membrane using Wecker eye scissors and thin forceps.
- Transfer the CAM-explant to fixative solution (3.7% PFA in PBS) with a skimmer. Euthanize the chicken embryos without removing them from the egg by making a precise cut in the neck region of the embryo with the help of large scissors.
- Assess the organ formation in paraffin sections of the CAM-derived explants by conventional histology and immunohistochemistry.
Representative Results
The above described protocol details a method that allows the investigation of both early- and late-stages of organogenesis, often limited by complex cellular and molecular interactions.
This method was previously employed in Figueiredo et al.5 to unravel the role of Notch and Hh signaling in the avian parathyroid/thymus common primordium development.
Herein, new results are shown in Figure 1 and Figure 2 using the same model of organogenesis. Figure 1A depicts the experimental design used to explore the early-stages of thymus and parathyroid formation. The quail embryonic territory comprising the prospective organ rudiments (3/4PAR) was isolated and grown in vitro for 48 h in an organotypic system.
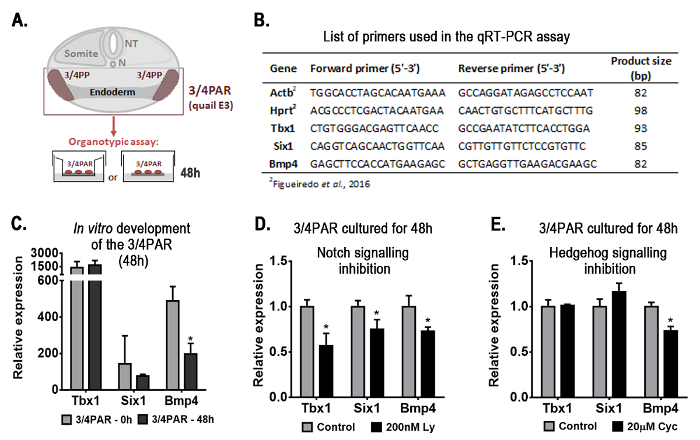
Figure 1. Representative results obtained with the organotypic culture assay: gene-expression analysis of the embryonic region containing the presumptive territories of the thymus and parathyroids (3/4PAR) developed in vitro for 48 h. Schematic representation of the transversal section of the embryo at the region of interest and the experimental design (A). Briefly, the 3/4PAR at qE3 was mechanically isolated and grown in vitro for 48 h. The expression of the 3/4PAR-related genes, Tbx1, Six1, and Bmp4, was examined by qRT-PCR using the primers in the table (B). The expression of Tbx1, Six1, and Bmp4 was analyzed in freshly isolated (3/4PAR-0 h) and cultured (3/4PAR-48 h) tissues (C). The expression of PAR-related genes was analyzed in tissues grown in vitro for 48 h in the presence of 200 nM Ly411575 (D) and 20 µM cyclopamine (E), which are pharmacological inhibitors of Notch and Hedgehog signaling pathways, respectively. Expression of each transcript was measured as a ratio against the mean of the β-actin and hypoxanthine-guaninephosphoribosyltransferase transcript expression levels and expressed in arbitrary units (each transcript in the control = 1). Means and standard deviations were determined with a software for biostatistics analysis and scientific graphic design. Error bars represent standard deviations of the mean. Two-tailed unpaired Student's t-test was used and results were considered significantly different when the p-value was less than 0.05 (p < 0.05). β-actin, Actb; cyclopamine, Cyc; Hypoxanthine-guaninephosphoribosyltransferase, Hprt; LY-411.575, Ly; N, Notocord; NT, Neural Tube; PAR, pharyngeal arch region; PP, pharyngeal pouch. Please click here to view a larger version of this figure.
The expression of genes known to be involved in the formation of PAR structures (PAR-related genes), i.e., Tbx116,17, Six118, and Bmp415,17, was evaluated during the normal development. Quantitative real time PCR (qRT-PCR) was performed as previously described5 (primers are listed in Figure 1B). Transcripts of the three genes were detected in freshly isolated (3/4PAR-0 h) and in 48 h-cultured tissues (3/4PAR-48 h) (Figure 1C). Only Bmp4 expression levels were significantly decreased after 48 h of culture.
To evaluate the role of Notch and Hh signaling pathways in the early-stages of thymus and parathyroid development, pharmacological inhibitors were added to the culture medium during in vitro development. Inhibitor doses are described in Figueiredo et al.5 The expression levels of the three genes analyzed were significantly reduced in the 3/4PAR grown in the presence of Notch inhibitor, when compared to control conditions (without drug) (Figure 1D). Conversely, only Bmp4 transcripts were significantly reduced in the 48 h-cultured tissues when Hg signaling was blocked (Figure 1E).
To study the late-stages of thymus and parathyroid gland organogenesis, cultured tissues were then grafted onto CAMs and allowed to further develop for 10 days (see the experimental design in Figure 2A).
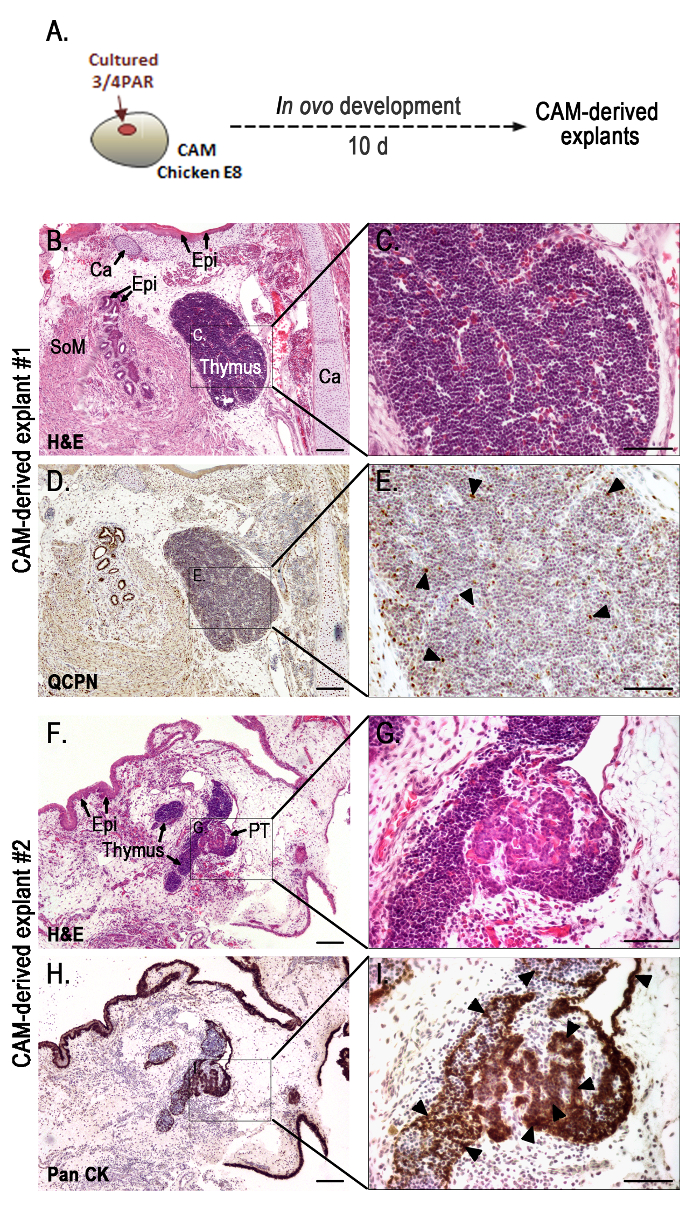
Figure 2. Representative results obtained with the in ovo assay: morphological analysis of the grafts grown for 10 days in the chorioallantoic membrane. Schematic representation of 48 h-cultured PAR grafted onto the CAM and developed for 10 days (A). Serial sections of CAM-derived explants (B–I) slides stained with H&E (B, C, F, and G), immunodetected with QCPN (D and E) and anti-Pan CK (H and I) antibodies, and counterstained with Gill's hematoxylin. Black arrow heads indicate strong immunostaining for QCPN (E) and Pan CK (I). A transverse section of a chimeric thymus with lymphoid cells of host origin and quail-derived thymic epithelial cells with strong QCPN+ signals (black arrowheads) (E). Strong Pan CK+ signals (black arrowheads) in the epithelia of the thymus and parathyroid glands (I). Images were collected using imaging software and a microscope with a camera (see Table of Materials). Ca, cartilage; CAM, chorioallantoic membrane; Epi, epithelium; PAR, pharyngeal arch region; PT, parathyroid glands; SoM, smooth muscle; 10 d, ten days. Scale bars, 50 µm (B, D, F, and H) and 100 µm (C, E, G, and I). Please click here to view a larger version of this figure.
Morphological analysis of organs developing on CAM-derived explants was performed by conventional histology and immunohistochemistry (Figure 2B–I), as previously described5. CAM-derived explants showed fully formed chimeric thymus (Figure 2B–E) with quail-derived (QCPN+) thymic epithelium colonized by lymphoid progenitor cells of donor origin (chicken) (Figures 2D, E). Serial sections of CAM-derived explants further processed for immunocytochemistry with anti-pan cytokeratin (anti-pan CK) antibody (an epithelial cell marker), showed thymic and parathyroid epithelia with normal morphological features (Figure 2H, I). The thymic epithelial cells displayed a reticular architecture while parathyroid parenchymal cells were globular, arranged in clusters and encircled by numerous capillaries. Additionally, other PAR-derived structures from the respiratory apparatus could be observed in the grafts. Cartilage, respiratory epithelium, and smooth muscle associated to the mucosa were easily distinguished in Figure 2B.
Discussion
A crucial aspect for the success of this method is the quality of both the chicken and quail eggs. Considering the long incubation periods, particularly during the in ovo assay, a good quality of chicken eggs improves viability rates (up to 90%) by the end of the procedure. To achieve this, test eggs from different suppliers. Incubate unmanipulated eggs for long periods (up to 16-17 days) and check their development. To be considered a good quality batch, more than 80% of the embryos should present normal development. It is also important to ensure that each incubation step provides reproducible synchronous developmental stages to guarantee reliable and truly representative results at the end. Due to egg shell porosity, maintain a humidified atmosphere in the incubator for all egg incubation steps. To avoid environmental contamination, antibiotics can be added to the PBS solutions in the procedure (optional step).
This method starts by isolating quail organ rudiments and growing them in an organotypic system for 48 h. This first-step, already used to study thymus and parathyroid early-development5, can also be applied to other organs if the assay limitations are taken into account. Small explants of organ rudiments (less than 3 mm) and short periods of in vitro incubation (up to 48 h) are advised to prevent inefficient diffusion of nutrients and drying of the tissues, which usually occurs when explants reach larger dimensions.
This method also allows the modulation of signaling pathways, which bypasses complex genetic manipulation by the use of soluble reagents, such as pharmacological inhibitors5,7. For this procedure, increasing doses of the drug should be tested to identify the physiological/toxic culture conditions. The inhibitory actions can be measured by gene expression analysis of the signaling pathway target-genes.
In step-two of this procedure, cultured tissues are grafted onto the CAM to study the late-stages of organ formation. The CAM assay has been used in other contexts of organogenesis like skeletal development and feather formation by direct grafting of the organ rudiments onto CAM9,10,11. Additionally, CAM engraftment was also successfully applied in mice-into-chicken xenografts to study testes maturation19. Although the CAM assay is a powerful research tool to study late-stages of organ formation, it is important to be aware of its limitations.One of the most critical steps of the protocol is the CAM preparation for grafting. It is important to target only the smaller vessels for vascular lesions. However, if only a few of those are lesioned, the subsequent angiogenic response may not be sufficient to promote invasion of grafted tissues by new vessels originating from the CAM. Consequently, the transplanted tissues will not have enough nutrients or gas exchanges to sustain growth. On the other hand, if the integrity of large vessels is compromised when preparing the wounded area, the embryo has to be discarded.
An important limitation of in ovo development using the CAM is the anatomical displacement of formed organs, due to three-dimensional constraint of growing explants. This often results in the incomplete separation of thymus and parathyroid glands (Figure 2F–I), and in inadequate thymic segmentation, with reduction of the normal number of organs formed5.
Another constraint of the CAM system may be a sub-optimal accessibility of pharmacological reagents5, even with daily drug administration, thus limiting the analysis of explant late-stage development. As an example, previous studies showed that cyclopamine successfully inhibit Hh signaling in ovo, while Notch signaling inhibitor, Ly411575, showed no inhibitory properties in ovo5.
Beyond these limitations, this method provides important experimental approaches to investigate the early- and late-stages of organ formation using the avian model. In addition, developing tissues can be manipulated and harvested at any time-window of the in vitro and in ovo development making the method also suitable for longitudinal studies in organogenesis.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors are grateful to António Cidadão, Isabel Alcobia, and Leonor Parreira for the critical reading of the manuscript, to Padma Akkapeddi for video narration, and to Vitor Proa from the Histology Service of the Instituto de Histologia e Biologia do Desenvolvimento, Faculdade de Medicina de Lisboa, Universidade de Lisboa, for technical support. We are particularly indebted to Paulo Caeiro and Hugo Silva from the Unidade de audiovisuais (Audiovisual Unit), Faculdade de Medicina de Lisboa, Universidade de Lisboa for their outstanding commitment to the production of this video. We acknowledge Leica Microsystems for kindly providing a stereoscope equipped with video system and to Interaves – Sociedade Agro-Pecuária, S.A for contributing with quail fertilized eggs. This work was supported by Faculdade de Medicina de Lisboa, Universidade de Lisboa (FMUL).
Materials
| Chicken fertilized eggs (Gallus gallus) | Pintobar, Portugal | Poultry farm | |
| Quail fertilized eggs (Coturnix coturnix) | Interaves, Portugal | Bird farm | |
| 15 mL PP centrifuge tubes | Corning | 430052 | |
| 50 mL PP centrifuge tubes | Corning | 430290 | |
| 60 x 20 mm pyrex dishes | Duran group | 21 755 41 | |
| 100 x 20 mm pyrex dishes | Duran group | 21 755 48 | |
| Polycarbonate Membrane Insert | Corning | 3412 | 24 mm transwell with 0.4 mm Pore Polycarbonate Membrane Insert |
| Membrane filter | Millipore | DTTP01300 | 0.6 mm Isopore membrane filter |
| 6-well culture plates | Nunc, Thermo Fisher Scientific | 140675 | |
| Petri dish, 35 x 10 mm | Sigma-Aldrich | P5112 | |
| Pyrex bowls | from supermarket | ||
| Transfer pipettes | Samco Scientific, Thermo Fisher Scientific | 2041S | 2 mL plastic pipet |
| Glass pasteur pipette | normax | 5426015 | |
| Whatman qualitative filter paper | Sigma-Aldrich | WHA1001090 | Filter paper |
| Clear plastic tape | from supermarket | ||
| Cytokeratin (pan; acidic and basic, type I and II cytokeratins), clone Lu-5 | BMA Biomedicals | T-1302 | |
| Cyclopamine hydrate | Sigma-Aldrich | C4116 | Pharmacological inhibitor of Hh signalling |
| Fetal Bovine Serum | Invitrogen, Thermo Fisher Scientific | Standart FBS | |
| Paraformaldehyde | Sigma-Aldrich | P6148 | |
| Penicillin-Streptomycin | Invitrogen, Thermo Fisher Scientific | 15140-122 | |
| Phosphate-Buffered Saline (PBS) | GIBCO, Thermo Fisher Scientific | 10010023 | |
| QCPN antibody | Developmental Studies Hybridoma Bank | QCPN | |
| RPMI 1640 Medium, GlutaMAX Supplement | GIBCO, Thermo Fisher Scientific | 61870010 | |
| Bluesil RTV141A/B Silicone Elastomer 1.1Kg Kit | ELKEM/Silmid | RH141001KG | To prepare the back base for petri dish |
| Stemolecule LY411575 | Stemgent | 04-0054 | Pharmacological inhibitor of Notch signalling |
| TRIzol Reagent | Invitrogen, Thermo Fisher Scientific | 15596026 | Reagent for total RNA isolation |
| Dumont #5 Forceps | Fine Science Tools | 11251-30 | Thin forceps |
| Extra fine Bonn scissors, curved | Fine Science Tools | 14085-08 | Curved scissors |
| Insect pins | Fine Science Tools | 26001-30 | |
| Micro spatula | Fine Science Tools | 10087-12 | Transplantation spoon |
| Minutien Pins | Fine Science Tools | 26002-20 | Microscalpel |
| Moria Nickel Plated Pin Holder | Fine Science Tools | 26016-12 | Holder |
| Moria Perforated Spoon | Fine Science Tools | 10370-17 | Skimmer |
| Wecker Eye Scissor | Fine Science Tools | 15010-11 | |
| Camera | Leica Microsystems | MC170 HD | |
| Stereoscope | Leica Microsystems | Leica M80 | |
| Microscope | Leica Microsystems | DM2500 |
Referanslar
- Le Douarin, N. M. The Nogent Institute-50 years of embryology. Int J Dev Biol. 49 (2-3), 85-103 (2005).
- Chuong, C. M., Wu, P., Plikus, M., Jiang, T. X., Bruce Widelitz, R. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr Top Dev Biol. 72, 237-274 (2006).
- Davey, M. G., Tickle, C. The chicken as a model for embryonic development. Cytogenet Genome Res. 117 (1-4), 231-239 (2007).
- Nowak-Sliwinska, P., Segura, T., Iruela-Arispe, M. L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis. 17 (4), 779-804 (2014).
- Figueiredo, M., Silva, J. C., et al. Notch and Hedgehog in the thymus/parathyroid common primordium: Crosstalk in organ formation. Dev Biol. 418 (2), 268-282 (2016).
- National Research Council. Chapter 6 – Recent Advances in Developmental Biology. Scientific Frontiers in Developmental Toxicology and Risk Assessment. , (2000).
- Moura, R. S., Coutinho-Borges, J. P., Pacheco, A. P., Damota, P. O., Correia-Pinto, J. FGF signalling pathway in the developing chick lung: expression and inhibition studies. PLoS One. 6 (3), e17660 (2011).
- Hamburger, V., Hamilton, H. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92 (1951).
- Takahashi, Y., Bontoux, M., Le Douarin, N. M. Epithelio-mesenchymal interactions are critical for Quox 7 expression and membrane bone differentiation in the neural crest derived mandibular mesenchyme. EMBO J. 10 (9), 2387-2393 (1991).
- Maeda, Y., Noda, M. Coordinated development of embryonic long bone on chorioallantoic membrane in ovo prevents perichondrium-derived suppressive signals against cartilage growth. Bone. 32 (1), 27-34 (2003).
- Ishida, K., Mitsui, T. Generation of bioengineered feather buds on a reconstructed chick skin from dissociated epithelial and mesenchymal cells. Dev Growth Differ. 58 (3), 303-314 (2016).
- Le Douarin, N. M., Jotereau, F. Tracing of cells of the avian thymus trough embryonic life in interspecific chimeras. J. Exp. Med. 142, 17-40 (1975).
- Farley, A. M., et al. Dynamics of thymus organogenesis and colonization in early human development. Development. 140, 2015-2026 (2013).
- Gordon, J., Bennett, A. R., Blackburn, C. C., Manley, N. R. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech Dev. 103, 141-143 (2001).
- Neves, H., Dupin, E., Parreira, L., Le Douarin, N. M. Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev Biol. 361 (2), 208-219 (2012).
- Jerome, L. A., Papaioannou, V. E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 27 (3), 286-291 (2001).
- Nie, X., Brown, C. B., Wang, Q., Jiao, K. Inactivation of Bmp4 from the Tbx1 expression domain causes abnormal pharyngeal arch artery and cardiac outflow tract remodeling. Cells Tissues Organs. 193 (6), 393-403 (2011).
- Zou, D., Silvius, D., Davenport, J., Grifone, R., Maire, P., Xu, P. X. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev Biol. 293 (2), 499-512 (2006).
- Uematsu, E., et al. Use of in ovo. chorioallantoic membrane engraftment to culture testes from neonatal mice. Comp Med. 64 (4), 264-269 (2014).

