Assessing Spatial Memory Impairment in a Mouse Model of Traumatic Brain Injury Using a Radial Water Tread Maze
Özet
Here we present a protocol for a mouse-specific test of cognition that does not require swimming. This test can be used to successfully distinguish controlled cortical impact-induced traumatic brain injury mice from sham controls.
Abstract
Despite the recent increase in use of mouse models in scientific research, researchers continue to use cognitive tasks that were originally designed and validated for rat use. The Radial Water Tread (RWT) maze test of spatial memory (designed specifically for mice and requiring no swimming) has been shown previously to successfully distinguish between controlled cortical impact-induced TBI mice and sham controls. Here, a detailed protocol for this task is presented. The RWT maze capitalizes on the natural tendency of mice to avoid open areas in favor of hugging the sides of an apparatus (thigmotaxis). The walls of the maze are lined with nine escape holes placed above the floor of the apparatus, and mice are trained to use visual cues to locate the escape hole that leads out of the maze. The maze is filled with an inch of cold water, sufficient to motivate escape but not deep enough to require that the mouse swim. The acquisition period takes only four training days, with a test of memory retention on day five and a long-term memory test on day 12. The results reported here suggest that the RWT maze is a feasible alternative to rat-validated, swimming-based cognitive tests in the assessment of spatial memory deficits in mouse models of TBI.
Introduction
Memory impairments are among the most common symptoms reported by patients following traumatic brain injury (TBI)1,2. Accurate identification and assessment of analogous memory deficits in animal models of TBI, therefore, are essential to our understanding of this condition and its management. Here, we present a protocol to test spatial memory in a mouse model of TBI using a Radial Water Tread (RWT) maze. This apparatus was previously shown to assess cognitive deficits in mouse models of controlled cortical impact (CCI)-induced TBI3, and represents a potential alternative to rat-validated, swim-based tests of cognition.
The growing diversity and availability of transgenic mouse models has led to a recent increase in the use of mice over rats in scientific research4. Despite this shift, researchers continue to rely on behavioral and cognitive tasks that were originally designed and validated for rat use. The most common tests currently used to assess cognition in mice, the Morris Water Maze (MWM) and the Barnes circular maze, were specifically designed to capitalize on instinctual behaviors found in rats5,6. Considering the genetic, neuroethological, and cognitive differences that exist between these two species4, it is unsurprising that mice consistently underperform on these tasks7,8.
Species-dependent differences in testing ability are particularly concerning in swimming-based cognitive tests, such as the MWM. While both rats and mice are proficient swimmers, researchers have identified several mouse strains that perform remarkably poorly on swimming-based cognitive tasks9,10,11,12,13. Even in wild-type animals, rats generally outperform mice7,8. While this could be interpreted as a species-specific difference in spatial memory, analogous follow-up testing using a dry-land maze revealed no species-dependent differences in cognitive performance8. A number of factors unrelated to cognition could account for this finding, including species-dependent differences in either swimming ability or search strategy. Indeed, factor analysis of mouse-specific search strategies in the MWM show that noncognitive factors (in particular, thigomotaxis and passivity [i.e., floating]) may play a more significant role in MWM performance than spatial learning14.
Here, we demonstrate the use of a cognitive test designed to capitalize on the instinctual behavior of mice, and which does not require swimming, to measure spatial memory impairment in a mouse model of CCI-induced TBI. While the RWT maze (Figure 1A-B) was conceived as a novel hybrid of the MWM and Barnes circular maze, it was specifically designed to take advantage of thigmotactic behavior instinctual to mice15,16. The apparatus consists of a 32 inch diameter galvanized steel tub in which nine evenly spaced exit holes have been bored. The holes are centered 2-1/4 inches above the floor of the tub and are sized to fit commonly available 1-1/2 inch ABS DWV SPG x SJ trap adapters. Eight of the exits are capped from the outside and blinded to a depth of 1 inch with rubber stoppers. The ninth is connected by a 90° acrylonitrile butadiene styrene (ABS) elbow to an opaque plastic box from which the mouse can be easily removed after testing. Over the course of a brief acquisition period, the mouse is trained to use the unique visual cues lining the maze to locate this escape box. During testing, the maze is filled with an inch of cold water (12-14 °C), sufficiently aversive enough to promote escape, but not deep enough that the mouse is required to swim.
The RWT maze represents a low-cost, low-maintenance alternative to the MWM, and has been used successfully in aged and transgenic mice15,17,18,19, and CCI-induced mouse models of TBI3. The protocol outlined here represents a simple and effective method for measuring spatial memory impairment requiring no pre-injury training, and could be easily modified to suit the particular needs of a research laboratory.
Protocol
All procedures and animal handling were conducted in accordance with the animal care guidelines issued by the National Institutes of Health and by the University of Washington Animal Care and Use Committee.
1. Surgery
- Anesthetize the mouse at 5% isoflurane in an induction box until unconscious. Confirm anesthesia by a reduction in the breathing rate and the absence of a withdrawal reflex following toe-pinch.
- Maintain anesthesia via nose cone at 2-2.5% throughout surgery. Monitor the breathing rate throughout surgery to ensure the mouse remains unconscious.
- Place the mouse prone on a heating pad and position the mouse in the stereotactic device using ear bars, ensuring that the head is secure and flat.
- Remove hair from the scalp using hair removal cream. Rinse the scalp thoroughly with saline.
- Clean the surgical site with an alternating iodine and 70% ethanol wash.
- Administer a subcutaneous injection of Lidocaine and Bupivacaine (1 mg/kg) at the scalp.
- With surgical scissors, make a longitudinal midline incision, and retract the skin to reveal the skull.
- Using a 5 mm trephine saw preform a craniotomy over the left frontoparietal cortex with center point at 2.5 mm behind bregma and 2.5 mm left of the midline. Carefully remove the circle of bone to expose the brain.
- Set the impactor device to a velocity of 6 m/s and 200 ms dwell time.
- Position the impactor device until the 3 mm convex impact tip is lightly touching the surface of the brain at 2.5 mm behind bregma and 2.55 mm left of the midline. Retract the impact tip, and lower by 1 mm (impact depth). When ready, fire the device, generating the desired impact.
- Cover the craniotomy with a sterile polypropylene disc cemented to derma with tissue adhesive, and suture the incision closed.
- Remove the mouse from anesthesia, and give IP injection of Buprenorphine (0.5 mg/kg).
- Allow the mouse to recover in a clean cage, warmed by a heating pad. The mouse should be monitored for signs of pain or distress over the next 24 h.
NOTE: Sham controls should receive identical treatment as above, with steps 1.8-1.9 omitted.
2. Radial Water Tread Maze Construction
- Bore 9 exit holes, large enough to accommodate 1-1/2 inch ABS DWV SPG x SJ trap adapters, at equal intervals around the circumference of a 32 inch diameter galvanized steel tub. Center these exit holes roughly 2-1/4 inches above the floor of the tub.
- Fit a 1-1/2 inch ABS DWV SPG x SJ trap adapter into each of the exit holes, and secure with the included ring nuts.
- With rubber stoppers, cap eight of the nine exits from the exterior of the apparatus. The final, uncapped exit will serve as the escape route. It does not matter which exit is designated as the escape route.
- Attach a 90° ABS elbow to the exterior end of the remaining exit. The 90° bend serves to prevent test subjects from visually determining the correct escape route from inside the maze.
- Construct the escape box from any opaque box capable of being sanitized and roughly 30 cm x 15 cm x 15 cm in size. Cut a hole on the side of the box, directly above the floor, large enough to accommodate a the 90° ABS elbow.
- Attach the escape box to the terminal end of the 90° ABS elbow.
- Slightly elevate the escape box (less than one inch) above the surface of the floor. This allows ample room for an electric heating pad, or other heating source, to be placed beneath the escape box.
- Print and laminate at least 5 unique, visual cues. Use simple, high contrast images that can be easily discerned from within the apparatus. Black and white clipart shapes (triangle, square, circle) are recommended.
- Using magnets, adhere the visual cues to the inner walls of the apparatus. Cues should be roughly equal distance apart, around the circumference of the apparatus.
3. Radial Water Tread Maze Protocol
NOTE: Water maze testing should begin only after the surgical site has healed (roughly one week post-surgery).
- Preparing for testing.
- Allow mice to acclimate to the testing room for at least 30 min prior to commencing testing.
- Sanitize the apparatus using a 70% ethanol spray.
- Fill the apparatus with roughly 1 inch of cold (12-14 °C) water.
- Place an electric heating pad, or other heating source, directly underneath the escape box. Keep the escape box dark and warm throughout the duration of testing.
- Position a bright light source over the apparatus.
NOTE: If using a lamp that might be visible to research animals from the apparatus itself, take care to ensure that the lamp is placed in the same position each day. The lamp itself might represent another visual cue for the mice to use to locate the escape box, and moving it drastically from day to day might complicate results.
- Testing Protocol
- Remove the mouse from its cage gently by the tail, and place in the center of the apparatus.
- As soon as the animal is in in the apparatus, begin timing.
- Once the animal has found the correct exit, and has located/entered the escape box, stop timing and record the number of seconds required to find the correct path.
- If the animal attempts to climb into a terminating hole and does not spontaneously reenter the maze after 10 s, guide the animal back into the center of the maze by hand.
- If the animal fails to find the correct path to the escape box within 3 min (180 s), score the trial as a failure and record as 180 s. Carefully guide the animal toward the correct path by hand.
- Allow the mouse to remain in the escape box for a 1 min, inter-trial rest.
- Once the 1 min rest has passed, remove the animal from the escape box and return to its home cage.
- Thoroughly sanitize the escape box and exits with a 70% ethanol spray to prevent the mouse from using olfactory cues to locate the correct escape route. This step should take no more than a few seconds.
- Return the mouse to the maze for the next trial.
- Repeat Steps 3.2.1-3.2.9 until the mouse has completed a total of three trials, and has either located or been led to the escape box three times.
- Following the final 1 min rest, return the mouse to its home cage.
- Drain and replace the water in the apparatus between animals to ensure consistent temperature throughout testing.
- Repeat steps 3.2.1-3.2.12 for each mouse to be tested.
- On the following day, repeat preparation steps 3.2.1-3.2.5. Take special care in ensuring that visual cues remain in consistent positions between testing days.
- Test animals using the above daily testing protocol for three trials a day for four days (training period), with a final three-trial test for memory retention on the fifth day. A sixth three-trial test (Long Term Memory retention) can be given on day twelve.
- Do not perform any tests between testing days five and twelve.
- Analysis
- If a mouse fails to complete the maze in under 180 s during any two-day period (i.e., a total of consecutive 6 trials all scored at 180 s), consider the mouse insufficiently motivated by the testing conditions, and remove from analysis.
- Calculate the mean latency to complete the maze for each subject by testing date by averaging their three daily trials for that day.
- Obtain group differences in memory retention using standard t-tests to compare groups at the fifth day test and long-term memory testing date. If more than two groups are being compared, one-way ANOVA followed by appropriate post-hoc analysis (such as Tukey's test) to follow up on any significance obtained should be employed instead.
- Obtain group differences in the acquisition period (days 1-4) via repeated measure analysis of variance.
Representative Results
The RWT maze (Figure 1) was used to investigate injury-dependent spatial memory deficits in mice randomly assigned to receive either controlled cortical impact-induced TBI or sham surgery. The injury was generated using a solenoid-driven cortical impact with a 3 mm convex tip and the following injury parameters: 6 m/s strike velocity, 1 mm depth of penetration, and 200 ms contact time. Mice received cognitive testing starting at 35 days post-surgery, and were given four days of training (acquisition period) followed by a test of memory retention on day 5 and a test of long-term memory on day 12, as outlined in the above protocol. Figure 2 shows a clear group distinction in latency to complete the maze over time between TBI mice and sham controls. Analysis of the data presented here revealed that latency was significantly reduced in sham controls compared to TBI mice on both day 5 and day 12 (Figure 2). No subjects met the criteria to be considered insufficiently motivated by the testing conditions, and thus no mice were removed from analysis.
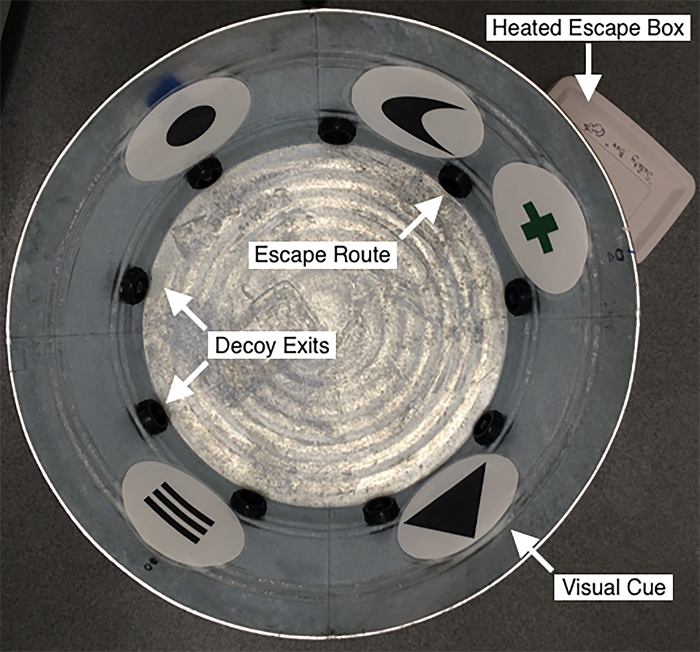
Figure 1: Radial Water Tread Maze.
The maze consists of a 32-inch galvanized steel tub with nine exits, each 2-1/4 inch above the apparatus floor. Of these exits, eight terminate after roughly 1 inch (decoy exits), and one leads to a heated escape box (30 cm x 15 cm x 15 cm) hidden behind a 90° angle bend to prevent visual confirmation of escape route. Upon reaching the escape box, subjects received a 1-minute, inter-trial rest. The apparatus is filled with one inch of cold water (12 – 14 °C) to motivate escape behavior, and lined with five unique visual cues for spatial orientation. Please click here to view a larger version of this figure.
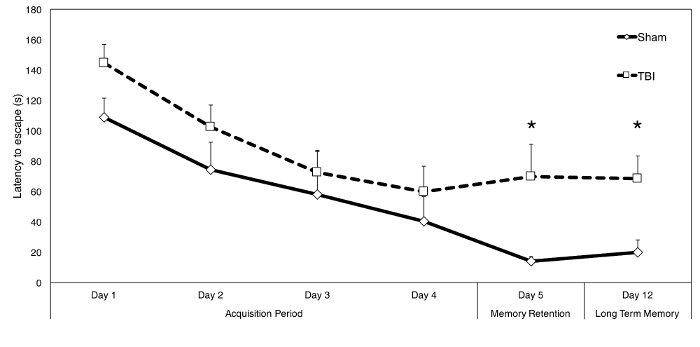
Figure 2: Representative Results of the Radial Water Tread Maze.
C57BL/6J mice, 10 weeks of age, received either controlled control impact (n=11) or sham (n=6) surgery. Injury parameters were as follows: 3 mm convex impact tip, 6 m/s velocity, 1 mm depth of penetration and 200 ms dwell time. Mice began receiving RWT maze testing 35 days post-injury. Testing protocol consisted of three trials per day for four days (acquisition period), followed by a three-trial test of memory retention on day five, and a three-trial long term memory test on day 12. Repeated measure analysis of variance found no group differences during the acquisition period (days 1-4) (F[1,15]=1.844, p>0.05). Latency to complete the maze was significantly elevated in TBI mice compared to sham controls on both day 5 (t[15]=1.907, p< 0.05) and day 12 (t[15]=2.242, p< 0.05). Data points represent group means (± SEM). Significance was determined by standard t-test (one-tailed, based on a priori hypothesis of group differences) and is reported as p< 0.05 (*) Please click here to view a larger version of this figure.
Discussion
The RWT maze protocol presented here successfully distinguishes between CCI-induced TBI mice and sham controls, and represents a feasible, mouse-centric, alternative to the MWM and Barnes circular maze. While the results reported here speak only to the use of the RWT maze in a TBI mouse model, this apparatus has been used successfully in aged and transgenic models where stress-induced noncompliance resulting from swim-based testing made using the MWM impractical15,17,18,19. Other mouse models in which noncompliance or motor deficits are potential research concerns may also benefit from this cognitive task.
In addition to the design advantages previously discussed, one of the benefits of the RWT maze is its simplicity, both in terms of construction and use. The apparatus itself is easily constructed using relatively low-cost materials and can be sanitized without damaging its components, making it ideal for specific pathogen free (SPF) facilities. The acquisition period requires only four days of testing, with no pre-injury training necessary. Daily testing involves minimal time commitment (~10 min/animal), and requires little experience before mastery. Because of its simplicity in construction and use, the RWT maze is ideal for a laboratory constrained by a relatively tight budget and with little to no behavioral or cognitive testing experience.
There are several steps researchers can take to reduce potential variance when using the protocol we have outlined here. Some recommendations to achieve consistent, quality results include testing at similar times of day across cohorts, using the same person/persons to conduct testing when possible, maintaining a quiet and calm testing environment, and extensive handling of mice prior to testing. It should also be emphasized that while the water temperature listed in this protocol resulted in successful testing conditions for male C57BL/6J mice, temperature preference and hypothermia are heavily strain and gender dependent20. Labs should conduct their own preliminary testing if using other strains or female mice to determine an effective temperature range that does not induce hypothermia. Finally, visual cues should be simple, easily distinguishable, and visible to subjects during testing. Basic black and white shapes (laminated, or sheathed in plastic, so that they can be sanitized) are preferable.
While the testing protocol described here is relatively simple, it could easily be adapted to give researchers a plethora of information beyond latency to escape. Animal tracking software can be employed to collect a wealth of additional parameters, and could be used to identify group specific differences in search behavior. Such software is not inherently necessary for testing, however, as demonstrated here. Additionally, probe trials, in which the exit to the escape box is blocked or the visual cues have been rotated to indicate exit in a terminating hole, could be used to supplement the protocol outlined here. While a four-day acquisition period was all that was necessary in order to generate the representative results presented here, we encourage researchers testing other TBI parameters/models or genetic strains to conduct their own pilot testing, and shorten or extend the training period as needed.
There are limitations with this testing protocol which deserve mention. First, replacing the cold water and sanitizing the apparatus between subjects can be both time intensive and physically demanding. To minimize researcher effort and time, testing should be conducted in a room with an available floor drain to ease water drainage, and easy access to a cold water sink with attached hose. Second, the use of hand timing without video recording introduces a risk of human error. As tracking software can be prohibitively expensive for some labs, however, such a risk is unavoidable if hand timing must be used. In addition, as with the MWM, spatial memory cannot be retested in the same subjects using the RWT maze (i.e., once the maze has been learned, it cannot be unlearned to allow for further spatial memory testing). Also, there may be effects from TBI-related motor deficits that could alter the ability of TBI-mice to perform the maze compared to shams. With that in mind, however, it may be that all rodent spatial memory tests which require movement would have a similar limitation. Motion tracking software could be employed with the RTM to assess total path length and speed and to quantify such differences. Finally, researchers should be aware that the RWT maze described here does not represent the only non-swimming test available for testing cognition in mouse models of TBI. Other tests, such as the y-maze, have been used to successfully distinguished sham from TBI mice21. Researchers should weigh the pros and cons of each test before deciding which to use in their lab.
The RWT maze protocol described here represents a novel, mouse-specific alternative to the rat-validated cognitive tests currently used in mouse-model research, and does not require swimming. As the use of mouse models in scientific research continues to rise, the eventual adoption of mouse-validated research tools could lead to more accurate research results.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Institute for Translational Health Sciences pilot project grant opportunity (UL1TR000423), the University of Washington Center on Human Development and Disability, and the University of Washington Animal Behavior Core and Brain Imaging Core. We would like to acknowledge Dr. Warren Ladiges for his role in the development and dissemination of the original Radial Water Tread maze design and protocol presented here. We also thank Toby Cole for his assistance with this project.
Materials
| 35 Gal. Hot Dipped Steel Round Tub | Home Depot | Internet #206638142 | Needed: 1 |
| 1-1/2 in. ABS DWV SPG x SJ Trap Adapter | Home Depot | Internet #100344703, Store SKU #188956 | Needed: 9 |
| 1-3/4 in. x 1-7/16 in. Black Rubber Stopper | Home Depot | Internet #100114974 Store SKU #755844 | Needed: 8 |
| 1-1/2 in. ABS DWV 90 Degree Hub x Hub Elbow | Home Depot | Internet #100346663 Store SKU #188603 | Needed: 1 |
| HDX 10 Gal. Storage Tote |
Home Depot | Internet #202523587 Store SKU #258804 Store SO SKU #258804 | Needed: 1 |
| Impact One Stereotaxic Impactor for CCI | Leica Biosystems | 39463920 | Needed: 1 |
| Vernier Stereotaxic w/ Manual Fine Drive Stereotaxic Instrument for Small Animals | Leica Biosystems | 39463001 | Needed: 1 |
Referanslar
- Levin, H. Neurobehavioral outcome of closed head injury: Implications for clinical trials. J. Neurotrauma. 12 (4), 601-610 (1995).
- Schretlen, D., Shapiro, A. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 15 (4), 341-349 (2003).
- Cline, M. M., et al. Novel application of a radial water tread maze can distinguish cognitive deficits in mice with traumatic brain injury. Brain Res. 1657, 140-147 (2017).
- Ellenbroek, B., Youn, J. Rodent models in neuroscience research: Is it a rat race?. Dis. Model. Mech. 9 (10), 1079-1087 (2016).
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci Methods. 11 (1), 47-60 (1984).
- Barnes, C. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psych. 93 (1), 74-104 (1979).
- Frick, K., Stillner, E., Berger-Sweeney, J. Mice are not little rats: Species differences in a one-day water maze task. Neuroreport. 11 (16), 3461-3465 (2000).
- Whishaw, I., Tomie, J. Of Mice and Mazes: Similarities Between Mice and Rats on Dry Land But Not Water Mazes. Physiol Behav. 60 (5), 1191-1197 (1995).
- Francis, D., Zaharia, M., Shanks, N., Anisman, H. Stress-induced disturbances in Morris water-maze performance: Interstrain variability. Physiol Behav. 58 (1), 57-65 (1995).
- Wahlsten, D., Rustay, N., Metten, P., Crabbe, J. In search of a better mouse test. Trends Neurosci. 26 (3), 132-136 (2003).
- Crawley, , et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. (Berl). 132 (2), 107-124 (1997).
- Wahlsten, D., et al. Different data from different labs: lessons from studies of gene-environment interaction. J. Neurobiol. 54 (1), 283-311 (2002).
- Rogers, D. C., et al. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 105 (2), 207-217 (1999).
- Wolfer, D. P., Stagljar-Bozicevic, M., Errington, M. L., Lipp, H. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact?. Physiology. 13 (3), 118-123 (1998).
- Koopmans, G., Blokland, A., Vannieuwenhuijzen, P., Prickaerts, J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 79 (4-5), 683-693 (2003).
- Deacon, R., Rawlins, N. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci. 116 (3), 472-478 (2002).
- Pettan-Brewer, C., et al. A novel radial water tread maze tracks age-related cognitive decline in mice. Pathobiol Aging Age Relat Dis. 3, 1-4 (2013).
- Wiley, J., Pettan-Brewer, C., Ladiges, W. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell. 10 (3), 418-428 (2011).
- Enns, L., et al. Disruption of Protein Kinase A in Mice Enhances Healthy Aging. PLoS ONE. 4 (6), (2009).
- Ivonen, H., Nurminen, L., Harri, M., Tanila, H., Puolivali, J. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 141 (2), 207-213 (2003).
- Shultz, S. R., et al. Granulocyte-macrophage colony-stimulating factor is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 31 (10), 976-983 (2014).

