Production of Humanized Mouse via Thymic Renal Capsule Grafting, CD34+ Cells Injection, and Cytokine Delivery
Özet
Humanized mouse models provide a more accurate representation of the human immune microenvironment. This manuscript describes the process in which these models are created through a renal graft of human thymus, injection of human CD34+ cells, and the targeted delivery of human cytokine transgenes to promote CD34+ cell proliferation and differentiation.
Abstract
Animal models provide a vital translation between in vitro and in vivo biomedical research. Humanized mouse models provide a bridge in the representation of human systems, thereby allowing for a more accurate study of pathogenesis, biomarkers, and many other scientific queries. In this method described, immune-deficient NOD-scid IL2Rγnull (NSG) mice are implanted with autologous thymus, injected with liver-derived CD34+ cells followed by a series of injected cytokine deliveries. In contrast to other models of a similar nature, the model described here promotes an improved reconstitution of immune cells by delivering cytokines and growth factors via transgenes encoded in AAV8 or pMV101 DNA-based vectors. Moreover, it offers long-term stability with reconstituted mice having an average lifespan of 30 weeks after CD34+ injections. Through this model, we hope to provide a stable and impactful method of studying immunotherapy and human disease in a murine model, thus demonstrating the need for predictive preclinical models.
Introduction
While animal models have created a deeper understanding of cellular and molecular systems, the challenge remains in elucidating the intricacies of species-specific systems, such as immunity, physiology, and other areas of pathology. Non-human primates (NHP), such as chimpanzees, have historically been used to compensate for the wanting gaps in model research; however, the NHP model can be quite costly and inaccessible, particularly as their use has been banned in Europe1.
Following a successful grafting procedure, the murine system replicates the human immune system, as demonstrated through the repopulation of the lymphoid organs. The development of mice with functional human immune systems provides the opportunity to conduct translational research on human immunity in a variety of contexts. Immunodeficient mice engrafted with human cells and tissues that can successfully replicate an operative human immune system facilitate the study of hematopoiesis, immunity, gene therapy2, infectious diseases3, cancer4, and regenerative medicine5. Our group and some of our collaborators have published results using this model that demonstrates preclinical models of cutaneous melanoma6. This model is versatile enough to be applied to innumerable fields beyond the context of melanoma and immunotherapy research.
Peripheral blood mononuclear cells (PBMCs) are commonly used for humanization as they result in robust reconstitution of T cells, which have established roles in immune tolerance; however, because of their low rate of self-renewal and their high rate of mature lineage-committed cells, PBMCs are often replaced with human-stem-cell (HSC)-based products, which can be derived from fetal liver7. In combination with these derived HSC products, the addition of implanting the human thymus under the kidney capsules of NSG mice creates a system capable of supporting human T cell development. This model, known as the bone marrow-liver-thymus (BLT), is highly advantageous because it allows for multilineage hematopoiesis, T-cell education in the autologous thymus, and HLA restriction8.
The model proposed in this manuscript is a modified BLT model with additional cytokine delivery. Proinflammatory cytokines have been shown to bolster the abilities of effector immune cells, specifically through IL-15 based immunotherapies9. CD45+ lymphocytes are observed in the peripheral blood of humanized mice (Hu-mice) approximately 8-12 weeks after human CD34+ cell injection, displaying an evident increase in reconstitution compared to circulating blood of regular NSG mice. Using the Adeno-Associated vector to deliver human IL-3, IL-7, and GM-CSF, the levels of human CD45+ cells increased in circulation compared to those mice that do not receive the cytokines. The addition of the DNA Combo II cytokines (SCF, FLT3, CKIT, and THPO) improves T cells and myeloid cell differentiation10. The addition of cytokine delivery distinguishes this method amongst the other Hu-mice models, as is supported by published data10.
The development of this model with an innate and adaptive human immune response has allowed to publish data regarding therapy resistance and the tumor microenvironment10. Provided laboratories can access tissue samples to utilize this method; this Hu-mice model has great potential for other labs to study similar fields as well as expand into other areas of immunotherapy and preclinical studies.
Protocol
All protocols involving the use of animals are closely monitored by the Wistar Institute's Institutional Animal Care and User Committee (IACUC). The laboratory adheres to the guidelines set by this committee and the attending veterinarian to ensure the health, safety, and wellbeing of the animals involved. Prior to following this protocol, veterinarian and IACUC approval are required, and individuals may have variations in the specific surgical techniques and animal handling compared to the protocol per the advice of those aforementioned parties involved in animal welfare.
NOTE: Tissue samples can be frozen until ready for use. Additionally, CD34+ isolation can be performed the same day or the following day as the renal graft surgery. This information will inform when Busulfan will be injected: if intending to conduct surgery on the same day as isolation, Busulfan should be injected preemptively in preparation of receiving tissue. Treat thirty female 5-7 week-old NSG mice with 30 mg/kg (100 µL, PBS 1x) of a freshly made myelo-depleting drug; Busulfan injected IP 24 h before surgery.
1. CD34+ cells isolation
- Prepare the digestion solution by dissolving 100 mg of Collagenase/Dispase in 50 mL of RPMI1640. Use a 0.22 µm vacuum filter to sterilize the digestion solution.
- Fetal liver (>20 weeks of age) is provided in 50 mL of RPMI1640 medium. Aspirate and discard the media, leaving 10 mL inside the tube containing the liver.
- Wash the tissue with 45 mL of RPMI1640 + 100 µg/mL of the antibiotic Primocin twice in a 50 mL conical tube, vacuuming off the media between each wash. Be sure to let the tissue settle to the bottom of the tube before vacuuming off the media.
- Place a sterile tissue sieve in a 200 mL sterile beaker.
- Pour the fetal liver through the sieve and use the end of a 10 mL sterile plastic syringe to grind the tissue.
- Rinse the liver cells remaining in the sieve using 50 mL of RPMI1640 + 100 µg/mL of the antibiotic Primocin followed by 50 mL of RPMI1640 Collagenase/Dispase media (2 mg/mL). The 200 mL beaker now contains the RPMI1640 with Primocin, RPMI1640 collagenase/dispase, and homogenized liver.
- Aliquot the mixture into four 50 mL conical tubes and incubate them in a water bath (37 °C) for 1 h. Shake the conical tubes every 15 min.
- Add 15 mL of Ficoll in four 50 mL conical tubes.
- Carefully layer the homogenized liver solution on top, distributing approximately 35 mL between each tube. Do not disturb the interface.
- Place the tubes in the centrifuge and spin at 800 x g for 1 h with minimal acceleration/brake.
- Remove the tubes from the centrifuge and collect the cells from the interface (10-15 mL) into two 50 mL conical tubes.
- Add PBS 1x up to 50 mL to wash the pellet, and centrifuge at 300 x g for 5 min. Discard the supernatant.
- Resuspend the pellet in 10 mL of ACK (Ammonium-Chloride-Potassium) lysis buffer and incubate for 5-10 min. Fill the tube with 1x PBS.
- Manually count the cells under the microscope using trypan blue and a hemocytometer. Centrifuge the cells at 300 x g for 5 min.
NOTE: The liver mononuclear cells can be injected directly from here if desired, for example, if the cell count is lower than 50 million cells. - Vacuum off the PBS and resuspend the cell pellet in 300 μL of the separation buffer (2 mM EDTA, 5 µg/mL of BSA in 1x PBS) for 100 x 106 cells or less. For more than 100 x 106 cells, use a ratio based on the above quantity of cells to determine the amount of buffer (i.e., double the cells, double the buffer).
- Add 100 µL of FcR blocking reagent and incubate 2-5 min.
- Add 100 µL of CD34 microbead antibodies, mix well and incubate on ice for 30 min; mechanically shake by hand after 15 min.
- Add 7-10 mL of the separation buffer and filter through 100 µm, 70 µm, and 40 µm cell strainers in that order.
- Place a column-based cell-separator in a magnetic separator. Place a 15 mL conical tube underneath the separator to collect the flow-through and buffer.
- Fill the column with 500 µL of the separation buffer.
- Pour the cell suspension through the column.
- Fill the column with the flow-through and buffer that has been collected in the conical tube.
- Wash the column 3 times with 500 µL of the separation buffer.
- Discard the flow-through and replace the tube with a 15 mL polystyrene tube.
- Fill the column with 1 mL of the separation buffer, remove the column from the magnet and flush the plunger down in one push.
- Count the cells and resuspend in 1x PBS for injection. Place on ice until use.
2. Thymic processing
- Wash the autologous thymus (>20 weeks of age) by adding 5-8 mL of RPMI1640, allow the tissue to settle. Carefully remove the medium by vacuum while avoiding contact with the thymus. Repeat this once more.
- Suspend in 10 mL of room temperature RPMI1640 with Primocin (100 µg/mL).
- Transfer thymus to a tissue-treated Petri dish. Ensure the thymus is fully coated in Primocin-media.
- Incubate for 30-45 min at room temperature.
- Vacuum off the Primocin-media. Wash the thymus with 5-8 mL of RPMI1640, remove the media by vacuum, and repeat this once more.
- Suspend the thymus in 5-8 mL of RPMI1640.
- Prepare the incubated thymus for grafting by slicing 1 mm x 2 mm segments using two scalpels.
NOTE: This will be grafted into the mice; therefore, ensure that the thymus slices can be vacuumed both in and out of the syringe and the attached 22 G Hamilton needle with relative ease.
3. Subrenal capsule grafting and CD34 cells injection
- Examine the mice for their general health before the surgery. Shave the mice to expose an area (approximately 3" x 2") on the left lateral body.
- Anesthetize the mice via isoflurane (induction 5%, maintenance 2%-3%) in the chamber and then transfer individual mice from the chamber to a surgical stage with a nose cone.
- Sanitize the shaved area with Chlorohexidine and alcohol prep swabs. Ensure individual anesthetization via pinch test.
- Make a large incision (5-7 mm) posterior, lateral to the spine of the mouse, and fold back the skin.
- Ensure that at this point, the spleen is visible beneath the fascia. Make a smaller ~2 mm incision in the fascia. Ensure that the incision is as small as possible while allowing the kidney to be fully exposed.
- Determine the location of this incision, visually identifying the spleen and using it as a marker for the kidney, which is not visible.
NOTE: The murine kidney is located behind a grouping of fat in the northeast quadrant to the spleen. - From here, position the hand (surgeon) under the mouse's side opposite the incision and push to expose the kidney. Reposition any fat to fully expose the kidney.
- Utilize the fingers on the same hand to stabilize the exposed kidney. If necessary, use sterile 1x PBS to lubricate the exposed kidney.
NOTE: It is important to note that the surgeon must use different fingers than those that handled the mouse's side to hold the kidney to maintain sterility. Consult with the institute's veterinarian regarding proper maintenance of sterility. - Simultaneously, let the assistant prepare a blunt syringe with the fetal thymus tissue. Gently suck up 2-3 medium-sized thymus chunks (1 mm x 2 mm) with minimal media to the tip of the needle and then hand it over to the surgeon to perform the procedure.
NOTE: The number of chunks of thymus depends on the size of the tissue, usually 2 chunks are enough to get humanized mice. - Let the surgeon insert the needle through the caudal end of the kidney capsule until the distal end is reached (~2 mm), to the point where the needle is visible, but it does not break through the cranial side of the kidney capsule. Let the assistant plunge the syringe.
- Place the kidney, along with any exposed fat, back in the peritoneum. Place one absorbable suture (size 4) and close the superficial incision with a vet bond glue.
- Postoperatively, administer a dose of the analgesic drug, 25 µL of sustained-release buprenorphine (1 mg/mL) via subcutaneous injection (1 mg/kg).
- Check that mice begin to recover within 5 min post-operation.
- Between cages, change the gloves and sterilize the instruments in a glass bead sterilizer.
- From 1-3 h after surgery, inject 100 µL of human CD34+ cells (approximately 100,000 cells) into the mouse by intravenous (IV) injection.
- Conduct a general health check on the mice after concluding the surgery (usually 2 h after the first cage), then 24 h and 48 h afterward.
- If there are any abnormalities in disposition or vital signs, monitor the mice further. Once they have cleared those checkpoints, monitor the mice less strictly (2x-3x per week).
- One week after surgery, inject (IV) the mice with 100 µL of AAV8 human cytokine (IL-3, IL-7, and GM-CSF; 2 x 109 genome copies/mL).
- Two weeks after surgery, perform IM injections of the plasmid DNA Combo II.
- Use an electroporation machine to perform IM injections of plasmid DNA Combo II: SCF, FLT3, CKIT, and THPO. For each mouse, combine 25 µL of SCF (2 mg/mL), 25 µL of THPO (2 mg/mL), 25 µL of FLT3 (2 mg/mL), and 25 µL of CKIT (10 mg/mL).
- Perform one injection per hind leg, i.e., two total, with 50 µL per leg, followed by electroporation on each injection site.
Representative Results
Following successful surgery and appropriate postoperative injections, CD34+ differentiation can be confirmed via flow cytometry. Approximately 8 weeks after surgery, mice are bled in preparation for FACS, recurring every 2 weeks until a specific threshold of human immune cells is met as is described previously10. Briefly, 100 mL of blood was collected in blood collection tubes coated with lithium and heparin. After the lysis of red blood cells using ACK lysis buffer, the cells were washed with FACS buffer (Phosphate buffered saline, 2% FBS, and 0.1% sodium azide) and centrifuged. The cell pellet was then resuspended in 100 mL of FACS buffer and incubated with a panel of antibodies (anti-mouse CD45, anti-human CD45, anti-CD3, anti-CD4, anti-CD8, and anti-CD20). To identify live and dead cells, cells were stained with DAPI, and the lymphocytes were gated based on the FSC-A and SSC-A parameters. A gate was generated to select the live cells and from those the single cells. From those single, live lymphocytes population, we first distinguished the human from the mouse CD45+ cells. CD20+ and CD3+ cells were identified from the human CD45+ cells, and finally, CD4+ and CD8+ cells were identified from the CD3+ cells (T cells). To elaborate, Hu-mice are ready to be used for immunotherapy experiments when they hit the threshold of >25% HuCD45+ in the total lymphocytes and >8%-10% CD8+ in the human CD3+ cells (Figure 1). In the early stages of reconstitution, CD20+ levels are high, and CD3+ levels are low. As cells proliferate and reconstitute with time, the CD20+ levels drop as CD3+ levels rise. This correlates with when the mouse thymus, mouse spleen, and the renal capsule-grafted Hu-thymus get repopulated with human lymphoid precursor cells that undergo differentiation.
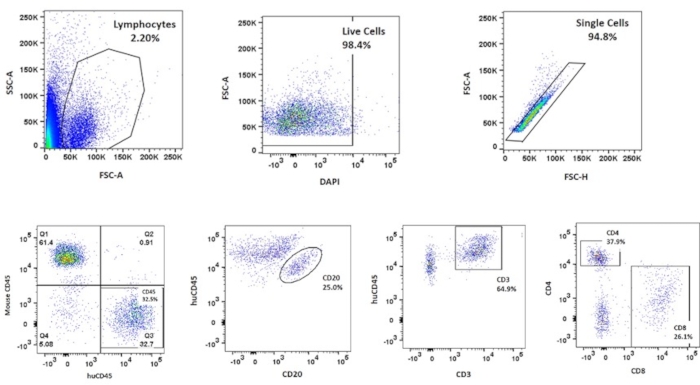
Figure 1: Flow cytometry analysis of a reconstituted mouse. The analysis is gated to isolate the different populations of cells, beginning with the total population of lymphocytes, then distinguishing between live cells, single cells, and various stem cell populations with specific attention paid to the CD45+ and CD8+ to ascertain proximity to the thresholds, as is previously reported10. Please click here to view a larger version of this figure.
Discussion
This manuscript has herein described generating humanized mice via human fetal thymus grafted under the renal capsule and subsequent CD34+ injection to recreate a human immune system.
While the protocol functions to create the best model possible, certain steps are essential to viability. For example, during the CD34+ isolation, it is essential that one looking through the microscope can identify CD34+ cells. Though it may seem redundant, automatic counting machines do not always identify these cells due to their morphology, and the machine itself may misidentify the debris as positive cells. Therefore, it is crucial to identify these cells manually. Moreover, it is essential to confirm that the thymus is truly entering the kidney capsule during renal graft surgery. This can be visualized or palpated: the surgeon should be able to feel the thymus pieces entering the kidney.
Importantly, the process has a few caveats that can determine the success of the model. The first being, the quality of the fetal tissue must be individually assessed to determine whether the isolated cells could potentially be toxic to the mouse. For example, tissue aged above 20 weeks appears firmer and cuts more precisely during preparation. There is generally less degradation. These qualities are general indicators of better caliber tissue. A second caveat of the technique regards the quantity and quality of CD34+ cells; the number of cells produced is variable and sometimes may not provide enough for injections. In these instances, it is possible to circumvent the problem by injecting the liver mononuclear cells isolated earlier in the protocol. Third, after the implementation of a successful model, the mice may begin to develop graft-versus-host disease (GVH) around 25 weeks of age. While the model can last up to 30 weeks, it is around 25 weeks that the investigators should pay careful attention to the mice-looking changes in body score, loss of hair, and facial distress. Around this point, one should always consult with the attending veterinarian, determine the quality of life, and attend to the mice as dictated or euthanize.
The main difference between this protocol and other humanized models is the use of cytokines to improve the proliferation and stabilization of hematopoietic cells first and then enhance the differentiation of immune cells. The effects of the use of the cytokines is addressed by the increased levels of human CD45+ cells in mouse peripheral blood circulation and aided in T-cell and myeloid cell differentiation compared to those mice with no cytokines, as previously reported10.
In summary, the described model provides a humanized mouse with a stable lifespan and functional recapitulation of the human immune cells. The model can be recreated by others in an effort to study a myriad of questions relating to immunotherapy, viral research, regenerative medicine, and numerous fields beyond these contexts.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
Thanks to Wistar Flow-Cytometry, Molecular Screening, Vector Core, and Animal Facility for their support. This work was made possible with support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Materials
| ACK lysis buffer | Life Technologies Corporation | ||
| BD Microcontainer | BD | blood collection tubes | |
| Busulfan | Sigma | B2635-25g | Irradiating drug; light sensitive |
| CD34 Microbeads | Miltenyi Biotec | 130-046-702 | antibody beads kit; stored at 4 °C |
| CKIT | Aldevron | custom | cytokine; stored at -20 °C |
| Collagenase/Dispase | Roche Diagnostics | 11097113001 | Stored at 4 °C |
| FcR Blocking reagent | Miltenyi Biotec | 130-046-702 | antibody beads kit; stored at 4 °C |
| Fetal tissue (liver and thymus) | Advanced Bioscience Resources | Delivered same day or overnight | |
| Ficoll | GE Healthcare | 17-1440-03 | Stored at room temperature |
| FLT3 | Aldevron | 125964 | cytokine; stored at -20 °C |
| Forceps | Various | Various | |
| Hamilton syringe needle | Various | Various | 22 G; 3 point; 2" length |
| Hemostats | Various | Various | |
| MS columns | Miltenyi Biotec | 130-042-201 | magnetic separator |
| PBS | Gibco | 14190-136 | Stored at room temperature |
| Primocin | Invivogen | amt-pm1 | antibiotic; stored at 4 °C |
| RPMI | Corning | 10-040-CM | Stored at 4 °C |
| SCF | Aldevron | 125962 | cytokine; stored at -20 °C |
| Surgical scissors | Various | Various | |
| THPO | Aldevron | 125963 | cytokine; stored at -20 °C |
| Tissue treated petri dish | Corning | 430167 | |
| VetBond glue | 3M | 1469SB | glue |
| Visorb suture | Stoelting Co | 5046 | absorbable suture, size 4, 19 mm cutting |
Referanslar
- Fujiwara, S. Humanized mice: A brief overview on their diverse applications in biomedical research. Journal of Cellular Physiology. 233 (4), 2889-2901 (2017).
- Singh, K. S., et al. IspH inhibitors kill Gram-negative bacteria and mobilize immune clearance. Nature. 589 (7843), 597-602 (2020).
- Thomas, T., et al. . High-throughput humanized mouse models for evaluation of HIV-1 therapeutics and pathogenesis. Methods in Molecular Biology. 1354, 221-235 (2016).
- Shultz, L. D., Ishikawa, F., Greiner, D. L. Humanized mice in translational biomedical research. Nature Reviews Immunology. 7 (2), 118-130 (2007).
- Walsh, N. C., et al. Humanized mouse models of clinical disease. Annual Review of Pathology: Mechanisms of Disease. 12 (1), 187-215 (2017).
- Rebecca, V. W., Somasundaram, R., Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nature Communications. 11 (1), (2020).
- Adigbli, G., et al. Humanization of immunodeficient animals for the modeling of transplantation, graft versus host disease, and regenerative medicine. Transplantation. 104 (11), 2290-2306 (2020).
- Akkina, R. Humanized mice for studying human immune responses and generating human monoclonal antibodies. Microbiology Spectrum. 2 (2), (2014).
- Wege, A. K., et al. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget. 8 (2), 2731-2744 (2016).
- Somasundaram, R., et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nature Communications. 12 (1), (2021).

.
