A Complex Diving-For-Food Task to Investigate Social Organization and Interactions in Rats
Özet
This protocol describes a method of examining social hierarchy in a rat model. Rats perform a complex diving-for-food task in which they form a distinct hierarchy according to their willingness to dive underwater and swim to obtain a food pellet. This method is used to understand decision making and social relationships among highly social animals in small groups.
Abstract
For many species, where status is a vital motivator that can affect health, social hierarchies influence behavior. Social hierarchies that include dominant-submissive relationships are common in both animal and human societies. These relationships can be affected by interactions with others and with their environment, making them difficult to analyze in a controlled study. Rather than a simple dominance hierarchy, this formation has a complicated presentation that allows rats to avoid aggression. Status can be stagnant or mutable, and results in complex societal stratifications. Here we describe a complex diving-for-food task to investigate rodent social hierarchy and behavioral interactions. This animal model may allow us to assess the relationship between a wide range of mental illnesses and social organization, as well as to study the effectiveness of therapy on social dysfunction.
Introduction
Rats are highly social animals, making them an ideal model for understanding social behavior and how it relates to decision making. Rats divide themselves into hierarchical groups based on dominant and submissive relationships. Rats can be trained for tasks that express cooperation, risk management, deceptive behavior, and behaviors that change depending on the decisions of other rats1,2. Studies with rat models expressing these behaviors prove helpful in understanding social structure and its relationship to decision making with relevance for human psychology.
As a necessary resource, access to food is a major reason for social organization among rats3. Naïve rats have been observed engaging in social interaction and differentiation in situations where access to food was limited1,2,4,5,6,7,8. In one study, adult rats were required to cross a tunnel underwater to access the food and then bring the food back through the tunnel to the cage9. Individual rats within each group were able to be categorized according to their method of obtaining food. Two behavioral profiles have emerged: the first are the "carriers", who dive down and swim underwater to the feeder, obtain a pellet, and hold the pellet in its mouth as they swim back to the cage. The second group are the "non-carriers", who do not dive and obtain food only by stealing from the carriers. In groups of six rats, approximately one-half were carriers and the other half were not9. All of the rats were observed to be carriers when they were trained individually in the diving apparatus10.
Similar animal behavioral tasks involve competition for food or space and have been employed with chickens11, rodents12,13,14,15, and pigs16. In the tube test, two mice are sent through a narrow tube from opposite ends, with one mouse necessarily ceding right of way to the other. This test assists in measuring social dominance17,18,19. A behavioral test referred to as the warm spot test has mice compete for a position in a small warm spot in an otherwise cold cage19,20.
A subsequent diving-for-food task that is more complex allows carrier rats to have access to a second cage, away from non-carriers, where they could consume their food separately4. In this protocol, we present a diving-for-food task as an alternative model for social hierarchy and behavior in rats. This diving-for-food task provides a method for rats to avoid the social groups of the main cage and therefore escape aggression and the social interactions of other rats. This task introduces the option of avoidant social behavior in rats that can elucidate our understanding of social aggression.
Social functioning, which describes the ability to engage in normal social roles, can be affected by conditions such as depression3. Depressed individuals often struggle with unemployment, have few social contacts, and scarcely engage in leisure activities3. Effective treatment of depression is often measured by improvement in social and interpersonal function21. Antidepressant treatments, however, vary in their efficacy in treating impairments in social functioning related to depression3.
In this methodology, we induced a depressive-like condition in rats through the Chronic Stress test and evaluated the rats' level of anhedonia, one of the features of a depression-like state, with a sucrose preference test. Anhedonic rats, as well as anhedonic rats who were administered anti-depressants, were monitored through the diving-for-food task in comparison to a control group.
The previously-mentioned diving-for-food tasks resemble food competition tests that often use only one pair of animals or one dichotomy as a point of comparison, such as carriers and non-carriers and a single analysis that compares submission to dominance15,17,22. Our method defines more complex interactions between rats through divisions into multiple types of behavior, including: carriers and non-carriers, those who fight for food and those who do not, and rats who share food or go to separate cages. We believe that this protocol is the only type that uses a hierarchy to assess a complex structure of social interaction in a group of animals, rather than in pairs. It will be helpful for studies that test dominance based on food preference, as well as studies that aim to clarify more hierarchical relationships that are not limited to a dominant-submissive model.
In this protocol, we describe in detail the complex diving-for-food task to investigate social organization and interactions in rats with changes in individual behavior, particularly after the development of anhedonia. This animal model may also be utilized to study other psychiatric conditions associated with changes in social behavior and hierarchy.
Protocol
The experiments were conducted in accordance with recommendations of the Declarations of Helsinki and Tokyo and the Guidelines for the Use of Experimental Animals of the European Community. The experiments were approved by the Animal Care Committee of Ben-Gurion University of the Negev. The authorization code for this experiment was IL-55-8-12.
1. Rat preparation
- Obtain approval for experiments from Institutional Animal Care and Use Committee (IACUC).
- Select adult Sprague Dawley rats. Exclude animals that exhibit abnormal physical traits, such as seizures or other motor deficits.
NOTE: For this protocol, we used adult male rats, weighing 300−350 g, aged 4-8 months old. Female rats may be used as well. - Maintain rats at room temperature (22 °C ± 1 °C), with 12 h light and 12 h dark cycles. Provide rat chow and water ad libitum. House 3 rats per cage.
NOTE: All rats housed in the same cage must be in the same experimental group. - Randomly place 120 rats into one of three experimental groups. Use Group 1 (n=60) w as a control group. Induce the experimental group, Group 2 (n=30), with stressors as detailed below. Induce Group 3, the experimental group with treatment (n=30), with anhedonia and subsequently administer antidepressant treatment. The timeline for the experimental protocol can be found in Figure 1.
- Mark rats with colored pens at the beginning of the experiment to allow for individual identification.
- Perform all experiments between 6:00 a.m. and 12:00 p.m.
- Weigh rats daily throughout procedure for possible weight loss. Weight loss above 20% will exclude rats from the study. See section 3.1.3.
2. Induction of anhedonia in rats
- Chronic Unpredictable Stress model
- Induce rats from the experimental group and the experimental group with treatment with features of a depressive-like state by the Chronic Unpredictable Stress model, as previously detailed23.
- Chronic Unpredictable Stress model
- Induce rats from the two experimental groups with depressive-like behaviors by the Chronic Unpredictable Stress model, as previously detailed23.
NOTE: Rats are exposed to 2 of the 7 stressors daily in a random order; one in the daytime and the second at night for 5 weeks24,25. - Introduce the following stressors in random order:
- House rats with 6 animals per cage instead of 3 for 18 h.
- Tilt cage placement 45° along the vertical axis for 3 h.
- Deprive animals of food for 18 h.
- Deprive animals of water for 18 h and then introduce an empty water bottle.
- Maintain a soiled cage for 8 h with 300 mL of water spilled in the bedding.
- Keep continuous lighting and reverse the light/dark cycle for 48 h per week.
- Heat the environment to 40 °C for 5 min.
- Confirm the development of anhedonia, one of the features of a depression-like state, by performing a sucrose preference test. See section 4.
- Induce rats from the two experimental groups with depressive-like behaviors by the Chronic Unpredictable Stress model, as previously detailed23.
- Anti-depression therapy
- Administer 20 mg/kg imipramine hydrochloride (tricyclic antidepressant) intraperitoneally once per day for 3 weeks to rats from the experimental group26,27,28.
NOTE: A sub-group (n=3 in each set of rats) of the experimental group is administered 0.9% saline (placebo) intraperitoneally once per day for the 3-week duration, at the same volume as the antidepressant treatment group.
- Administer 20 mg/kg imipramine hydrochloride (tricyclic antidepressant) intraperitoneally once per day for 3 weeks to rats from the experimental group26,27,28.
3. The social organization test (complex diving-for-food task)
NOTE: The experimental apparatus was described in previous studies9,29,30 with minor modifications. All parts of the apparatus should be composed of transparent plexiglass.
- Prepare apparatus and acclimate rats.
- Connect two cages (50 cm x 50 cm x 50 cm) to an aquarium (130 cm x 35 cm x 50 cm) via tunnels (45 cm x 15 cm x 15 cm) (Figure 2). Ensure that there is no access from one cage to another without diving into the aquarium25. Maintain water temperature at 25 °C.
- Place tubes with food pellets (one food pellet in each tube) at one end of the aquarium.
NOTE: A reduction in the accessibility of food pellets in the cage of departure should gradually encourage the rat, who would otherwise develop a habit of stealing, to dive to reach the food. - On day 1 of the experiment, introduce each group of 6 rats to experimental apparatus without water for 3-hour sessions. Return rats to standard cage after session.
- Restrict rat food access to the 3-hour sessions, with no other access to food during the rest of the day.
- Remove rats that lost more than 20% of their baseline weight from the experiment together with their social group, and give ad libitum food and water.
- Manually towel dry rats or provide access to a heat source until dry and before placing them back in their regular housing to avoid hypothermia.
- Repeat these sessions for days 2-3.
NOTE: Video record rats in the apparatus continuously for 3-hour sessions. Ensure that the camera is set to high definition (720p) and the auto-focus is turned off.
- Perform diving-for-food task
- On days 4-17, add water progressively until maximum water level is reached, as described previously4. On days 17-21, maintain maximum water level.
- Observe rats diving for pellet access.
- Record the following parameters for each rat:
- Assess frequency of entry into the tunnel.
- Count each attempt to dive for food.
NOTE: The travel from cage to aquarium back to a cage should not exceed 5-6 seconds, which will ensure that the pellet remains edible. - Assess the number of times that food is obtained by attack between rats that do swim for food and rats that do not.
- Record the number of times food is carried by a rat who swims.
- Record the time that rats spent in separate cages compared to time spent in the original cage.
NOTE: All the data was obtained by continuous visual observation.
4. Assessment of anhedonia: The sucrose preference test
- Assess anhedonia by the sucrose preference test with minor modification, as previously described23,25,31,32,33. Perform this test on days -6, 0, 35, 41, 62 and 68 of the procedure (see Figure 1 for protocol timeline).
- Allow rats to consume sucrose solution for 24 h by free access to the two bottles in each cage, containing 100 mL of sucrose solution (1%, w/v).
- After 24 h, replace one of the bottles with water for an additional 24 h.
- Deprive rats of water for 12 hours34.
- Give rats both bottles (one with water and one with sucrose). After 4 h, record the volume of both the consumed sucrose solution and water.
- Calculate sucrose preference as sucrose preference (%) = sucrose consumption (mL)/(sucrose consumption (mL) + water consumption (mL)) × 100%.
NOTE: When utilizing the Chronic Unpredictable Stress model in conjunction with a social organization test, we recommend not only recording the mean sucrose and water consumption, but also to note changes in behavior of each individual rat. This will allow for a more specific understanding of behavioral changes within the individual instead of within the group when confronted with a hierarchical model such as the diving-for-food task.
5. Statistical analysis
- Determine comparisons between groups using the Kruskal-Wallis followed by Mann-Whitney for nonparametric data or a one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test or the Student's t-test for parametric data.
NOTE: Results are considered statistically significant when p < 0.05, and highly significant when p < 0.01.
Representative Results
Body weight changes
A one-way ANOVA did not show any differences in changes in body weight between experimental groups for the 21 days of the diving-for-food task. From days 2 to 21, there were changes in body weight for all 3 groups (p<0.01, Table 1).
Sucrose preference test
At the start of the experiment (day 0), there was no difference in the percent of sucrose preference between the experimental group of rats induced with anhedonia (85.6% ± 18.6), the experimental group treated with antidepressant therapy (85.1% ± 18.8), and the control group (85.7% ± 9.9). On day 35, compared to the control group (84.13% ± 12.3), there was a significantly lower percent sucrose preference in the experimental group (62.69% ± 17.7, p<0.01) and in the experimental group with treatment (68.48% ± 13.9, p<0.01, Figure 3A). There were not yet any differences between the experimental group and the experimental group with treatment. On day 62, the experimental rats had a lower percent sucrose preference (68% ± 15) than the control group (78.5% ± 16) and the experimental group with treatment (77% ± 16, p<0.05, Figure 3B). There were no differences between the treatment group and the control group at this time. Data is presented as percent sucrose preference ± standard deviation.
Diving-for-food task
The social activity of rats in a situation of restricted access to food is illustrated in Figure 4. Rats in the experimental group demonstrated an increase in frequency of entries into the tunnel (113% ± 3.7, p<0.01, Figure 4A), diving for food (141% ± 7, p<0.01, Figure 4B), food obtained by carrying (168% ± 12, p<0.01, Figure 4C), time spent in separate cages (123% ± 7.9, p<0.01, Figure 4D), and food obtained by attack (232% ± 26, p<0.01, Figure 4E) compared to the experimental group with treatment (44% ± 7, 53% ± 6, 54% ± 5, 55% ± 4.7, 67% ± 3.4, respectively). The differences between the experimental group of rats and the experimental rats treated with antidepressants were statistically greater than the difference between the experimental group and the control group in all 5 parameters of the diving-for-food test (p<0.05). Data is presented as an average percentage compared to controls ± standard error of the mean.
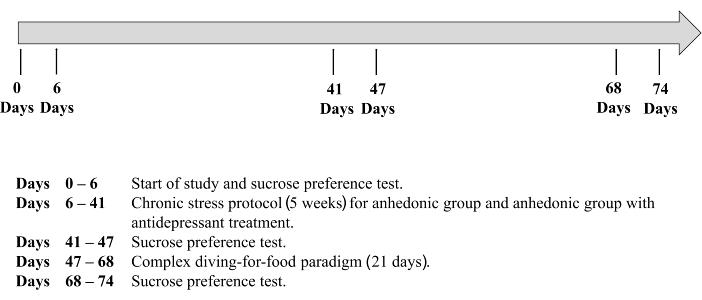
Figure 1. A timeline of the experimental protocol. Please click here to view a larger version of this figure.
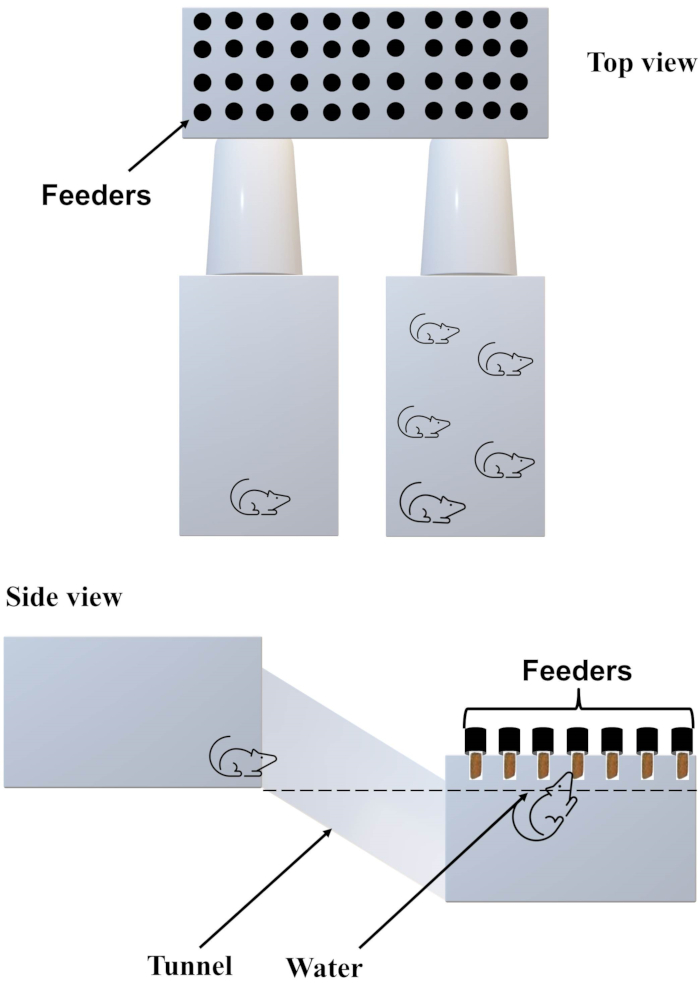
Figure 2. Illustration of the diving-for-food apparatus. Please click here to view a larger version of this figure.

Figure 3. The sucrose preference test (A) after 35 days and (B) after 62 days. There was no difference in sucrose consumption at the beginning of the experiment. (A) By day 35 of the experiment, the anhedonic group (p<0.01) and the anhedonic group treated with antidepressant therapy (p<0.01) had a significantly lower percent sucrose preference than the control group. (B) On day 62, the rats induced with anhedonia had a lower percent sucrose preference compared to both the control and the anhedonic group treated with antidepressant treatment (p<0.05). Please click here to view a larger version of this figure.
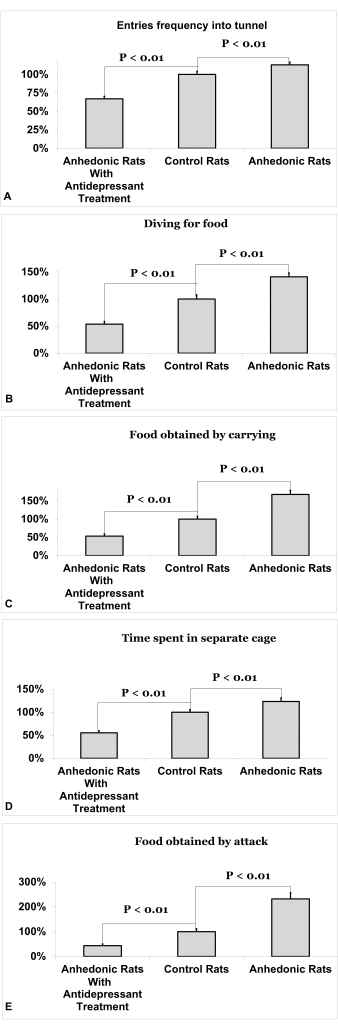
Figure 4. Social activity of rats in a situation of restricted access to food. (A) Frequency of entries into the tunnel. (B) Diving for food. (C) Food obtained by carrying. (D) Time spent in separate cages. (E) Food obtained by attack. Data is presented as an average percentage compared to mean control values + standard error of the mean. Please click here to view a larger version of this figure.
| Change in rats’ body weight | |||||||||||||||||||||
| Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| Control Group | |||||||||||||||||||||
| AVER. | 0 | -0.02 | -0.04 | -0.04 | -0.06 | -0.06 | -0.07 | -0.08 | -0.09 | -0.1 | -0.11 | -0.12 | -0.12 | -0.13 | -0.14 | -0.15 | -0.16 | -0.18 | -0.19 | -0.2 | -0.21 |
| SD | 0 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| Experimental Group with Treatment | |||||||||||||||||||||
| AVER. | 0 | -0.02 | -0.03 | -0.04 | -0.05 | -0.06 | -0.07 | -0.08 | -0.09 | -0.1 | -0.1 | -0.12 | -0.12 | -0.14 | -0.14 | -0.15 | -0.16 | -0.17 | -0.18 | -0.19 | -0.2 |
| SD | 0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 |
| Experimental Group | |||||||||||||||||||||
| AVER. | 0 | -0.01 | -0.03 | -0.04 | -0.05 | -0.05 | -0.07 | -0.07 | -0.08 | -0.1 | -0.11 | -0.12 | -0.13 | -0.14 | -0.15 | -0.15 | -0.17 | -0.18 | -0.19 | -0.2 | -0.21 |
| SD | 0 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 |
Table 1. Changes in body weight (as a percentage) during the diving-for-food task. There were no differences between the 3 experimental groups for changes in body weight during the 21 days of the task. From days 2 to 21, there was an overall effect between days expressed as a change in body weight (p<0.01).
Discussion
Social hierarchies determine the behavior of many species, including humans, and are often defined by relationships based on aggression and submission. These relationships often depend on environmental factors in additional to social structures35. Social formations based on dominance and submission are multifaceted36,37. Among humans, aggression is described as consisting of behaviors ranging from non-physical bullying to war and violence38,39,40. These formations can be influenced by depression and other impaired conditions3,21.
In the complex diving-for-food task, rats have the option to avoid aggression and return to a cage without other rats from their small social group. The apparatus' design obligates rats into diving and swimming under water for about 1 meter. The placement of the feeder necessitates that the rats return to a cage to eat their food. Access to the alternative cage is possibly only through swimming in the aquarium. Therefore, the carrier rats decide if they will consume their food in the home cage or in the alternative cage.
The experiment allows for rat acclimatization. On the first three days, the animals learn the spatial features of the aquarium and the location of the food without water inside. From days 4-17, water is progressively added. After day 17, the water level is high enough that animals must dive to obtain their food from the feeder. They dive for food from days 17-21. We observed that the rats' behavioral groups did not emerge until day 11 of the experiment, which suggests that results are most significant starting on that day. By day 21, the rats critically lose weight, and this appears to be the last day to feasibly collect data. Rats began attacking for food in all groups at days 9 or 10.
There are several critical steps of this protocol. The exclusion of weight loss is important to ensure the method will give the best data. Typically, a weight loss of 20% is considerable enough to remove the rats from the experiment41,42,43,44. Rats should be weighed at least once a day. Similarly, there should be ample pellet tubes so that rats can easily acquire a pellet. There is a high likelihood of a rat losing a pellet on the way, and this ensures that they can try again quickly. The water level must be high enough so that the rats cannot touch the floor when crossing the tunnel. We also stress that a video recording is necessary even if the researchers are observing the rats' behavior in real time, to allow for additional data collection.
In groups of six rats, a common pattern revealed behavioral groups of 5 carrier rats and 1 non-carrier rat25. Other diving-for-food tasks used different numbers of rats in each group with similar proportions of carriers to non-carriers. In the task presented here, 1 or 2 of the carrier rats remained in the alternative cage, using it as a new base to swim in and out to access food, in order to avoid other rats. Out of the carrier rats that returned to the main cage with their pellets, 1-2 were less active when attempting to protect their food in the common cage.
In another study, the group of six rats consisted of only non-carriers determined from a previous experiment1. The division of behavioral roles maintained the proportions of a typical group: one rat did not swim, and five rats were carriers. This suggests that rats change their behavioral roles depending on the situation and the rats around them. This is echoed in humans, in which behavior is altered by one's situation and through social instability45,46.
The results of this protocol suggest that rats with anhedonia, one of the features of a depression-like state, and without treatment are more aggressive and prone to obtain food themselves, whether via attack, diving, or carrying, and more likely to remain in separate cages. It appears that the anhedonic rats are more willing to alter their social relationships, and to engage in activities that rats would consider dangerous, such as swimming. It is possible that a relationship exists between inability to regulate risk-taking behaviors and to perform expected social roles.
In order to reduce the labor involved in analyzing the video recordings of the rat behaviors, we attempted to use video software (e.g., Ethnovision). However, the software was not suitable for this behavioral task and could not identify individual rats out of a group. We believe that it would be possible to use special software to analyze the video, or to mark each rat visually or place a capsule under the rat's skin for the computer program to differentiate between individual rats. Another possible limitation of the protocol involves the long duration of the training period and the procedure.
There are other options that may improve the technique, including an alternative method that involves one or two cells in the apparatus1,25. We identified an unpredictable stress model as a method to induce differing behavior in hierarchical relationships, though other models may work as well.
In conclusion, this diving-for-food task allows for the investigation of rodent social hierarchy and behavioral interactions. Our protocol significantly tests a group of rats rather than only a pair of rats and allows for an analysis of more multilayered hierarchical relationships. We see two main uses for the technique described here. It can be applied to study the pathophysiology of mental illness in rat models, as well as testing for new treatments for illnesses related to anxiety-depressive diseases. This animal model may also allow us to assess the relationship between a wide range of mental illnesses and social organization, as well as to study the effectiveness of therapy on social dysfunction.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
We would like to thank Professor Olena Severynovska, Anastasia Halinska and Maryna Kuscheriava of the Department of Physiology, Faculty of Biology, Ecology, and Medicine, as well as Oles Honchar of Dnipro University, Dnipro, Ukraine, for their help in analyzing video recordings of the social organization test.
Materials
| Alcohol | Pharmacy | 99% pharmaceutical alcohol diluted to 5% and used for cleaning the open field test box before the introduction of each rat | |
| Bottles | Techniplast | ACBT0262SU | 150 mL bottles filled with 100 mL of water and 100 mL of 1% (w/v) sucrose solution |
| Equipment for Diving for Food Task (Plexiglas) | self made in Ben Gurion University of Negev | Two cages (50 x 50 x 50 cm) to an aquarium (130 x 35 x 50 cm) via tunnels | |
| Imipramine hydrochloride | SIGMA | Lot# SLBB9914V | (Tricyclic antidepressant) 20 mg/kg intraperitoneally once per day for 3 weeks |
| Purina Chow | Purina | 5001 | Rodent laboratory chow given to rats, mice and hamster is a life-cycle nutrition that has been used in biomedical researc for over 5 |
| Rat Cages | Techniplast | 2000P | Conventional housing for rodents. Was used for housing rats throughout the experiment |
| Video Camera | Canon | Digital video camera for high definition recording of rat behavior under plus maze test |
Referanslar
- Colin, C., Desor, D. Differenciations comportementales dans des groupes de rats soumis a une difficulte d’acces a la nourriture. Behavioural Processes. 13 (1-2), 85-100 (1986).
- Boyko, M., et al. The effect of depressive-like behavior and antidepressant therapy on social behavior and hierarchy in rats. Behavioural Brain Research. 370, 111953 (2019).
- Hirschfeld, R. M., et al. Social functioning in depression: a review. Journal of Clinical Psychiatry. 61 (4), 268-275 (2000).
- Grasmuck, V., Desor, D. Behavioural differentiation of rats confronted to a complex diving-for-food situation. Behavioural Processes. 58 (1-2), 67-77 (2002).
- Grasmuck, V., Desor, D. Behavioural differentiation of rats confronted to a complex diving-for-food situation. Behavioural Processes. 58 (1-2), 67-77 (2002).
- Thullier, F., Desor, D., Mos, J., Krafft, B. Effect of group size on social organization in rats with restricted access to food. Physiology & Nehavior. 52 (1), 17-20 (1992).
- Schroeder, H., Toniolo, A., Nehlig, A., Desor, D. Long-term effects of early diazepam exposure on social differentiation in adult male rats subjected to the diving-for-food situation. Behavioral Neuroscience. 112 (5), 1209 (1998).
- Helder, R., Desor, D., Toniolo, A. -. M. Potential stock differences in the social behavior of rats in a situation of restricted access to food. Behavior Genetics. 25 (5), 483-487 (1995).
- Thullier, F., Desor, D., Mos, J., Krafft, B. Effect of group size on social organization in rats with restricted access to food. Physiology & Behavior. 52 (1), 17-20 (1992).
- Krafft, B., Colin, C., Peignot, P. Diving-for-food: a new model to assess social roles in a group of laboratory rats. Ethology. 96 (1), 11-23 (1994).
- Lee, Y. -. p., Craig, J., Dayton, A. The social rank index as a measure of social status and its association with egg production in White Leghorn pullets. Applied Animal Ethology. 8 (4), 377-390 (1982).
- Timmer, M., Sandi, C. A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrinology. 35 (10), 1543-1552 (2010).
- Ujita, W., Kohyama-Koganeya, A., Endo, N., Saito, T., Oyama, H. Mice lacking a functional NMDA receptor exhibit social subordination in a group-housed environment. The FEBS journal. 285 (1), 188-196 (2018).
- Merlot, E., Moze, E., Bartolomucci, A., Dantzer, R., Neveu, P. J. The rank assessed in a food competition test influences subsequent reactivity to immune and social challenges in mice. Brain, Behavior, and Immunity. 18 (5), 468-475 (2004).
- Cordero, M. I., Sandi, C. Stress amplifies memory for social hierarchy. Frontiers in Neuroscience. 1, 13 (2007).
- Hessing, M., Tielen, M. The effect of climatic environment and relocating and mixing on health status and productivity of pigs. Animal Science. 59 (1), 131-139 (1994).
- Fan, Z., et al. Using the tube test to measure social hierarchy in mice. Nature Protocols. 14 (3), 819-831 (2019).
- Lucion, A., Vogel, W. H. Effects of stress on defensive aggression and dominance in a water competition test. Integrative Physiological and Behavioral Science. 29 (4), 415-422 (1994).
- Zhu, H., Hu, H. Brain’s neural switch for social dominance in animals. Science China Life Sciences. 61, 113-114 (2018).
- Zhou, T., et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 357 (6347), 162-168 (2017).
- Bech, P. Social functioning: should it become an endpoint in trials of antidepressants. CNS Drugs. 19 (4), 313-324 (2005).
- Saxena, K., et al. Experiential contributions to social dominance in a rat model of fragile-X syndrome. Proceedings of the Royal Society B. 285 (1880), 20180294 (2018).
- Zeldetz, V., et al. A New Method for Inducing a Depression-Like Behavior in Rats. Journal of Visualized Experiments. (132), e57137 (2018).
- Boyko, M., et al. Establishment of an animal model of depression contagion. Behavioural Brain Research. 281, 358-363 (2015).
- Boyko, M., et al. The effect of depressive-like behavior and antidepressant therapy on social behavior and hierarchy in rats. Behavioural Brain Research. 370, 111953 (2019).
- Castagné, V., Moser, P., Roux, S., Porsolt, R. D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current Protocols in Neuroscience. 55 (1), 11-14 (2011).
- Elgarf, A. -. S. A., et al. Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacology Biochemistry and Behavior. 126, 152-162 (2014).
- Ismail, B., et al. Behavioural, metabolic, and endothelial effects of the TNF-α suppressor thalidomide on rats subjected to chronic mild stress and fed an atherogenic diet. Canadian Journal of Physiology and Pharmacology. 92 (5), 375-385 (2014).
- Helder, R., Desor, D., Toniolo, A. M. Potential stock differences in the social behavior of rats in a situation of restricted access to food. Behavior Genetics. 25 (5), 483-487 (1995).
- Deviterne, D., Peignot, P., Krafft, B. Behavioral profiles of adult rats in a difficult food supply social situation, related to certain early behavioral features. Developmental Psychobiology. 27 (4), 215-225 (1994).
- Kuts, R., et al. A Middle Cerebral Artery Occlusion Technique for Inducing Post-stroke Depression in Rats. Journal of Visualized Experiments. (147), e58875 (2019).
- Boyko, M., et al. The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cognitive, Affective, & Behavioral Neuroscience. 13 (4), 847-859 (2013).
- Boyko, M., et al. The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Research. 1491, 109-116 (2013).
- Boyko, M., et al. Establishment of an animal model of depression contagion. Behavioural Brain Research. 281, 358-363 (2015).
- Malatynska, E., Pinhasov, A., Crooke, J. J., Smith-Swintosky, V. L., Brenneman, D. E. Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: technical considerations. Journal of Neuroscience Methods. 165 (2), 175-182 (2007).
- Chase, I. D., Tovey, C., Spangler-Martin, D., Manfredonia, M. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proceedings of the National Academy of Sciences of the United States of America. 99 (8), 5744-5749 (2002).
- Cordero, M. I., Sandi, C. Stress amplifies memory for social hierarchy. Frontiers in Neuroscience. 1 (1), 175-184 (2007).
- Lewon, M., Houmanfar, R. A., Hayes, L. J. The Will to Fight: Aversion-Induced Aggression and the Role of Motivation in Intergroup Conflicts. Perspectives on Behavior Science. 42 (4), 889-910 (2019).
- Ingram, K. M., Espelage, D. L., Davis, J. P., Merrin, G. J. Family Violence, Sibling, and Peer Aggression During Adolescence: Associations With Behavioral Health Outcomes. Frontiers in Psychiatry. 11, 26 (2020).
- Semenyna, S. W., Vasey, P. L. Bullying, Physical Aggression, Gender-Atypicality, and Sexual Orientation in Samoan Males. Archives of Sexual Behavior. 46 (5), 1375-1381 (2017).
- Gauthier, C., Griffin, G. Choosing an appropriate endpoint in experiments using animals for research, teaching and testing. Alternatives to Laboratory Animals. 27, 374 (1999).
- Organisation for Economic Co-operation and Development. ENV/JM/MONO, 2000. Organisation for Economic Co-operation and Development. , (2000).
- Stokes, W. S. Humane endpoints for laboratory animals used in regulatory testing. ILAR Journal. 43, 31-38 (2002).
- Savvas, I., Anagnostou, T., Kazakos, G. Choosing an appropriate endpoint in experiments using animals. Archives of Hellenic Medicine. 26 (6), 778-786 (2009).
- Vives, A., et al. Employment precariousness in Spain: prevalence, social distribution, and population-attributable risk percent of poor mental health. International Journal of Health Services. 41 (4), 625-646 (2011).
- Bossarte, R. M., Blosnich, J. R., Piegari, R. I., Hill, L. L., Kane, V. Housing instability and mental distress among US veterans. American Journal of Public Health. 103, 213-216 (2013).

