Operative Technique and Nuances for the Stereoelectroencephalographic (SEEG) Methodology Utilizing a Robotic Stereotactic Guidance System
Özet
The SEEG methodology is simplified and made faster with a stereotactic robot. Careful attention must be paid to the registration of the preoperative volumetric MRI to the patient prior to use of the robot in the OR. The robot streamlines the procedure, leading to decreased operative times and accurate implantations.
Abstract
The SEEG methodology has gained favor in North America over the last decade as a means of localizing the epileptogenic zone (EZ) prior to epilepsy surgery. Recently, the application of a robotic stereotactic guidance system for implantation of SEEG electrodes has become more popular in many epilepsy centers. The technique for the use of the robot requires extreme precision in the pre-surgical planning phase and then the technique is streamlined during the operative portion of the methodology, as the robot and surgeon work in concert to implant the electrodes. Herein is detailed precise operative methodology of using the robot to guide implantation of SEEG electrodes. A major limitation of the procedure, namely its heavy reliance on the ability to register the patient to a preoperative volumetric magnetic resonance image (MRI), is also discussed. Overall, this procedure has been shown to have a low morbidity rate and an extremely low mortality rate. The use of a robotic stereotactic guidance system for the implantation of SEEG electrodes is an efficient, fast, safe, and accurate alternative to conventional manual implantation strategies.
Introduction
Medically refractory epilepsy (MRE) is estimated to afflict fifteen million people world-wide1. Many of these patients, therefore, may well be treated with surgery. Epilepsy surgery relies on the precise localization of the theorized epileptogenic zone (EZ) in order to guide surgical resections. Jean Tailarach and Jean Bancaud developed the stereoelectroencephalography (SEEG) methodology in the 1950s as a method for more accurately localizing the EZ based on the in situ electrophysiology of the epileptic brain in both cortical and deep structures2,3. However, only recently has the SEEG methodology started to gain favor across North America4.
Various techniques and technologies are used throughout the world as part of the SEEG methodology, based on the clinical experience of different professionals and epilepsy centers5,6,7. Recently, however, there has been an evolution of the surgical techniques used to implant SEEG electrodes, beyond the classical use manual headframe based strategies. Specifically, the use of robotic stereotactic guidance systems has been shown to be an accurate alternative for SEEG implantation8. Robotic implantation can be safely and effectively used by those with surgical expertise who are looking for a faster, more automated, approach to electrode implantation.
Herein is discussed the specific steps undertaken when employing the use of a robotic stereotactic guidance system for the implantation of SEEG electrodes. Though the SEEG methodology has previously been described, herein particular attention is given to the surgical technique employed with the use of the robot9.
Protocol
All devices used herein are FDA approved and the protocol contained herein constitutes the standard of care at our institution. As such, no IRB approval was needed for the detailing of this protocol.
1. Pre-implantation phase
- Create an anatamo-electro-clinical (AEC) hypothesis.
NOTE: Creation of the AEC hypothesis relies on the coordination of multiple non-invasive techniques for identifying the potential EZ. A team of experts, including epileptologists, radiologists, and epilepsy surgeons will typically convene a meeting to discuss the clinical data for each patient in order to create the AEC hypothesis, which serves as the initial hypothesis for the patient’s EZ. The details of how this is accomplished is beyond the scope of this article. - Identify the best methodology for invasive monitoring depending on the location of the AEC hypothesis. Table 1 lists the different scenarios for which SEEG is preferred over subdural grids (SDG) with or without depth electrodes for invasive monitoring.
- After a patient is deemed a candidate for SEEG evaluation, create an implantation strategy.
NOTE: The implantation strategy should adequately cover the area identified as a part of the AEC hypothesis as well as the wider epileptogenic network in general and neighboring areas of eloquent cortex. This monitoring aids the surgeon in defining the borders of the resection.- Obtain a pre-operative volumetric MRI and CTA.
- Transfer the images in DICOM format to the stereotactic robot’s native planning software and perform imaging fusion (T1+Gadolinium MRI fused with CTA).
NOTE: Imaging fusion is performed automatically by the robot’s software. One need only select the studies that need to be fused. - Plan the trajectory of each individual electrode array within the 3D reconstruction of the MRI-CTA fusion, making sure to maximize sampling from a multitude of areas, including superficial, intermediate, and deep cortical and subcortical areas within the AEC hypothesis.
- Define each trajectory by manually selecting the surface entry point and deep target point for each electrode.
NOTE: Generally, it is best to initially use a working distance of 150 mm from the drilling platform to the deep target point and then adjust the depth to maximally reduce the working distance in order to improve implantation accuracy.
- Define each trajectory by manually selecting the surface entry point and deep target point for each electrode.
- Verify each implantation trajectory.
- Review each electrode in the 3D MRI-CTA fusion reconstruction individually to make sure that the trajectory does not compromise any vascular structures, adjusting any trajectories as needed.
- Review the overall implantation schema in the 3D MRI reconstruction, assessing for any trajectory collisions.
- Verify that the surface entry points are all at least 1.5 cm apart on the skin surface, as anything closer than this would be prohibitive to implantation later.
2. Operative technique
- In the OR, prepare the patient and place them supine while preparing the stereotactic robot for surgery.
- Intubate under general anesthesia according to the recommendations of the anesthesiologist. Use propofol for sufficient anesthesia and verify by adequate electrophysiological recordings as certified by a clinical epileptologist.
- Fix the patient’s head using a three-point fixation head holder.
NOTE: This is a standard 4-point Lexell frame. Occasionally one of the front posts will be removed in order to facilitate registration of the robot to the patient, as described later. Therefore, the fixation is referred to as 3-point. - Position the robot at the head of the patient, such that the distance between the base of the robotic arm and the midpoint of the cranium is 70 cm. Lock the robot into position and secure the three-point head-holder to the robot.
NOTE: Do not make any more adjustments to the position of the patient or the robot after this time. Any further adjustment after this point will potentially result in implantation inaccuracies. - Use the semi-automatic laser based facial recognition system to register the preoperative volumetric MRI with the patient, following all prompts given by the robot.
- Calibrate the laser using the set distance calibration tool.
- Select the preset anatomical facial landmarks manually with the laser. Registration is then completed as the robot automatically scans the facial surface.
- Confirm the accuracy of the registration by correlating additional independent surface landmarks with the registered MRI.
NOTE: The planned trajectories are then automatically verified by the robot software.
- Prepare and drape the patient in standard sterile fashion.
- Drape the robotic working arm using sterile plastic.
- Attach the drilling platform, with a 2.5 mm working cannula, to the robotic arm.
- Implant the bolts along their designated trajectories.
- Select the desired trajectory on the touch screen of the robot.
- Step on the robot pedal to initiate movement of the robotic arm to the correct trajectory. When the correct position is reached, the arm is automatically locked by the robot.
- Insert a 2 mm drill through the working cannula and use it to create a pinhole through the entire thickness of the skull.
- Open the dura with an insulated dural perforator using monopolar cautery at a low setting.
NOTE: Opening the dura can be particularly challenging in small children. Because the dura is not completely adherent to the internal layers of the skull, it is very easy to displace rather than open the dura without noticing. - Screw guide bolt firmly into each pin hole.
- Measure the distance from the drilling platform to the guide bolt using a sterile ruler.
NOTE: This is a fixed distance related to the length of the drilling adapter.- Subtract this measured distance from the value of the distance “platform to target” used in planning the trajectory.
NOTE: Remember that the recommendation is to always use the standard 150 mm platform to target distance unless a need arises to change this distance. Using this standard will simplify this step in the OR. - Record and note the result as it will be used later as the final length of the implanted electrode.
- Subtract this measured distance from the value of the distance “platform to target” used in planning the trajectory.
- Measure and note the final length of the electrode and ensure that it matches the newly calculated length for the bolt. Ensure the electrode and bolt have matching labels to prevent confusion later during electrode implantation.
- Repeat steps 2.2.1 – 2.2.7 for every bolt (i.e., implant all bolts) and mark all electrodes accordingly.
- Change surgical gloves and open a new sterile field.
- Implant all electrodes to the target depth via the implanted bolts.
- Insert a 2 mm diameter stylet through the guide bolt to the intended depth of the final electrode as calculated after implantation of the bolt previously.
- Immediately insert the electrode through the bolt after removing the stylet and screw the electrode into the bolt for fixation.
- Ensure that the electrode is appropriately labeled.
- Repeat steps 2.4.1 – 2.4.3 for every electrode.
- Connect the electrodes to the clinical electrophysiology hardware.
- Wrap the patient’s head using standard head bandaging technique.
Representative Results
The absolute indicator of success following use of the SEEG methodology is seizure freedom for the patient, which ultimately follows successful electrode implantations, successful electrophysiological recordings, as well as successful resection of the EZ. Such a case is shown in Figure 1. Panels A and B of Figure 1 show two tests (single positron emission computed tomography (SPECT) and magnetoelectroencephalography (MEG), respectively) that help in the creation of the AEC hypothesis. However, discussion of the identification of the EZ and the completion of the subsequent resection is outside the scope of this article. However, when SEEG evaluation demonstrates that a patient is a poor surgical candidate for any number of reasons (AEC overlaps with eloquent cortex, multifocal epiliptogenicity, etc.) helping a patient to avoid surgery may certainly be classified as a successful study. Here the focus is instead on the successful anatomical placement of the electrodes and the absence of complications as the indicator for success using this methodology. As such, Figure 1C demonstrates the positioning of an electrode in the frontal opercular and dorsal insular area. Figure 1D shows the resection of the right operculum and insula in a post-operative T1 MRI image.
Figure 2 demonstrates the appropriate OR setup, successful bolt placement, and successful electrode implantation for the SEEG methodology. In a study of 200 patients who underwent a total of 2,663 SEEG electrode implantations at our center only 5 patients experienced complications. The rates of wound infection, hemorrhagic complications, and transient neurological deficit were 0.08%/electrode, 0.08%/electrode, and 0.04%/electrode for a total morbidity rate of 2.5%/patient and a mortality rate of 0%/patient.
| Clinical Scenario | Method of Choice | Second Option |
| Lesional MRI: Potential epileptogenic lesion is superficially located, near or in the proximity of eloquent cortex. -OR- Non-lesional MRI: Hypothetical EZ located in proximity of eloquent cortex |
SBG | SEEG |
| Lesional MRI: Potential epileptogenic lesion is located in deep cortical and subcortical areas. -OR- Non-lesional MRI: Hypothetical EZ is deeply located or located in non-eloquent areas. |
SEEG | SBG with depths |
| Need for bilateral explorations and/or reoperations | SEEG | SBG with depths |
| After subdural grids failure | SEEG | SBG with depths |
| When the AEC hypothesis suggests the involvement of a more extensive multilobar epileptic network. | SEEG | SBG with depths |
| Suspected frontal lobe epilepsy in non-lesional MRI scenario. | SEEG | SEEG |
Table 1. Selection criteria for SDG (with or without depth electrodes) vs. SEEG for invasive monitoring of patients with medically refractory focal epilepsy.
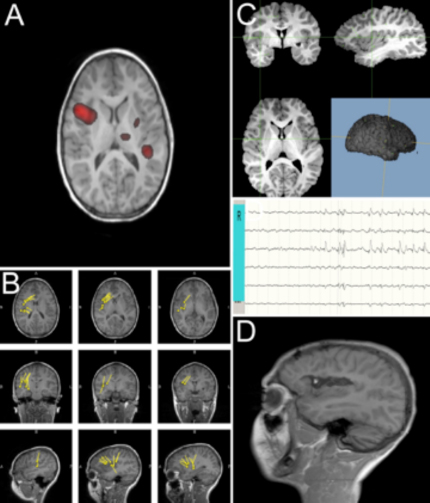
Figure 1: Components of the STEREO-ELECTRO-ENCEPHALOGRAPHY methodology. Panels A and B are showing non-invasive pre-implantation localization testings (as ictal SPECT – A, and MEG scan – B) demonstrating potential epileptogenicity located in the right opercular-insular areas. Panel C depicts the location of the R electrode, in the frontal opercular and dorsal insular area, from which epileptic activity was demonstrated by local field potentials. Panel D depicts post-operative T1 MRI image (sagittal view), demonstrating right opercular and insula resection. Please click here to view a larger version of this figure.
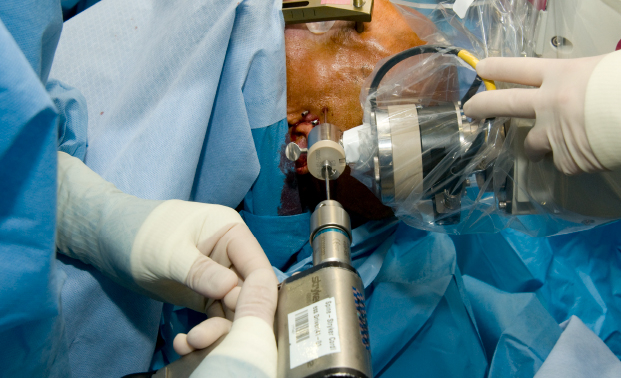
Figure 2: STEREO-ELECTRO-ENCEPHALOGRAPHY robotic method. The figure represents an intra-operative digital picture of the robotic technique, during the drilling phase. The robotic arm precisely guides the drilling step, allowing (after opening the dura and the position of the guiding bolt) the final implantation of the depth electrode. The robotic arm is equipped with a 2.55 mm adapter, which allows precise alignment of the 2.5 mm drill bit. Please click here to view a larger version of this figure.
Discussion
Meticulously defining of the AEC hypothesis coupled with particularly detailed attention to the design of the implantation strategy is ultimately what will determine the success of the SEEG methodology for each individual patient. As such, careful pre-surgical planning of the procedure is critical and makes for a relatively simple, low-risk surgery. Generally it is best to orient the trajectories orthogonally to the sagittal midline, thereby facilitating an easier anatomo-electrophysiological correlation in the future and also obtaining higher precision during implantation. However, in some cases oblique trajectories can be used. Specifically, when an oblique trajectory allows for the sampling of multiple targets within the AEC hypothesis, this may be preferable as it will reduce the total number of electrodes that must be implanted for adequate sampling. The implantation strategy should therefore account for the three-dimensional, dynamic, multidirectional spatiotemporal organization of epileptic activity and the pathways it follows.
Because the use of the stereotactic robot is so critical to the entire operative technique outlined herein, it is recommended that a surgeon gain hands-on experience in working with one of these intraoperative robots before using it in the OR. Familiarity with the workings of the hardware and software associated with the stereotactic guidance system will not only improve patient safety, but also increase the speed of the procedure and facilitates a streamlined operative experience. Furthermore, as detailed in the protocol, it is important that the surgeon and all assistants change surgical gloves and open a new sterile field following the implantation of all bolts and prior to the implantation of the electrodes. This is done to prevent infection.
A caution to this methodology is the importance of accurately registering the patient to 3D reconstruction of the preoperative MRI. Any variance in the registration, or deviation therefrom, will manifest in decreased implantation accuracy for each electrode. It is therefore crucial that the registration be meticulously checked throughout the implantation procedure to make sure it starts off correct and remains as such. Any concern of an inaccurate implantation should be met with verification of the registration and, if necessary, reregistration.
Ultimately, there are many ways of completing the stereotactic implantation of these depth electrodes, but in the experience of the authors, the use of the stereotactic robot provides a much preferable (efficient and precise) operative experience, as well as a very low morbidity rate and an extremely low mortality rate. Additionally, a previous study of the implantation accuracy achieved with this protocol has shown high levels of implantation accuracy10. The results and conclusions herein are congruent with previously published literature regarding the morbidity of the SEEG methodology11,12,13,14,15.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| 2 mm drill bit | DIXI | KIP-ACS-510 | For opening the cranium |
| Coagulation Electrode Dura | DIXI | KIP-ACS-600 | for opening and coagulating the dura |
| Cordless driver | Stryker | 4405-000-000 | to drive the drill bit |
| Leksell Coordinate Frame G | Elekta | 14611 | For head fixation |
| Microdeep Depth Electrode | DIXI | D08-**AM | SEEG electrodes that are implanted, complete with: guide bolt and stylet, as described in manuscript. |
| ROSA | Medtech | n/a | stereotactic guidance system with robotic arm, complete with: robotic arm, calibration tool, registration laser, head frame attachment, and software, as described in the manuscript. |
| Stylet | DIXI | ACS-770S-10 | for creating a path through the parenchyma for the electrode |
Referanslar
- World Health Organization. . Epilepsy. , (2018).
- Talairach, J., Bancaud, J. Stereotaxic approach to epilepsy. Progress in neurological surgery. 5, 297-354 (1973).
- Bancaud, J., Talairach, J. Functional organization of the supplementary motor area. Data obtained by stereo-E.E.G. Neurochirurgie. 13, 343-356 (1967).
- Jehi, L. The Epileptogenic Zone: Concept and Definition. Epilepsy Currents. 18 (1), 12-16 (2018).
- Nowell, M., et al. A novel method for implementation of frameless StereoEEG in epilepsy surgery. Operative Neurosurgery. 10 (4), 525-534 (2014).
- Abel, T. J., et al. Frameless robot-assisted stereoelectroencephalography in children: technical aspects and comparison with Talairach frame technique. Journal of Neurosurgery: Pediatrics. 1, 1-10 (2018).
- van der Loo, L. E., et al. Methodology, outcome, safety and in vivo accuracy in traditional frame-based stereoelectroencephalography. Acta neurochirurgica. 159 (9), 1733-1746 (2017).
- González-Martínez, J., et al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery. 78 (2), 169-180 (2015).
- Mullin, J. P., Smithason, S., Gonzalez-Martinez, J. Stereo-electro-encephalo-graphy (SEEG) with robotic assistance in the presurgical evaluation of medical refractory epilepsy: a technical note. Journal of visualized experiments. , 112 (2016).
- Jones, J. C., et al. Techniques for placement of stereotactic electroencephalographic depth electrodes: Comparison of implantation and tracking accuracies in a cadaveric human study. Epilepsia. 59 (9), 1667-1675 (2018).
- Mullin, J. P., et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. 57 (3), 386-401 (2016).
- Serletis, D., et al. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. Journal of Neurosurgery. 121, 1239-1246 (2014).
- Taussig, D., et al. Stereo-electroencephalography (SEEG) in 65 children: an effective and safe diagnostic method for pre-surgical diagnosis, independent of age. Epileptic Disorders. 16, 280-295 (2014).
- Munyon, C., et al. The 3-dimensional grid: a novel approach to stereoelectroencephalography. Neurosurgery. 11, 127-133 (2015).
- Ortler, M., et al. Frame-based vs frameless placement of intrahippocampal depth electrodes in patients with refractory epilepsy: a comparative in vivo (application) study. Neurosurgery. 68, 881-887 (2011).

