Biomimetic Replication of Root Surface Microstructure using Alteration of Soft Lithography
Summary
Biomimetics has been previously used as a tool to study leaf-microorganism interactions. However, no such tool exists for roots. Here, we develop a protocol to form synthetic surfaces mimicking root surface microstructure for the study of root-environment interactions.
Abstract
Biomimetics is the use of chemistry and material sciences to mimic biological systems, specifically biological structures, to better humankind. Recently, biomimetic surfaces mimicking the microstructure of leaf surface, were used to study the effects of leaf microstructure on leaf-environment interactions. However, no such tool exists for roots. We developed a tool allowing the synthetic mimicry of the root surface microstructure into an artificial surface. We relied on the soft lithography method, known for leaf surface microstructure replication, using a two-step process. The first step is the more challenging one as it involves the biological tissue. Here, we used a different polymer and curing strategy, relying on the strong, rigid, polyurethane, cured by UV for the root molding. This allowed us to achieve a reliable negative image of the root surface microstructure including the delicate, challenging features such as root hairs. We then used this negative image as a template to achieve the root surface microstructure replication using both the well-established polydimethyl siloxane (PDMS) as well as a cellulose derivative, ethyl cellulose, which represents a closer mimic of the root and which can also be degraded by cellulase enzymes secreted by microorganisms. This newly formed platform can be used to study the microstructural effects of the surface in root-microorganism interactions in a similar manner to what has previously been shown in leaves. Additionally, the system enables us to track the microorganism’s locations, relative to surface features, and in the future its activity, in the form of cellulase secretion.
Introduction
Replication of leaf surface microstructure is a known method in the biomimetics research field1,2,3,4. The earliest replications of the leaf surface microstructure were performed using nail polish and rubber materials applied on the leaf surface for better visualization of microstructure, specifically stomata5,6,7,8,9,10. The method was then improved, and advanced polymers were used to mimic leaf surface microstructure using soft lithography, especially in the context of biomimetics of super hydrophobic surfaces2,3,4,11,12. In recent years, this method was proven as a useful tool in the study of the interaction between the leaf surface and microorganisms residing on the surface whether they are pathogenic13,14 or beneficial, as part of the natural leaf phyllosphere15. Simplification of the natural system was proven extremely useful in the study of surface-microorganism interactions even when purely synthetic systems were used as surfaces15,16,17,18.
While replication of leaf surface microstructure was shown to be a useful tool for studying the interaction occurring on the surface of the leaf with different microorganisms, no such tool exists for plant roots. Plant roots are harder to study since they reside below the ground and all interactions occur within the soil. Similar to leaves, root surface microstructure is likely to play a role in root-microorganism interactions. However, currently no method exists to isolate the specific role of root surface microstructure in the complex root-microorganism interactions. The most studied root surface microstructural feature is the root hairs19,20,21. Root hairs have an important role in increasing the surface area and by that allowing more efficient intake of nutrients and water22, however their involvement as a structural feature in root-microorganism interactions has never been tested.
The most widely used polymer for soft lithography in leaves is polydimethyl siloxane (PDMS). PDMS properties resemble those of the leaf cuticle15,23. However, in plant roots, the most abundant material is cellulose24,25 which has different properties than those of PDMS26,27,28. Using PDMS to build a synthetic platform for studying the surface microstructure effects in root-environment interactions is, hence, less than ideal.
The protocol presented here enables the formation of synthetic root surface microstructure replica from various materials. Like the method for leaf surface microstructure replication this is a two-step process. The first step uses the biological tissue (root) as a source for molding into a polyurethane mold (a negative replica). The polyurethane mold, which represents the negative image of the root surface microstructure, can then be used as a base to generate the positive replication of the root surface microstructure from a variety of materials, including PDMS and cellulose derivatives. This root surface replication can later be used as a platform to understand the surface structure role in root-microorganism interactions.
Protocol
1. Growing the plants and root preparation
- Option 1: Prepare adventitious roots from stem.
- Take a rooting tray for growing plants.
- Fill the tray with soil.
- Add one seed of M82 tomato cultivar to each cell in the tray.
- Cover the seeds with a little soil.
- Water the tray from the bottom with a dropper as the water fills the bottom of the tray and the soil absorbs water.
- Add 2 mL of fertilizer per 1 L of water to the bottom of the tray once a week.
- Grow in a growing chamber at 25 °C.
- Use lighting conditions of 9 h light (7:00-16:00) alternated with 15 h of darkness.
- After 3 weeks remove the plant from the soil.
- Cut the root system from the plant at the point of interaction with the stem.
- Put the rootless plant in a beaker filled with water.
- After a few days, cut the adventitious roots that emerge from the stem and use them for replication.
- Option 2: Prepare seed germinating roots.
- Wet a Petri dish sized filter paper with water.
- Put several M82 seeds (no more than 10) on the paper, inside a Petri dish.
- Incubate the plate at 25 °C.
- Hydrate the paper every day.
- After germinated roots are long enough (approximately 5 days), remove the seeds and use the roots for replication.
2. Preparation of the root negative replica from polyurethane
- To generate negative replica solution, add 9.49 g of diurethane dimethacrylate to a 20 mL vial.
- Add 1.45 mL of ethyl methacrylate to the vial.
- Stir at room temperature (RT) until the solution looks clear and becomes homogeneous.
NOTE: Approximately 2 h is sufficient to reach a homogeneous solution. - Add 3 mL of the plasticizer, diethyl phthalate, and stir for 1 h at RT.
NOTE: Diethyl phthalate is miscible in acrylate monomer. - Add 300 µL of the photo initiator, 2-hydroxy-2-methylpropiophenone, and stir overnight at RT. Continue stirring until all bubbles are removed.
NOTE: The protocol can be paused here. The solution can be kept at RT.
- To generate the negative replica of the root, take a clean glass slide and pour 1 mL of the negative replica solution on it.
- Place 2‒3 roots over the solution. Do not allow the roots to be fully covered by the solution.
- Keep the slide under 8 W ultra violet (UV) lamp for 8‒10 min. Do not keep the solution under UV light for too long.
NOTE: It is important not to keep the solution under the UV light for too long as it makes the polyurethane too hard, making it impossible to remove the root. - Switch off the UV lamp, remove the replica from the glass slide and put it in a Petri dish filled with ethanol, to remove unreacted monomer.
- To obtain the negative replica, remove the root from the replica very slowly using forceps.
3. Prepare the root positive replica from PDMS.
- To generate the mixture for the positive replica, place 10 g of dimethyl siloxane in a paper cup.
- Add 1 g of curing agent and mix thoroughly.
- Keep the mixture in a desiccator under vacuum for 2 h to remove air bubbles.
- To generate the positive replica, place the polyurethane negative replica in a Petri dish.
- Pour the PDMS mixture on top of the negative replica.
- Apply vacuum for 2 h to assure coverage of the microstructure.
- Keep the Petri dish overnight at RT.
- Separate the cured positive replica from the negative replica by hand.
4. Prepare the root positive replica from ethyl cellulose.
- To generate the ethyl cellulose solution, put 1.32 ml of diethyl pthalate as a plasticizer in a 100 ml cup.
- Add 20 mL of ethanol and stir at RT for 2 h.
- Add 3.3 g of ethyl cellulose and stir overnight.
- To generate the positive replica, place the polyurethane negative replica in a Petri dish.
- Pour the ethyl cellulose solution on top of the negative replica.
- Keep the Petri dish overnight at RT under the hood.
- Remove the positive replica from the negative replica very slowly by forceps.
Representative Results
To form the root surface microstructure replication, a root must be chosen for molding. We grow tomato plants in soil, making the use of the natural root from the root system extremely challenging. Removal of soil from the root system can be difficult and additionally, the root system roots are fragile and can break upon molding attempts. We therefore suggest to first use more rigid roots, to establish the protocol in the lab. The formation of such roots is described in Figure 1A. The plant root system is removed after the plant was grown for 3 weeks and the rootless plant is placed in water for about a week until adventitious roots emerge from the stem. Those roots can be used for replication during the protocol establishment. Once the protocol has been well established, a more realistic root surface structure is desired. Here we suggest avoiding roots grown in soil as the full removal of soil in extremely challenging. Instead we suggest the use of germinating roots, supplying valuable information on the root surface microstructure of a genetically specific plant. The growth of such roots is described in Figure 1B. The seeds are placed on a wet filter paper and incubated at 25 °C. After approximately 5 days, during which the filter paper is kept moist, the germinated roots are long enough for replication. Those roots are more fragile than the previously suggested roots and require more delicate care.
The production of the root surface microstructure replica is a two-step process. In the first step the natural root is being molded into a polyurethane based mold (the negative replica). The advantage of this step is that all materials for the polyurethane mold are being prepared and the root is placed on top of the prepared solution at the very end for a 10 min exposure to UV. As a result, the biological tissue is not exposed to harsh conditions for too long and can be gently handled at the end of the process. If all protocol steps are followed, a good negative replica is generated. This replica will show the cell structure of the root surface as well as holes representing the location of the root hairs (Figure 2A). If some critical steps in the protocol are not being followed, the procedure will fail. One such step is the placement of the root on the polyurethane solution prior to curing. The root must be placed very gently to avoid the submergence of it in the polyurethane solution. Such submergence, of any part of the root, will cause the entrapment of the root in the hard polymer with no ability to remove it. If such an event occurs, the root will remain within the negative replica after it is cured (Figure 2B). Another crucial step is regarding the curing time by UV light. The recommended curing time is 8‒10 min. Going past 10 min will result in an extremely hard polyurethane mold, making it impossible to remove the root without breaking it within the polyurethane mold. The breakage of the root can sometimes be visible to the naked eye, e.g., when a large piece is broken (Figure 2C, top, marked with purple arrows). However, sometimes small root pieces are left in the material which are difficult to spot by the naked eye and a microscope has to be used (Figure 2C, bottom, marked with purple arrows). We recommend carefully examining the polyurethane negative replica with a microscope prior to the continuation of the protocol to make sure no residual root is present.
Once the polyurethane negative replica is prepared; many materials can be used for the preparation of the positive replica. The preparation of the positive replica, using the polyurethane negative replica as a mold, is straight forward and depends completely on the quality of the polyurethane negative replica. To generate the positive replica we have used both PDMS—as it is well known in the field of soft lithography (Figure 3A)—and ethyl cellulose as a material that better mimics the properties of the root surface which is mostly composed of cellulose (Figure 3B). The SEM image of the PDMS replica shows the root hairs very clearly. The hairs are in the elongation zone, where they begin to emerge. Hence, the length of root hairs varies along the root surface as they become longer, much like in the natural root (Figure 3A). Ethyl cellulose generates harder and less flexible film than PDMS. Hence, the removal of it from the negative mold requires more care. However, some hairs and the surface microstructure are visible under the light microscope (Figure 3B). We used those two materials to generate the positive replica, however, any material that can form a film will be a good candidate for the positive replica, using the polyurethane negative replica.

Figure 1: Tomato plant roots for replication. (A) A tomato (M82) plant is grown at 25 °C with 9 h of light and 15 h darkness. After 3 weeks, the plant is removed from the soil and the root system is cut off. The rootless plant is put in water until adventitious roots emerge from the stem after about a week. These roots do not show the exact structure as the roots from the root system, but they represent a good model. Those roots are less fragile than the root system roots and hence are preferred to work with when establishing the technique in the lab. (B) Tomato (M82) seeds are put on a wet filter paper in a Petri dish and incubated at 25 °C. The paper is hydrated every day and the seeds are germinating. The roots are growing and after approximately 5 days are long enough to be used for replication. These roots are gentler and should be used once the method is well established. Please click here to view a larger version of this figure.
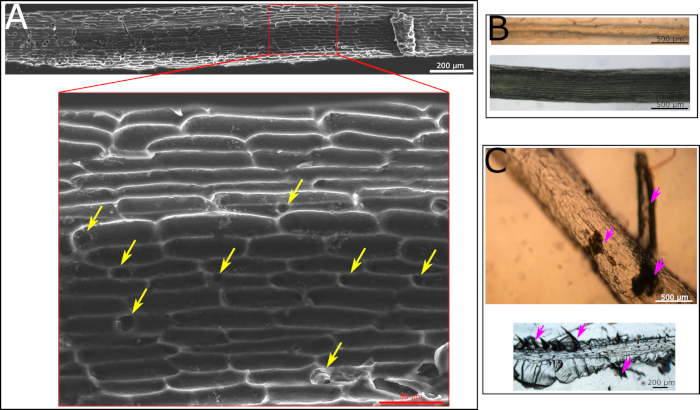
Figure 2: Microscopy images of polyurethane negative replica. (A) SEM image of polyurethane negative replica made according to a protocol following all the steps. Cell structure is clearly visible. Yellow arrows point at holes formed by the hairs in the root. (B) Light microscopy images of polyurethane negative replica with a root inside of it as it was fully covered with the solution and the removal of it was impossible. The polyurethane negative was cured with the root inside. The root is visible by eye and using light microscopy. It is impossible to remove this root from the cured replica. (C) Light microscopy images of polyurethane negative replica that was kept under UV light for too long. As a result, root could not be fully removed from the polymer with either large particles visible by eye (top image, marked with purple arrows) or small fractions visible only by microscope (lower image, marked with purple arrows). Please click here to view a larger version of this figure.
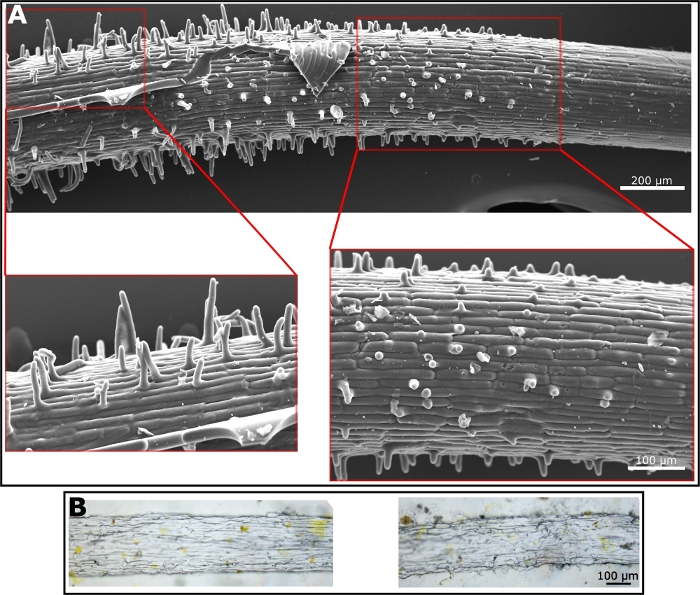
Figure 3: Microscope images of positive replica. (A) SEM micrograph of a positive replica made from PDMS. Enlargement shows root hairs. (B) Light microscopy images of a positive replica made from ethyl cellulose. Hairs are shown in the images on the right while surface texture is visible in the image on the left. Please click here to view a larger version of this figure.
Discussion
We present a novel method for the replication of root surface microstructure. This method relies on existing methods of leaf surface microstructure replication4. In order to develop this method, we had to tweak the existing method for leaves. We realized that the problematic step in copying the leaf replication method into roots involves the first step of the root molding. This is the most sensitive part of the method as it involves the biological tissue. As a result, we wanted to choose a polymer that would demand relatively gentle conditions for curing and hence causing minimal damage to the biological tissue. We chose polyurethane because it can be polymerized quickly (within 10 min) under UV light29. Additionally, it is very hard once polymerized30 and we hoped that this property would allow for the relatively easy removal of the root from the polyurethane mold.
The presented method is a two-step approach in which the negative image (negative replica) is formed in the first step and the replication is formed in the second step, based on the negative replica. This extends the range of materials we can work with. Leaf surface microstructure replication was mainly performed on PDMS or epoxy materials11,31. Some work was done with other materials, specifically materials supporting microorganism growth13,32. This is because in recent years this method has been used to study microorganism-surface interactions in the context of leaf surface structure. However, no cellulose-like materials have been used in this method in the context of leaves. We suggest the use of a polyurethane negative replica as a mold and a variety of materials for the positive replica. In other words, making the positive replica, from a variety of materials, is relatively easy once a good negative replica is made. We currently use cellulose derivatives, but are exploring the possibilities of using more relevant materials to root surface such as pectin and lignin33,34 in combination with cellulose derivatives.
The method also expands upon the existing method of leaf surface microstructure replication since the leaf is a 2D surface while the root surface is curved and hence is a 3D surface. Our method does not enable the replication of the whole surface since embedding the whole root in the polyurethane solution does not allow for its release. Therefore, one side of the root has to be chosen when replicating the root surface microstructure. The generated synthetic surface is curved and represents roughly half the surface, but not all of it. Our assumption is that the structural features of the root surface are mostly symmetrical about the axis along the root length. However, in studies where such symmetry is not assumed, one should be careful to choose the appropriate side root to replicate.
We present two options for roots to be used as molds. The first is the option of adventitious roots grown from the stem and the second is the option of germinated roots on paper. The first option is mostly meant to assist researchers in practicing the method as these roots are more robust and easier to work with. The second option represents the genetic differences that can be found between roots of different cultivars, regardless of the environmental conditions. These surfaces can be used as important research tools, however, one should be aware that the environment can have a strong influence on the root surface structure, specifically the soil in which the roots are grown35,36. Due to the mechanical stress inflicted by the soil, some morphological changes are bound to happen, in addition to wounds accruing on the surface as the root penetrates the soil37. Removal of roots from soil, as well as cleaning them, without damaging their structure is a very difficult task. Hence, we are not optimistic as to the ability to use this method to reliably mimic the root surface microstructure of roots grown in soil. However, for research that focuses on genetic differences or environmental differences where the change in microstructure is noticeably clear, this method can be used as a tool to study the influence of root surface microstructure.
Our method produces an inert surface mimicking of only the microstructural properties of the root surface. While this method is designed to separate the structural effects in root-environment interactions from all other effects, we cannot ignore the chemical compounds in those interactions. Some microorganisms may not survive or function on the surface without the addition of compounds, specifically nutrients. The next step in the development of this platform will be the controlled addition of chemical compounds to study their effects on the different interactions when combined with structure.
This method was developed as a first step in the development of a synthetic platform to study root-microorganism interactions. Here we mimic the microstructure of the root surface and this initial platform can be used to study the influence of surface microstructure on microorganism behavior. However, this platform is limited since it lacks many other elements from the natural system. This platform should be further developed with the use of the right materials to generate the surface and with the addition of other, critical, chemicals into the system. In a more advanced platform, we can also imagine spatial distribution of the chemicals. However, since currently no other method exists to isolate structural effects in root-microorganism interactions, we hope researchers could use this initial platform to ask structure-specific questions in those interactions.
Declarações
The authors have nothing to disclose.
Acknowledgements
Research was supported by seed funds from The Agricultural Research Organization to MK.
Materials
| 2-hydroxy-2-methylpropiophenone | Sigma | 405655 | |
| Diethyl phthalate | Across | 114520010 | |
| Diurethane dimetharylate | Sigma | 436909 | |
| Ethyl cellulose | Across | 232705000 | |
| Ethyl methacrylate | Sigma | 234893 | |
| Shaphir Solution | GAT fertilizer | 6-2-4 | |
| Sylgard 184 kit | Polymer-G | 510018400500 |
Referências
- Bhushan, B., Jung, Y. C., Niemietz, A., Koch, K. Lotus-Like Biomimetic Hierarchical Structures Developed by the Self-Assembly of Tubular Plant Waxes. Langmuir. 25, 1659-1666 (2009).
- Koch, K., Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 367, 1487-1509 (2009).
- Schulte, A. J., Koch, K., Spaeth, M., Barthlott, W. Biomimetic replicas: Transfer of complex architectures with different optical properties from plant surfaces onto technical materials. Acta Biomaterialia. 5, 1848-1854 (2009).
- Koch, K., Schulte, A., Fischer, A., Gorb, S., Barthlott, W. A fast, precise and low-cost replication technique for nano- and high-aspect-ratio structures of biological and artificial surfacese. Bioinspiration & Biomimetics. 3, 046002 (2008).
- Weyers, J. D. B., Johansen, L. G. Accurate Estimation of Stomatal Aperture From Silicone Rubber Impressions. New Phytology. 101, 109-115 (1985).
- Hilu, K. W., Randall, J. L. Convenient Method for Studying Grass Leaf Epidermis. Taxon. 33, 413-415 (1984).
- Sampson, J. A. Method of replicating Dry or Moist Surfaces for Examination by Light. Nature. 191, 932-933 (1961).
- Weyers, J. B. D., Travis, A. J. Selection and Preparation of Leaf Epidermis for Experiments on Stomatal Physiology. Journal of experimental botany. 32, 837-850 (1981).
- Groot, J. The Use of Silicone Rubber Plastic for Replicating Leaf Surfaces. Acta Botanica. Neerlandica. 18, 703-708 (1969).
- Wu, S., Zhao, B. Using Clear Nail Polish to Make Arabidopsis Epidermal Impressions for Measuring the Change of Stomatal Aperture Size in Immune Response. Plant Pattern Recognition Receptors. , 243-248 (2017).
- Wu, W., Guijt, R., Silina, Y., Koch, M., Manz, A. Plant leaves as templates for soft lithography. RSC Advances. 6, 22469-22475 (2016).
- Barthlott, W., Mail, M., Bhushan, B., Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nano-Micro Letters. 9, 23 (2017).
- Zhang, B., et al. Fabrication of biomimetically patterned surfaces and their application to probing plant-bacteria interactions. ACS Applied Materials and Interfaces. 6, 12467-12478 (2014).
- Szyndler, M. W., Haynes, K. F., Potter, M. F., Corn, R. M., Loudon, C. Entrapment of bed bugs by leaf trichomes inspires microfabrication of biomimetic surfaces. Journal of the Royal Society Interface. 10, 20130174 (2013).
- Doan, H. K., Leveau, J. H. J. Artificial Surfaces in Phyllosphere Microbiology. Phytopathology. 105, 1036-1042 (2015).
- Chung, K. K., et al. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases. 2, 89-94 (2007).
- Sirinutsomboon, B., Delwiche, M. J., Young, G. M. Attachment of Escherichia coli on plant surface structures built by microfabrication. Biosystems Engineering. 108, 244-252 (2011).
- Bhattacharjee, A., Khan, M., Kleiman, M., Hochbaum, A. I. Effects of Growth Surface Topography on Bacterial Signaling in Coculture Biofilms. ACS Applied Materials and Interfaces. 9, 18531-18539 (2017).
- Mancuso, S. . Measuring roots: an updated approach. , (2011).
- Schneider, K., Wells, B., Dolan, L., Roberts, K. Structural and genetic analysis of epidermal cell differentiation in Arabidopsis primary roots. Development. 1798, 1789-1798 (1997).
- Dolan, L., et al. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 2474, 2465-2474 (1994).
- Leitner, D., et al. A dynamic model of nutrient uptake by root hairs. New Phytology. 185, 792-802 (2010).
- Soffe, R., Bernach, M., Remus-emsermann, M. N. P., Nock, V. Replicating Arabidopsis Model Leaf Surfaces for Phyllosphere Microbiology. Scientific Reports. 9, 1-12 (2019).
- Sorieul, M., Dickson, A., Hill, S. J., Pearson, H. Plant fibre: Molecular structure and biomechanical properties, of a complex living material, influencing its deconstruction towards a biobased composite. Materials. 9, 618 (2016).
- Gibson, L. J. The hierarchical structure and mechanics of plant materials. Journal of the Royal Society Interface. 9, 2749-2766 (2012).
- Poletto, M., Pistor, V., Zattera, A. J. Structural characteristics and thermal properties of native cellulose. Cellulose-fundamental aspects. , 45-68 (2013).
- Moon, R. J., Martini, A., Nairn, J. A., Simonsen, J., Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties. Chemical Society Reviews. 40, 3941-3994 (2011).
- Johnston, I., McCluskey, D., Tan, C., Tracey, M. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. Journal of Micromechanics and Microengineering. 24, 035017 (2014).
- Yan-yan, W., Ying-wu, L., Bao-fang, L., Bo-geng, L. Water-soluble UV curable urethane methyl acrylate coating: preparation and properties. Journal of Zhejiang University-SCIENCE A. 5, 906-911 (2004).
- Bao, L., Huang, Y. Synthesis and Properties of UV Curable Waterborne Polyurethane Acrylate Based on Modified Castor Oil. The pharmaceutical and chemical journal. 4, 34-40 (2017).
- Sharma, V., Orejon, D., Takata, Y., Krishnan, V., Harish, S. Gladiolus dalenii Based Bioinspired Structured Surface via Soft Lithography and Its Application in Water Vapor Condensation and Fog Harvesting. ACS Sustainable Chemistry & Engineering. 6, 6981-6993 (2018).
- Soffe, R., Altenhuber, N., Bernach, M., Remus-Emsermann, M. N. P., Nock, V. Comparison of replica leaf surface materials for phyllosphere microbiology. PloS one. 14, 1-19 (2019).
- Whitehead, D. C., Buchan, H., Hartlay, R. D. Composition and decomposition of roots of ryegrass and red clover. Soil Biology and Biochemistry. 11, 619-628 (1979).
- Ververis, C., Georghiou, K., Christodoulakis, N., Santas, P., Santas, R. Fiber dimensions, lignin and cellulose content of various plant material and their suitability for paper production. Industrial crops and products. 19, 245-254 (2004).
- Croser, C., Bengough, A. G., Pritchard, J. The effect of mechanical impedance on root growth in pea (Pisum sativum). II. Cell expansion and wall rheology during recovery. Physiologia Plantarum. 109, 150-159 (2000).
- Lipiec, J., Horn, R., Pietrusiewicz, J., Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil and Tillage Research. 121, 74-81 (2012).
- Potocka, I., Szymanowska-Pulka, J. Morphological responses of plant roots to mechanical stress. Annals of botany. 122, 711-723 (2018).

