A Protocol for Collecting and Constructing Soil Core Lysimeters
Summary
A detailed method for extraction and assembly of intact soil core lysimeters and their use for study of leachate and associated loss of nutrients from surface applied poultry litter is demonstrated.
Abstract
Leaching of nutrients from land applied fertilizers and manure used in agriculture can lead to accelerated eutrophication of surface water. Because the landscape has complex and varied soil morphology, an accompanying disparity in flow paths for leachate through the soil macropore and matrix structure is present. The rate of flow through these paths is further affected by antecedent soil moisture. Lysimeters are used to quantify flow rate, volume of water and concentration of nutrients leaching downward through soils. While many lysimeter designs exist, accurately determining the volume of water and mass balance of nutrients is best accomplished with bounded lysimeters that leave the natural soil structure intact.
Here we present a detailed method for the extraction and construction of soil core lysimeters equipped with soil moisture sensors at 5 cm and 25 cm depths. Lysimeters from four different Coastal Plain soils (Bojac, Evesboro, Quindocqua and Sassafras) were collected on the Delmarva Peninsula and moved to an indoor climate controlled facility. Soils were irrigated once weekly with the equivalent of 2 cm of rainfall to draw down soil nitrate-N concentrations. At the end of the draw down period, poultry litter was applied (162 kg TN ha-1) and leaching was resumed for an additional five weeks. Total recovery of applied irrigation water varied from 71% to 85%. Nitrate-N concentration varied over the course of the study from an average of 27.1 mg L-1 before litter application to 40.3 mg L-1 following litter application. While greatest flux of nutrients was measured in soils dominated by coarse sand (Sassafras) the greatest immediate flux occurred from the finest textured soil with pronounced macropore development (Quindocqua).
Introduction
The Delmarva Peninsula borders the eastern shore of the Chesapeake Bay, and is the home to one of largest poultry production regions in the US. Roughly 600 million chickens and an estimated 750,000 tons of manure are generated from the production of these birds each year1. Most of the manure is used locally as a fertilizer amendment on agricultural fields. Because of historically high rates of manure application, nutrients such as nitrogen and phosphorus have accumulated in the soil and are now susceptible to off-site losses via subsurface leaching2. Much of the groundwater flow is directed toward an extensive network of ditches that ultimately drain to the Chesapeake Bay3. The nutrients carried to the Bay are linked to the decline in the Bay's health due to eutrophication4.
Connecting nutrient management with off-site losses of nutrients requires specialized tools to monitor hydrologic flows and associated nutrient transfers. Lysimeters represent a major category of instruments used to characterize and quantify the movement of nutrients through soils. Lysimeters have a long history of use in monitoring nutrient flow in percolating water5-7, from tension lysimeters that can be adjusted to counter soil matrix potential so that they better estimate plant available water, to zero-tension lysimeters that are more representative of processes occurring during free drainage. All approaches to lysimetery present inherent biases. For instance, some lysimeters are too small to fully represent spatially complex processes in natural soils, or are too large and expensive to provide good statistical replication of heterogeneous soils8. Further, pan lysimeters require soils above them to be saturated to collect leachate and are inefficient compared to tension lysimeters at measuring matrix flow9.
Closed lysimeter systems, such as zero-tension soil core lysimeters (also known as soil monolith lysimeters), greatly improve the confidence with which water budgets and associated pollutant budgets (e.g., nutrient budgets) are carried out10. These lysimeters are most representative when they contain intact cores of soil; lysimeters filled with repacked soils do not maintain the original structure, horizons and macropore connections that influence the transport of solutes and particulate compounds alike11,12. From an experimental stand point, approaches that facilitate greater replication of undisturbed soil conditions are advantageous, given the inherent spatial variability that exists in soil physical and chemical properties13.
Two preferred methods have been used for collecting intact soil core lysimeters: drop hammer and cutting head. The former has been more commonly performed, as it can be achieved with devices as simple as a sledge hammer (smaller lysimeters). When performed properly, soil core collection with a drop hammer has been shown to be relatively cost effective, especially when compared with other coring techniques. However, the sheer forces imposed by driving a lysimeter casing into the ground can cause smearing and compaction, producing conditions inside the lysimeter that are not representative of native soil and may even favor certain types of water movement (e.g., bypass flow, or flow along the soil core edge). As a result, some researchers have preferred the use of corers that cut away an intact soil with a drilling apparatus or other excavation device5.
Various materials have been used as casings for soil core lysimeters. Steel pipes and boxes are comparatively low cost, durable and readily available and can be used to collect larger lysimeters due to their strength14-17. However, while steel is satisfactory for evaluating the leaching of relatively unreactive compounds such as nitrate, the iron in steel reacts with phosphate and must therefore be coated or otherwise treated for the study of phosphorus leaching. Commonly, plastic casings are used to study phosphorus leaching, such as thick walled (Schedule 80) PVC pipe that can withstand the impact of a drop hammer (if used) and retain its structure when larger diameter soil cores are obtained (e.g., ≥30 cm)18-22.
In general, soil core lysimeters are analyzed ex situ. Once collected, soil core lysimeters may be installed in outdoor "lysimeter farms" where surrounding soil and above ground climates represent natural field conditions. For instance, in Sweden, the Swedish Agricultural University has maintained three separate lysimeter farms over the past three decades, analyzing pesticide fate-and-transport, long-term soil fertility trials, and management practices that can be scaled to 30 cm diameter intact cores23. Soil core lysimeters have also been subjected to indoor leaching experiments where there is greater control of climatic conditions24,25. Liu et al. used a rainfall simulator to regularly irrigate soil core lysimeters under an array of catch crops26. Kibet and Kun all employed hand irrigation techniques to study arsenic and nutrient leaching through soil cores27,28.
A variety of edaphic and hydrologic processes can be inferred from soil core lysimeters. Kun et al. (2015) used 30 cm diameter PVC column lysimeters to investigate nitrogen leaching after urea application28. By collecting leachate at different time intervals following an irrigation event, they were able to differentiate between rapid and gradual flows, with the former assumed to be dominated by macropore flow, and the later assumed to be dominated by matrix flow. Since urea is readily hydrolyzed when in contact with soil, they interpreted the presence of elevated urea concentrations in leachate collected shortly after urea application as evidence of macropore transport that bypassed the soil matrix. Over time, they detected elevated concentrations of different forms of nitrogen in leachate, tracking the transformation of applied urea to ammonium after initial hydrolysis, then the transformation of ammonium to nitrate with nitrification.
To illustrate considerations in designing, conducting and interpreting soil core lysimeter experiments, we carried out an investigation of four different soils found in the mid-Atlantic coastal plain of USA. The study measured leaching concentration and loss of nitrate before and after application of dry poultry manure (i.e., poultry "litter")28. Nutrient losses from the application of poultry litter to soils are a key concern to the health of the Chesapeake Bay, and understanding the interaction of applied poultry litter and agricultural soil properties is needed to improve nutrient management recommendations. We present here a detailed method for extracting intact soil core lysimeters, tracking soil moisture, and interpreting differential nitrate leaching losses from these soils.
This experiment is part of a larger study conducted to assess nutrient leaching from agricultural soils of the Delmarva Peninsula, USA27,28. Soil core lysimeters were collected from sites in Delaware, Maryland and Virginia in 2010. Here we present unpublished results from these studies. Although initial experiments were conducted to assess phosphorus leaching, nitrate leaching from theses soils was also monitored.
Four common agricultural soils from the Atlantic coastal plain of the Chesapeake Bay Watershed were sampled: Bojac (coarse-loamy, mixed, semiactive, thermic Typic Hapludult); Evesboro (mesic, coated Lamellic Quartzipsamment); Quindocqua (fine-loamy, mixed, active, mesic Typic Endoaquult); Sassafras (fine-loamy, siliceous, semiactive, mesic Typic Hapludult). For each soil, horizon morphology was described from the profiles exposed by the excavation of the columns (Table 1). Surface textures of the soils ranged from sand (Evesboro) to loamy fine sand/sandy loam (Bojac and Sassafras) to silt loam (Quindocqua). Although all soils had been historically fertilized with poultry litter, none had been applied in the 10 months prior to the study. All soils had been in no-till corn production for at least one season prior to soil core lysimeter collection.
Following collection, soil core lysimeters were transported to the USDA-ARS simulatorium facility in State College, PA. There they were subject to indoor irrigation experiments (22-26 °C) to assess nutrient leaching related to poultry litter application. Specifically, lysimeters were irrigated with 2 cm of water weekly for 8 weeks until nitrate in percolate was equilibrated between the soils. Poultry litter (dry poultry manure) was then applied to the surface of all soils at the rate of 162 kg ha-1 of total N. Irrigation was continued for 5 more weeks. Moisture sensors recorded volumetric moisture content at 5 minute intervals continuously, throughout the irrigation and leaching cycle. Leachate was collected after 24 hr and again 7 days later immediately prior to irrigation.
Leachate data from the soil core lysimeters were analyzed using simple descriptive statistics to illustrate differences in leachate quantity and quality between soils, as well as differences before and after litter application. Because soil moisture sensors were placed in only two of the replicate soil core lysimeters for each soil (Evesboro, Bojac, Sassafras, Quindocqua), statistics for soil moisture content were based on N = 2, whereas statistics for leachate depth, nitrate-N concentration and nitrate-N flux were derived from 10 soil core lysimeters for Evesboro, Bojac and Sassafras and 5 soil core lysimeters for Quindocqua. To assess the importance of replication within soils, coefficients of variation (CV) for leachate depth were calculated for different replicate numbers. A Monte Carlo simulation approach was used to repeatedly sample a subset of soil core lysimeters (N = 3) from the total number of replicates within each soil group (10 for Evesboro, Bojac, Sassafras; 5 for the Quindocqua).
Protocol
1. Preparing the Materials
- Cut the main body of the lysimeter from 30.5 cm (12 inch) diameter (ID; nominal) schedule 80 PVC; this has a wall thickness of 1.9 cm (0.75 inch) (Figure 1a). Cut the length of the lysimeter body depending on the thickness of the soil layer(s) to be studied; here, use a 53 cm (21 inch) long body. Rout a 0.63 cm deep by 45° bevel around the bottom end of the lysimeter to form a sharp leading edge on the inside wall of the lysimeter body to aid in cutting through the soil.
- Modify a 34.5 cm ID, flat bottom PVC cap by gluing a 15.3 cm tall 30.5 cm ID ring of schedule 80 PVC into the cap to allow for free drainage of water and provide storage capacity for leachate prior to collection (Figure 1b). Cut the ring from the same stock as the main body to serve as a coupling to join the cap to the body. The cap will be joined to the body with a flexible coupling and hose clamps (Figure 1c and 1d). Install a port for sample collection by drilling a 1.27 cm hole and tapping it with a 1.27 cm 14 NPT pipe tap and turn a 1.27 cm nylon barbed male adapter (Figure 1e) into the outer edge of the cap where the sidewall and bottom meet.
- Cut a 34 cm diameter disk from 1.27 cm thick flat stock PVC that will be used to cover the bottom of the lysimeters (Figure 1g). Drill 180, evenly spaced, 0.32 cm. diameter holes into the disk to allow drainage from the bottom of the soil-filled lysimeter to enter the cap. Glue ground cloth or other filter fabric to one side of the disk to prevent soil from passing through the bottom disk during leachate drainage.
- Build lifting scissors from 2.5 cm flat iron and 2.5 cm water pipe (Figure 2). Cut two of the 2.5 cm bands of flat iron to 50.0 cm lengths and bend into a semi-circle using the outside of the lysimeter body as a guide. Weld a 5 cm band to each end of each semi-circle band. Join each of the bands with a hinge pin. Weld the water pipe on the outer ring of the bands opposite of each other.
2. Driving Lysimeter Casing into Soil with the Drop Hammer
- Remove surface vegetation, rocks and other debris from collection area. Position 2 lysimeter bodies on level ground where lysimeters are to be taken (Figure 3a). Ensure that lysimeters are level so that soil within the column is of a uniform depth.
- Drive a specially designed, trailer-mounted, drop hammer into place over the lysimeter bodies. When the drop hammer is in place, deploy hydraulically powered outriggers to level the steel plate with the ground and top of the lysimeter bodies. The outriggers also provide stability for the drop hammer (Figure 3b).
- Partially hoist the 10.2 cm thick, 1.52 m by 1.52 m square steel plate weighing 1,180 kg up a 3 m tower with the aid of a mechanical winch (Figure 3b). Release the steel plate to hammer the columns into the soil.
- Repeat step 2.3 several times until the column rim is 2 cm above the soil surface (Figure 3c).
- Check for soil compaction inside the lysimeter by measuring the depth of soil inside and outside of the column. If the soil inside the column is more than 1 cm lower than soil outside the column, soils are compacted and are not suitable for research.
3. Removing the Soil Core
- Place a perforated PVC disk (Figure 1c) and flexible pipe coupling (Figure 1d) over the column to prevent contamination by soil and other debris during the excavation process.
- Dig a trench beside the soil core and slightly deeper than the column bottom with a backhoe (Figure 4a).
- Widen the hole with a shovel or pick (Figure 4b) and expose as much of the outside of cylinder as possible.
- Push a heavy metal digging bar down along the entire length of the side of the column so that it is between the soil and outside column wall (Figure 4c).
- Pry the digging bar back and forth until the soil interface at the bottom of the column is broken.
- Frame the lifting scissors around the top of the lysimeter (shown in Figure 2) in preparation for soil core removal. With a person holding each bar, pull up until the scissors close tightly around the column and lift the lysimeter out of the hole. Place the lysimeter on a flat working surface such as piece of plywood.
4. Preparing the Soil Core for Lysimeter Assembly
- Flip the soil core over so that the bottom side is up. The wooden plywood disk installed in step 3.1 will hold the soil in place.
- Gently, level the soil even with the rim of the PVC (Figure 5a) with a straight edge. Remove stones protruding above the plane of the rim with a pen knife or screwdriver.
- Fill any voids with chemically inert play sand and gently pack it (Figure 5b).
- Grade the sand even with the column bottom with a straight edge and remove any excess sand (Figure 5c and d).
- Clean any soil from the rim and the outer side walls of the lysimeters with a brush or lightly blow it off the edge and ensure that the rim is clean for adhesives to stick and for a snug fitting of the cap.
5. Assembling the Lysimeter
- Extrude a continuous round bead of clear silicon caulk around the rim of the lysimeter (Figure 6a). The caulk should be thick enough to seal the perforated disk bottom to the lysimeters and prevent leaking.
- Lay the perforated disk (Figure 1c) onto the rim with the filter fabric facing the sand and press down firmly to allow good contact of the plate and lysimeter.
- Drill eight evenly spaced pilot holes around the edge of the plate and fasten the perforated disk with 1.0 inch stainless steel screws with a drill driver (Figure 6b).
- Slip the flexible pipe coupling on to the lysimeter base so that about 2 cm of the coupling is projecting above the lysimeter rim (Figure 5c).
- Fit the modified PVC cap into the flexible pipe coupling (Figure 6c), and push the cap down until it makes contact with the lysimeter body. With a block of wood on top of the cap use a mallet to gently tap the cap in place.
- Place the fastening bands in the grooves of the coupling and secure lightly without constricting the coupling. Tighten the metal around the coupling with a hand held 1/4 inch hex driver until the lysimeter cap is firmly held in place. The lysimeter is ready to be flipped and transported to a climate controlled facility.
6. Installing Moisture Sensors
- Scribe a 5 cm long, horizontal line on the lysimeter wall at 5 and 25 cm depths. Measure from the soil surface and not the rim of the lysimeter.
- Drill a 1.0 cm diameter hole through the wall of the lysimeter at each end of the marked lines.
- Cut the remaining 3 cm of plastic between the drilled holes away with a rotary cutting tool.
- Chisel a 1 cm thick by 5 cm long slit into the soil to accommodate the casing of a moisture sensor (e.g., Decagon).
- Push the moisture sensor into the hole into the cleaned out slot until the sensor prongs are firmly buried in the soil and that only the wire is sticking out of the lysimeter.
- Clean soil from the walls of the slot with a brush or rag.
- Apply a thick bead of silicone caulk into the slot to prevent water from leaking out. After the caulk has dried, apply a second cycle of silicone to ensure that all gaps in the hole surrounding the sensor are sealed.
7. Preparing Lysimeters for Leachate Collection
- Seal gaps between the soil and lysimeter wall with caulk to reduce the risk of preferential flow down the inside walls of the lysimeter.
- Pierce and load a tube of clear silicone caulk into a standard caulk gun.
- Place tip of caulk tube between void in the soil to be filled and the inside face of the lysimeter body. Push the tip of the caulk gun below the soil about 2 cm. Squeeze the caulk out of the tube until it fills the void and oozes above the soil surface.
- Set lysimeters on top of a bench or flat surface and sturdy enough to handle the weight of several lysimeters and high enough to allow free drainage of water into a 4.0 L jug (Figure 7).
- Check that soil core lysimeters are leveled in all directions with a small (15 cm) spirit level. If necessary place shims underneath lysimeters until the soil surface is completely leveled.
- Wrap Teflon tape around the threaded nylon tube fitting (0.5 in NPT) and turn the fitting clockwise into the cap. Tighten the fitting with an adjustable wrench until none of the threads are visible.
- Push a 0.5 inch hose onto the barbed end of the nylon fitting and cut the hose so that it passes approximately 4.0 cm into the mouth of the collection jug.
- Set the container under the lysimeter and place the hose inside the collection jug.
8. Irrigating Lysimeters and Collecting Leachate
- Cover the soil surface with cheese cloth or other permeable, chemically inert fabric to protect and preserve soil aggregates and surface residue.
- Measure 1,450 ml of deionized water into a graduated cylinder and pour it into watering can, equipped with shower head. Gently and evenly sprinkle the water over the fabric at a rate that does not disturb the soil surface.
- Wait a period of time for water to infiltrate an percolate through the soil column into the cap and collection container.
- Tip lysimeter in the toward the outlet hole until all water is drained from the lysimeter reservoir cap into the collection vessel.
- Measure the mass of leachate collected with a scale and convert mass in grams to ml (assume that 1.0 g of water is equivalent to 1.0 ml). Pour the leachate sample into 350 ml sterile plastic sample bottle. Immediately filter 50 ml with a suction funnel equipped with 0.45 µm filter paper in preparation for nitrate analysis using colorimetry via flow injection analysis31.
- Store filtered and unfiltered portions of the samples in a refrigerator and 4 °C until analysis.
Representative Results
Soil moisture, leachate depth and leachate chemistry all illustrate variability across soils, revealing differences as a function of soil properties despite internal variability between replicate soil core lysimeters of a particular soil. The later point warrants particular note from the standpoint of experimental design, as inherent variability in soil moisture and leaching processes requires considerable replication to minimize type 2 statistical error. In the current study, coefficients of variation (CV) across all soils ranged from 0.02 to 0.38 for soil moisture, 0.02 to 0.06 for leachate depth, 0.22 to 0.55 for nitrate-N concentrations, and 0.23 to 0.54 for nitrate-N flux.
The effect of lysimeter replication on variance is illustrated by sampling leachate data from the replicates of individual soils (Bojac, Evesboro, Sassafras, Quindocqua), revealing a stronger influence of replication on some variables than others. In general, the CV clearly decreases as lysimeter replicates increase from three to ten (or, in the case of Quindocqua, three to five replicates). For leachate depth, CV decreased from 0.14 to 0.06 for the Bojac soil, from 0.12 to 0.06 for the Evesboro soil and from 0.08 to 0.03 for the Sassafras soil. In the case of the Quindocqua, for which only five replicates existed, the CV of N = 3 was 0.04 while the CV for N = 5 was 0.02. For nitrate-N concentration, the CV decreased from 0.88 to 0.34 for Bojac, from 0.39 to 0.17 for Evesboro, and from 0.26 to 0.12 for Sassafras. For Quindocqua, the CV of nitrate-N concentration declined from 0.35 with three replicates to 0.17 with five replicates. The effect of replication on the CV of nitrate-N flux was similar to that observed with nitrate-N concentration.
Soil moisture
Changes in soil water content at 5 cm and 25 cm depths following irrigation demonstrate differences in water transmission between coarser and finer textured soils (Figure 8). Moisture profiles indicate rapid movement of irrigation water through the coarser textured Evesboro sand and Sassafras sandy loam soils. Volumetric water content in these soils at both 5 and 25 cm depths increased to an average of 0.31 and 0.22 m3 m-3, respectively, within 1 h of irrigation and then returned to background levels (0.17 and 0.21 m3 m-3) by 9 hr after irrigation. In contrast, volumetric water content in the Bojac and Quindocqua soils did not return to background levels until at least 20 hr after irrigation.
Leachate depth
Weekly leachate depths ranged from 1.12 to 1.95 cm for the four soils over the course of the experiments (Figure 9). Irrigation water recoveries, expressed as percentage of irrigation water, followed a general trend related to soil texture, with recoveries from the sandy Evesboro (81%) and Sassafras (85%) soils being slightly more efficient than from the finer textured Bojac (77%) and Quindocqua (71%) soils. Most leachate was collected with the first sampling after irrigation (24 hr), equivalent to 80% of the total leachate collected for Bojac, 84% of the total leachate collected for Evesboro, 91% of the total leachate collected for Sassafras, and 99% of the total leachate collected for Quindocqua.
Nitrate-N concentrations and fluxes in leachate
Nitrate-N concentrations in leachate increased after litter application, but followed different temporal patterns between soils. In the week before manure application, nitrate-N concentration in leachate for the four soils averaged 27.1 mg L-1 (Figure 10). For the fine textured Quindocqua, concentration peaked immediately, with nitrate-N in the leachate samples from the first week averaging 39.9 mg L-1. In contrast, nitrate-N in leachate from the sandier textured soils increased more slowly, with peak nitrate-N concentrations occurring two weeks after litter addition for the Bojac soil (average of 37.3 mg L-1) and four weeks after litter addition for the Evesboro (average of 53.0 mg L-1) and Sassafras soils (average of 57.1 mg L-1).
Differences in leachate nitrate-N flux (kg ha-1) reflect not only trends in nitrate-N concentrations in leachate but also differences in leachate depths (Figure 11). Before litter application, weekly nitrate fluxes were 2.0-5.8 kg ha-1, with Sassafras > Evesboro > Bojac > Quindocqua. The greater leachate depths from Sassafras and Evesboro lysimeters (Figure 9) are evident in the nitrate-N fluxes before litter application. To assess the role of poultry litter application and leachate volume on nitrate-N flux, soil nitrate-N fluxes from before litter application were subtracted from subsequent weekly fluxes (Figure 12). The resulting pattern in flux changes visually and the range in nitrate-N flux among the soils is 1.1 to 4.7 kg ha-1. Nitrate-N flux from the Quindocqua soils after litter application spikes immediately and remains greater than fluxes from the other soils until week six. Nitrate-N fluxes from the coarser textured soils, again, is delayed with Bojac (3.7 kg ha-1) and Sassafras (3.8 kg ha-1) peaking in the second week after litter application and the Evesboro peaking at 3.0 kg ha-1, four weeks after litter application.
| Hydrologic | Particle size distribution | KCl Nitrate | ||||||
| Soil | Class | 0-5 cm | 15-30 cm | 45-50 cm | 0-5 cm | |||
| % sand | % clay | % sand | % clay | % sand | % clay | mg kg-1 | ||
| Bojac | B | 72.7 | 9.6 | 65.1 | 16.9 | 57.9 | 21.8 | 74 |
| Evesboro | A | 89.8 | 3.7 | 86.9 | 5.6 | 89.0 | 5.9 | 110 |
| Quindoqua | C | 30.2 | 17 | 29.2 | 24.8 | 33.9 | 23 | 341 |
| Sassafras | B | 82.0 | 5.7 | 74.4 | 9.7 | 88.4 | 7.9 | 103 |
Table 1: Chemical and physical properties of the soil core lysimeters.

Figure 1: Major Parts for Constructing Lysimeter. (a) Schedule 80 PVC lysimeter bodies; (b) PVC cap; (c) Flexible coupling; (d) Perforated disk; (e) Hose clamps; (f) Food grade tubing; (g) Threaded barbed hose fitting. Please click here to view a larger version of this figure.

Figure 2: Custom lifting scissors. Custom lifting scissors allow two people to lift and move heavy soil core lysimeters. Please click here to view a larger version of this figure.

Figure 3: View of Drop Hammer and insertion of Columns. (a) PVC columns placed level on the soil in preparation for the drop hammer. (b) Drop hammer pounding in cylinders. (c) Cylinders completely driven into soil. Please click here to view a larger version of this figure.

Figure 4: Preparation for removal of soil columns. (a) Hole being dug along-side of columns. (b) Soil being picked away from columns (note lysimeters protected from external soils with PVC cover and flexible coupling). (c) Soil-to-soil interface being broken with a digging bar. Please click here to view a larger version of this figure.

Figure 5: Preparation of lysimeter bottom for perforated plate and cap. (a) Leveling bottom and removal of protruding stones. (b) Filling voids with sterile sand. (c) Leveling sand. (d) Cleaned column with level sand. Please click here to view a larger version of this figure.

Figure 6: Installing bottom onto lysimeter. (a) Putting a ring of caulk on cleaned rim of lysimeter. (b) Fastening perforated disk onto lysimeter with stainless steel screws. (c) Putting cap on lysimeter and fastening tight with flexible coupling. Please click here to view a larger version of this figure.
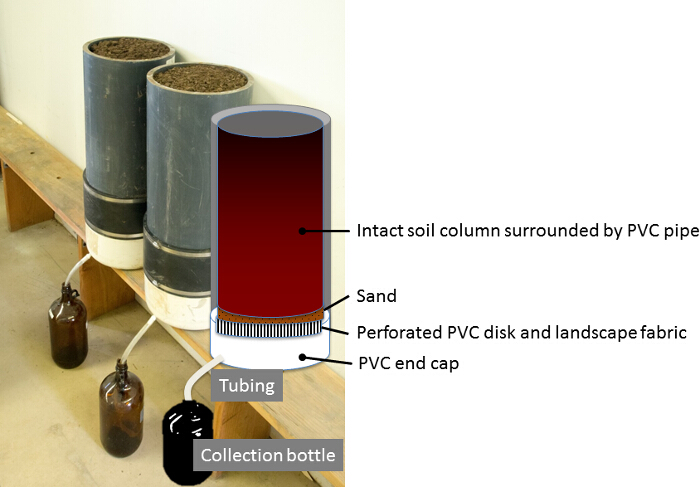
Figure 7: Completely assembled lysimeter. Assembled lysimeter with hose attached and glass bottles placed beneath for leachate collection (moisture sensors not installed). Please click here to view a larger version of this figure.
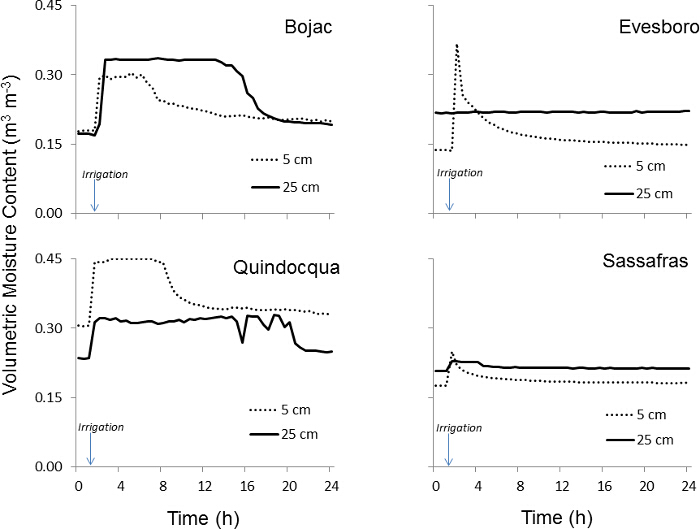
Figure 8: Volumetric water content. Volumetric water content (m3 m-3) within the soil core lysimeter at 5 cm and 25 cm depths over a typical 24 hr period following irrigation. Please click here to view a larger version of this figure.
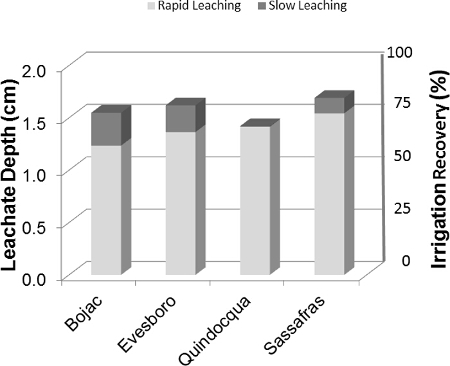
Figure 9: Leachate depth. The sum of weekly leachate depth (cm) collected from soil core lysimeters partitioned into rapid leaching (24 hr) and slow leaching (7 day) segments. Please click here to view a larger version of this figure.
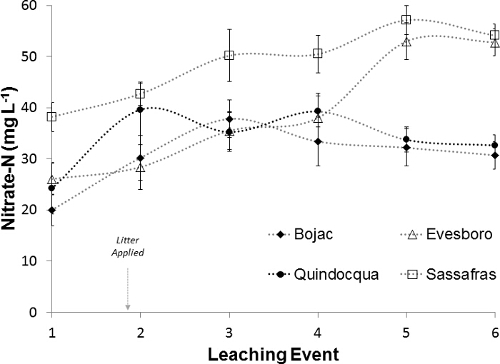
Figure 10: Nitrate-N concentration. Weekly nitrate-N concentration (mg L-1) in leachate collected from soil core lysimeters before and after poultry litter application. Plotted points represent the mean and error bars around the points represent standard error of the mean. Please click here to view a larger version of this figure.
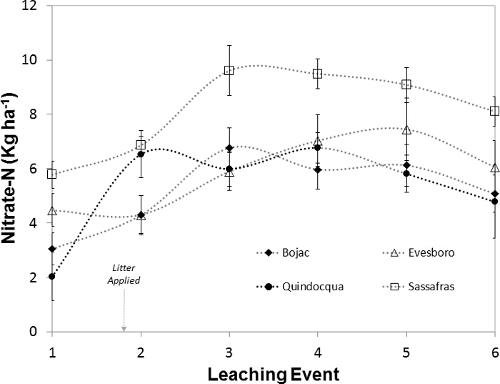
Figure 11: Nitrate-N Flux. The mass of nitrate-N (kg ha-1) in leachate collected from soil core lysimeters before and after poultry litter application. Plotted points represent the mean and error bars around the points represent standard error of the mean. Please click here to view a larger version of this figure.
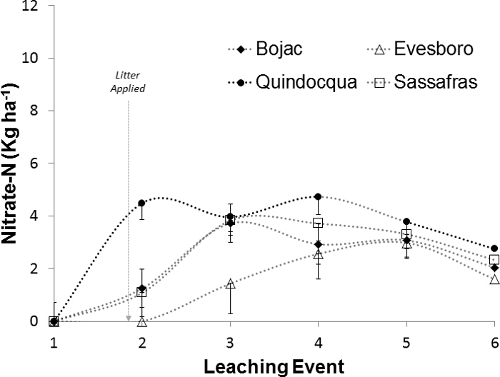
Figure 12: Estimated nitrate-N flux contribution from manure. Soil nitrate-N fluxes (kg ha-1) from before litter application were subtracted from subsequent weekly fluxes to assess the contribution of poultry litter nitrogen to soil core leachate. Plotted points represent the mean and error bars around the points represent standard error of the mean. Please click here to view a larger version of this figure.
Discussion
Important steps of lysimeter Collection
Leaching studies illustrate the influence of soil properties and manure management on nitrogen losses to shallow groundwater. Soil physical properties such as soil texture, aggregate structure and bulk density mediate the percolation of water and solutes. Accurately determining leachate volume and solute concentrations is dependent upon retaining the integrity of these soil physical properties during lysimeter collection by following these critical steps: 1) the lysimeter and the drop hammer must remain level while the column is being driven into the soil; 2) soil within the lysimeter must be checked for compaction; 3) the bottom of the soil column must be leveled and voids must be filled with inert sand before the drain cap is installed; and 4) all gaps including those between the lysimeter wall and soil must be sealed with silicone caulk to prevent preferential sidewall flow or leakage from the moisture sensor ports.
Importance of maintaining soil structure
Leaching studies need to accurately represent the volume of water moving through soil profiles in order to effectively determine mass loss of solutes. The average irrigation recovered from the four soils studied was 79% of the volume applied. Similar research comparing the efficiency unbound zero-tension pan lysimeters reported average irrigation collection efficiencies of 56% and 58%29,10. Although the soils in the aforementioned studies were different from the soils in this study, we attribute the increase in irrigation recovery efficiency to the soil core lysimeters ability to retain soil physical properties and encase the soil profile.
Importance of replication
This study points to the influence of replication on variance in leachate properties and the need to increase replication in order to draw significant inferences from soil core lysimeters. Variability in leachate properties was greatest for nitrate-N concentration and flux and lowest for leachate volume. For all leachate properties, increasing the number of replicate soil core lysimeters from three to 10 (Bojac, Evesboro and Sassafras or in the case of Quindocqua, from three to five), reduced the CV to 0.06 or less. From our experience, a minimum of four replications is needed in soil core lysimeter experiments18,28,29.
Importance of tracking soil moisture
Soil moisture trends at 5 cm and 25 cm depths, in combination with an understanding of soil morphology at these depths, can be used to explain hydrologic trends and steady state assumptions. For example, soil moisture trends reveal differences in leaching processes between the coarse textured Evesboro and Sassafras soils and the finer textured Bojac and Quindocqua soils. The coarser textured soils exhibited brief increases in volumetric water content when compared to finer textured soils which had more prolonged increases in soil moisture (Figure 8). These differences were also revealed when comparing 24 hr and 7 day leachate collections, but lacked the finer temporal resolution to refine hypotheses regarding rapid macropore flow. In the case of the Bojac soil, which yielded the largest proportion of leachate after the first 24 hr collection, soil moisture trends at 25 cm depth reveal a prolonged period of soil moisture saturation that would favor denitrification conditions and therefore diminish nitrate-N in leachate. Given the insight gained from soil moisture sensors, a premium should be place on installing sensors in as many soil cores lysimeters as possible to facilitate post hoc assessment of leaching processes.
Importance of calculating mass balance
In the current study, 8.5-19.6% of applied N was lost in leachate as nitrate-N over a 6 week period. Leaching losses are clearly a major component of the N budget for manured soils and minimizing these losses is important not only for environmental quality but also for nutrient use efficiency. An estimated 80.4-91.5% of the litter-applied N remained in the soil core lysimeters. Documenting the fate of this N could be improved with use of techniques such as labels or tracers. Thus, a clear benefit of soil core lysimeters is in budgeting of water and applied materials, something that is much more difficult with other types of lysimeter systems, such as pan lysimeters, that are not bounded and are known to be less efficient9.
Limitations of Design
Although the current design efficiently measures free draining gravitational water, it is believed that the lysimeters underestimate leaching volume from the smaller pore spaces of finer textured soils due to tensional forces. The average fraction of irrigation water recovered from the fine textured Quindocqua soil accounted for only 71% of the total applied. Further, less than 1% of this volume is attributed to 'slow leaching' through the finer pores in the soil matrix. Collection efficiencies have been increased by 50% or more with the addition of passive capillary fiberglass wicks to soil profiles9. The authors are currently investigating the efficacy of fiberglass wicks for use in the soil core lysimeter described in this manuscript.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors are grateful to the staff of USDA-ARS Pasture Systems and Watershed Management Unit. David Otto was important to both the design and construction of the custom made drop hammer (aka ‘The Intimidator’). Michael Reiner and Terry Troutman assisted in the collection and construction of the lysimeters reported in this study. Sarah Fishel, Charles Montgomery and Paul Spock performed all of the nutrient analyses reported in this manuscript.
Materials
| Schedule 80 PVC Pipe | Fry's Plastic | Call | Sold in 10 ft lengths |
| Fernco Fittings | Fry's Plastic | Call | 12 in. diameter |
| Type II PVC plates for perforated discs | AIN Plastic | Call | Sold in 4' x 8' sheets of PVC II Vintec II |
| Schedule 40 PVC Caps | Fry's Plastic | Call | 12 in. diameter |
| Stainless Steel Screws | Fastenal | 135716 | #8 Bugle Head Phillips Drive Sharp Point Grade 18-8 Stainless Steel |
| Silicone II Caulk | Lowe's | 447488 | |
| Nylon Tube Fitting | United State's Plastic Corp. | 61137 | 0.5 in. NPT |
| Foodgrade Tubing | Lowe's | 443209 | 0.5 in. vinyl |
Referências
- Patterson, P. H., Lorenz, E. S., Weaver, W. D., Schwart, J. H. Litter production and nutrients from commercial broiler chickens. J. Applied Poultry Res. 7 (3), 247-252 (1998).
- Cullum, R. F. Macropore flow estimations under no-till and till systems. Catena. 78, 87-91 (2009).
- Kladivko, E. J., et al. Nitrate leaching to subsurface drains as affected by drain spacing and changes in crop production systems. J. Environ. Qual. 33, 1803-1813 (2004).
- Persson, L., Bergstrom, L. Drilling method for collection of undisturbed soil monoliths). Soil Sci. Soc. Am. J. 55 (1), 285-287 (1991).
- Belford, R. K. Collection and evaluation of large soil monoliths for soil and crop studies. J. Soil Sci. 30 (2), 363-373 (1979).
- Dell, C. J., Kleinman, P. J. A., Schmidt, J. P., Beegle, D. P. Low disturbance manure incorporation effects on ammonia and nitrate loss. J. Environ. Qual. 41, 928-937 (2012).
- Owens, L. B. Nitrate-nitrogen concentrations in percolate from lysimeters planted to a legume-grass mixture. J. Environ. Qual. 19, 131-135 (1990).
- Zhu, Y., Fox, R. H., Toth, J. D. Leachate collection efficiency of zero-tension pan and passive capillary fiberglass wick lysimeters. Soil Sci. Soc. Am. J. , (2002).
- Jemison, J. M., Fox, R. H. Estimation of zero-tension pan lysimeter collection efficiency. Soil Sci. 154, 85-94 (1992).
- Corwin, D. L. Evaluation of a simple lysimeter-design modification to minimize sidewall flow. J. Contaminant Hydrology. 42 (1), 35-49 (2000).
- Havis, R. N., Alberts, E. E. Nutrient leaching from field decomposed corn and soybean residue under simulated rainfall. Soil Sci. Soc. Am. J. 57, 211-218 (1993).
- Bergstrom, L., Johanssson, R. Leaching of nitrate from monolith lysimeters of different types of agricultural soils. J. Environ. Qual. 20, 801-807 (1991).
- Lotter, D., Seidel, R., Liebhardt, W. The performance of organic and conventional cropping systems in an extreme climate year. Am. J. Alternative Agriculture. 18 (3), 146-154 (2003).
- Moyer, J., Saporito, L., Janke, R. Design, construction, and installation of an intact soil core lysimeter. Agronomy J. 88 (2), 253-256 (1996).
- Stout, W. L., et al. Nitrate leaching from cattle urine and feces in northeast US. Soil Sci. Soc. Am. J. 61, 1787-1794 (1997).
- Stout, W. L., Gburek, W. J., Schnabel, R. R., Folmar, G. J., Weaver, S. R. Soil-climate effects on nitrate leaching from cattle excreta. J. Environ. Qual. 27, 992-998 (1998).
- Kleinman, P. J. A., Srinivasan, M. S., Sharpley, A. N., Gburek, W. J. Phosphorus leaching through intact soil columns before and after poultry manure applications. Soil Sci. 170 (3), 153-166 (2005).
- Kleinman, P. J. A., Sharpley, A. N., Saporito, L. S., Buda, A. R., Bryant, R. B. Application of manure to no-till soils: Phosphorus losses by subsurface and surface pathways. Nutr. Cycling Agroecosyst. 84, 215-227 (2009).
- McDowell, R. W., Sharpley, A. N. Approximating phosphorus release to surface runoff and subsurface drainage. J. Environ. Qual. 30, 508-520 (2001).
- McDowell, R. W., Sharpley, A. N. Phosphorus losses in subsurface flow before and after manure application. Sci. Total Environ. 278, 113-125 (2001).
- Brock, E. H., Ketterings, Q. M., Kleinman, P. J. A. Phosphorus leaching through intact soil cores as influenced by type and duration of manure application. Nutr. Cycl. Agroecosyst. 77, 269-281 (2007).
- Svanback, A., et al. Influence of soil phosphorus and manure on phosphorus leaching in Swedish topsoils. Nutr. Cycling Agroecosyst. 96, 133-147 (2013).
- Feyereisen, G. W., et al. Effect of direct incorporation of poultry litter on phosphorus leaching from coastal plain soils. J. Soil Water Cons. 65 (4), 243-251 (2010).
- Williams, M. R., et al. Manure application under winter conditions: Nutrient runoff and leachate losses. Trans. ASABE. 54 (3), 891-899 (2011).
- Liu, J., Aronsson, H., Ulén, B., Bergström, L. Potential phosphorus leaching from sandy topsoils with different fertilizer histories before and after application of pig slurry. Soil Use Mgmt. 28, 457-467 (2012).
- Kibet, L. C., et al. Transport of dissolved trace elements in surface runoff and leachate from a coastal plain soil after poultry litter application. J. Soil Water Cons. 68 (3), 212-220 (2013).
- Han, K., et al. Phosphorus and nitrogen leaching before and after tillage and urea application. J. Environ. Qual. 44, 560-571 (2014).
- Day, P. R., Black, C. A. This chapter in Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling. American Society of Agronomy, Soil Science Society of America. , (1965).
- Kleinman, P. J. A., et al. Phosphorus leaching from agricultural soils of the Delmarva Peninsula, USA. J. Environ. Qual. 44 (2), 524-534 (2015).
- . Lachat Instruments. Determination of nitrate/nitrite in surface and wastewaters by flow injection analysis. QuickChem Method. , (2003).

