Simultaneous Focused Ultrasound Neuromodulation and Fiber Photometry Recording in Free-Moving Mouse
Summary
The protocol includes transducer manufacturing, parameters reporting, surgical procedure, and signal recording for the entire operational workflow of concurrent focused ultrasound neuromodulation and fiber photometry recording in free-moving mice.
Abstract
Focused ultrasound neuromodulation (FUN) represents a promising approach for non-invasive perturbation of neuronal circuits at deep brain regions. It is compatible with most of the existing modalities for monitoring brain functions in vivo. Integration with brain function recording modalities not only enables us to address orders and disorders of specific brain functions with closed-loop feedback but also provides us with mechanistic insights about FUN itself. Here, we provide a modified, simple, dependable, and robust protocol for the simultaneous application of FUN and fiber photometry GCaMP6s fluorescence recording in free-moving mice. This involves the fabrication of a well-sized single transducer and its temporary placement on the mice, along with the secure fixation of a fiber optical implant to facilitate the smooth passage of the transducer. The combination of FUN and fiber photometry provides for the optical recording of neural circuitry responses upon FUN in real-time in deep brain regions. To demonstrate the efficiency of this protocol, Thy1-GCaMP6s mice were used as an example to record the neuroactivity in the anterior thalamic nucleus during FUN while the mice are freely moving. We believe that this protocol can promote the widespread use of FUN in both the neuroscience field and the biomedical ultrasound field.
Introduction
Focused ultrasound neuromodulation (FUN) has emerged as a promising and versatile neuromodulation tool, enabling the exploration of brain function and organization with great potential1. FUN is able to deliver acoustic energy noninvasively to any position within the brain tissue with pinpoint precision2. Its ability to transiently and reversibly modulate neuroactivity in deep brain structure, with high spatiotemporal specificity, in a safe and noninvasive manner, presents an appealing attribute that complements existing clinical neuromodulation technique3. Demonstration of effective FUN has been confirmed in both human subjects4,5,6 and in various animal models, encompassing small7,8,9,10 and large species11,12,13,14,15,16,17.
By observing the effect of FUN on specific neural types through neuroactivity monitoring during FUN, we can delve into the mechanism behind this process18,19. Fiber photometry based on genetically encoded calcium indicators (GECIs) has become widely utilized in the past decade as a versatile method for tracking cell-type-specific population activity in vivo20,21,22,23,24. Hence, the simultaneous application of FUN and fiber photometry can significantly enrich our comprehensive understanding of FUN. Nevertheless, the use of bulky single transducers necessitates fixation to a frame, while animals need to undergo anesthesia and be immobilized in a stereotaxic frame7,19,25,26. This approach may not be suitable for certain types of experiments related to perception, cognition, and behavior evaluation. It is crucial to establish a protocol that facilitates the amalgamation of FUN and fiber photometry without impeding the mice's mobilization7.
In this study, we present a refined protocol used in our previous studies to seamlessly and gracefully complement the method for crafting a single transducer and its temporary fixation on to the mice, as well as the secure fixation of a fiber optical implant to facilitate the smooth passage of the transducer7,19,26. It allows researchers to record the neuroactivity modulated by ultrasound in unrestrained mice. We opted for a smoother envelope, such as a sinusoidal envelope, to reduce the auditory confound27. This protocol's feasibility is confirmed through the simultaneous recording of neuroactivity in the anterior thalamic nucleus of free-moving mice during FUN. It demonstrates that the transducer's energy is sufficient to achieve neuromodulation, and the fixation methods for the fiber optical implant and the transducer can ensure their stability.
Protocol
All procedures and animal handling complied with NSFC ethics guidelines and approved protocol requirements of the Institutional Animal Care and Use Committee of the Guangdong Institute of Intelligence Science and Technology.
1. Transducer preparation
- Prepare a piezoelectric plate with an inner diameter of 3 mm, an outer diameter of 7 mm, and a center frequency of 500 kHz.
NOTE: The outer diameter can be adjusted based on the specific brain region being targeted and should be maximized while maintaining stimulation accuracy and without exceeding the boundary of the mouse skull. - Attach the wire to the two sides of the piezoelectric plate using epoxy silver paste (Figure 1). After the epoxy silver paste has solidified, use a multimeter to measure the resistance at both ends of the wire to ensure that it is approximately 0.
- Apply a layer of double-sided tape onto a clean glass sheet surface. Adhere to the piezoelectric plate and copper ring, with a height of 8 mm, an outer diameter of 8 mm, and an inner diameter of 7.6 mm, tightly to the glass sheet.
NOTE: The inner diameter of the copper ring is determined by the size of the piezoelectric plate to ensure the plate is covered by the copper ring. - Securely insert the polypropylene pipe with an outer diameter of 3 mm into the center of the piezoelectric plate and firmly adhere it to the glass sheet (Figure 1).
- Prepare an appropriate amount of epoxy resin glue and vacuum it. Extract the epoxy using a disposable syringe and slowly inject it into the copper ring. Wait about 10 h until the epoxy has solidified (Figure 1).
- Solder the loose ends of two wires onto the bayonet nut connector using an electronic soldering iron. Remove the glass sheet. Clean the surface of the transducer with alcohol (Figure 1).
2. Reporting parameters for FUN
- Place the hydrophone and transducer in a water tank filled with deionized water (Figure 2A). Ensure that the central beam (Z-axis) of the positioning system is aligned with the transducer axis. This alignment can be achieved by firstly, discovering a field maximum in the focal plane through 2D scanning; secondly, identifying a field maximum in another plane with a clear maximum; thirdly, comparing the X and Y coordinates of the two maxima and then iteratively adjusting the position and/or orientation of the transducer if needed28.
- Adjust the tip of the hydrophone with the surface of the transducer at a distance of 1 mm, keeping this distance constant while positioning the hydrophone in the middle of the right edge of the transducer. Initiate the scanning program to capture the free acoustic field in the XZ plane (Figure 2B).
NOTE: The construction method of the hydrophone can be found at https://github.com/HQArrayLab/Hydrophone_system_control. - Move the hydrophone along the Z-axis to determine the depths associated with the spatial peak pressure. In this experiment, the spatial peak pressure appears at a distance of 3.4 mm from the surface of the transducer; maintain this distance when moving the hydrophone to the bottom right corner of the transducer at the XY plane.Power up and initiate the scanning program to capture the free acoustic field in the XY plane (Figure 2B).
- Place the transducer on the skull of a mouse that underwent surgery as described in step 4. Acquire the transcranial acoustic field on the XZ plane and XY plane (Figure 2D) through hydrophone scanning as described in 2.1-2.3.
- Read the pressure amplitudes at the focal point, which is the spatial peak region in the free acoustic field and transcranial acoustic field. The pressure amplitude at the focal point in the free acoustic field is 730k Pa, and in the transcranial acoustic field is 580k Pa. Read the focal dimensions at -3 dB (Figure 2C,E), and the position on the XY and XZ planes within the transcranial acoustic field to assess whether the acoustic field of this transducer can cover the target brain areas.
- Compute the Mechanical Index (MI), which is constrained by the FDA guidance document to be below 1.9 in order to mitigate cavitation. The calculation of MI is given by the equation:
 (1)
(1)
where pr,.3 represents the peak-rarefactional pressure in MPa adjusted by an attenuation coefficient of 0.3 dB cm-1 MHz-1, and f0 is the operating frequency in MHz. The transcranial-field peak rarefactional pressure measured is 580 kPa, , 3.4 mm from the transducer, the f0 is 500 kHz, so the derated pr,.3 is 576.6 kPa. The MI is 0.82. - Calculate the spatial-peak pulse-average intensity (Isppa), which is required to be below 190 W/cm2 in the plane direction according to the FDA guidance document. The calculation of intensity is given by the equation:
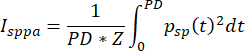 (2)
(2)
where psp (t) is the time-varying acoustic pressure at the spatial-peak location, Z is the characteristic acoustic impedance of the medium (approximately 1.5 x 106 Rayls for soft tissue), and PD is pulse duration. In the case of the square envelope, this reduces to the equation:
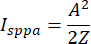 (3)
(3)
where A is the spatial-peak pressure amplitude. The A measured at the ultrasound focused location is 580 kPa, and the Z of the brain is about 1.58 x 106 Rayls, so the Isppa of the square envelope is 10.65 W/cm2 and the Isppa of the sinusoidal envelope is 10.65 W/cm2. - Calculate the spatial-peak time-average intensity (Ispta), which is constrained by the FDA guidance document to be below 430 mW/cm2 in the plane direction. The calculation of intensity is given by the equation:
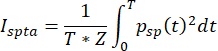 (4)
(4)
where T is the time period over which the average is calculated. In the case of the square envelope, this reduces to the equation:
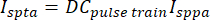 (5)
(5)
where DCpulse train is the duty cycle of pulse. Here, the DC pulse train is 1% because continuous waves were used, so the Ispta is equal to the spatial-peak pulse-average intensity, 106.5 mW/cm2 for a square envelope. The MI, Isppa, and Ispta can be calculated using the software (Figure 3A). A MATLAB-based code for easy use can be found at https://github.com/HQArrayLab/Ultrasound_Parameter_Caculation. - Report the pulse timing parameters, including Amax, pulse duration, pulse repetition interval, pulse train duration, and envelope (Figure 3B).
3. Preparing the animal for surgery
- Weigh 8-week-old male GCaMP6s transgenic mice, with an approximate weight of about 20 g. Prepare a solution containing ketamine at 10 mg/mL and xylazine at 2 mg/mL in sterile saline. Administer the ketamine/xylazine solution by intraperitoneal injection at a dosage of 100 mg/kg ketamine and 20 mg/kg xylazine using a 26G needle and 1 mL disposable syringe. Commence surgical preparation once the animal is unresponsive to painful stimuli, such as toe pinch.
- Use a fader to trim the hair on the animal's head and disinfect the area with 70% ethanol and povidone-iodine prior to the surgical procedure.
- Place the mouse in a prone position on the stereotaxic frame and ensure that the skull is level. Place a protective ophthalmic ointment over the animal's eyes to maintain moisture.
4. Surgical procedure
- Make an incision along the sagittal suture, starting from the occipital bone to the beginning of the nasal bone. Use surgical scissors to remove the skin covering both hemispheres.
- Use sterile saline to cleanse the skull and eliminate any remaining periosteum.
- Apply 3% hydrogen peroxide to the exposed cranium using a cotton swab for approximately 2 s-3 s to create micropores. Rinse with sterile saline thoroughly and ensure the area is completely dry.
- Create a 0.6 mm diameter burr hole craniotomy using a sterile, autoclaved drill bit above the brain area location as determined by the stereotaxic atlas aligned to bregma and lambda. Wash away any debris with sterile saline and ensure thorough drying. Be careful not to damage any tissue.
- Insert the fiber optical ferrule (implant) into the probe holder and connect it to the stereotaxic arm.
- Align the implant directly above the region of interest using the stereotaxic arm. When inserting the optical fiber into the brain tissue, advance the fiber slowly at a rate of approximately 2 mm/min.
- Mix the dental cement to achieve a viscosity that allows for easy application across the cranium. Use a sterile toothpick to spread a thin layer of dental cement over the cranium and onto the lower part of the implant. Allow it to dry completely.
- Detach the probe holder carefully. Prepare a polypropylene pipe with a height of 3 mm, an outer diameter of 3 mm, and an inner diameter of 2.6 mm, and then cut the pipe throughout its length.
- Attach the pipe to the bottom of the implant using tweezers. Pour the dental cement powder into the pipe ensuring sufficient length above the implant for recording the optical fiber signal. Add the required liquid and allow a few minutes for the dental cement to solidify.
- Locate the opening of the pipe and carefully clamp it to remove the pipe using tweezers. Prepare the dental cement mixture for application, ensuring an even and thin layer is spread across the cranium. Cover as much surface area on the cranium as possible with dental cement. Wait a few minutes for the dental cemental to solidify.
NOTE: Do not let the dental cement come into contact with the skin of the mouse. - Drill three holes (1 mm diameter) into the 3D-printed ring, evenly divided on the horizon, with a height of 7 mm, an outer diameter of 10 mm, and an inner diameter of 8.4 mm. Secure the screws (1 mm length) into their respective holes.
- Insert the top of the implant into the hole of the pre-manufactured transducer. Make sure the inner wall of the 3D-printed ring is smooth, and then place it around the transducer positioned on the mouse skull. Ensure that the transducer is centered within the ring.
- Apply dental cement to the junction between the ring and the skull, then wait a few minutes for dental cement to solidify. Avoid placing the dental cement on the connection between the transducer and the skull.
- Carefully remove the transducer and securely tighten the screws.Transfer the mouse to a warm cage and ensure it is monitored until fully recovered before returning it to its original cage.
- After surgery, administer subcutaneous Carprofen (2 mg/kg) for analgesia and continue every 24 h for 3 days to manage inflammation and pain. Monitor the animals daily for any signs of distress, abnormal weight loss, pain, or infection. Normally, by the 3rd day post-surgery, all mice should be exhibiting normal behavior. If any signs of distress or illness are observed in a mouse after the 3rd day, follow institutional guidelines for euthanasia.
5. Stimulation and signal recording
- At 7 days after surgery, turn on the oxygen supply to the gas anesthesia machine and adjust the oxygen flow regulator to set the gas flow to 300-500 mL/min.
- Place the mouse in the induction chamber and close the anesthetic gas delivery to the mask. Rotate the vaporizer dial to adjust the appropriate anesthetic concentration (2%- 2.5%).
- After the mouse is anesthetized, place it on the stereotaxic frame with an anesthetic mask. Close the induction line to let the anesthetic gas flow into the anesthetic mask. Adjust the appropriate maintenance anesthetic concentration (1%-1.5%).
- Clean the top surface of the implant with alcohol, and then insert the fiber optic patch cord into the center of the prepared transducer.
- Inject water into the space between the implant and the 3D-printed ring using a 26G needle and 1 mL disposable syringe to moisten the skull. Use paper towels to absorb excess water.
- Inject a coupling agent into the space between the implant and the 3D-printed ring using a 26G needle and 1 mL disposable syringe to facilitate the easy propagation of ultrasound from the transducer to the brain.
- Connect the implant to the fiber optic patch cord. Carefully insert the transducer into the area which is filled with a coupling agent and securely tighten the screws.
- Place the mouse in an open field and allow it to wake up. Attach the transducer to the ultrasonic excitation system and connect the fiber optic patch cord to the optical fiber recording system, enabling freedom of movement for the mouse.
NOTE: The fiber optic patch cord has a length of 2m and a diameter of 1.25 mm. The light intensity for the 405 channel is 20 µW, and for the 470 channel is 40 µW. - Activate both the ultrasonic excitation device and the optical fiber recording system in order to synchronize ultrasonic neural modulation with optical fiber signal recording.
Representative Results
The acoustic pressure distribution in the free acoustic field on the XY plane and XZ plane located 3.4 mm away from the transducer surface, corresponding to the position of the mouse's anterior thalamic nucleus, is shown in Figure 2B,C. These measurements were acquired through hydrophone scanning in the XY domain and XZ domain. The acoustic pressure distribution in the transcranial acoustic field on the XY plane and XZ plane located 3.4 mm away from the transducer surface is shown in Figure 2D,E. The free acoustic pressure measured is 730 kPa, and the transcranial acoustic pressure measured is 580 kPa for 500 kHz center frequency. The thickness of the skull measured is about 0.2 mm, on average. We assume that the dispersion relationship is approximately linear, so the skull has an attenuation coefficient of 19.98 dB/cmMHz. The lightweight transducer, weighing around 1.66 g, allows the mouse to move easily, facilitating the observation of the response behavior of the mouse under FUN and the motion trail.
The optical fiber signals were recorded under FUN (Figure 4B,D), with the envelope being square and sinusoidal, respectively. Five male mice were used in the experiment. The square lasted for 300 ms, while the continuous sinusoidal lasted for 471 ms, which can ensure the total energy is the same in two different FUNs (Figure 4A,C). An enhancement in optical fiber signal indicates an increase in neural activity. The neural response is rapid under the FUN, suggesting that the transducer has sufficient energy and excellent focusing capabilities.
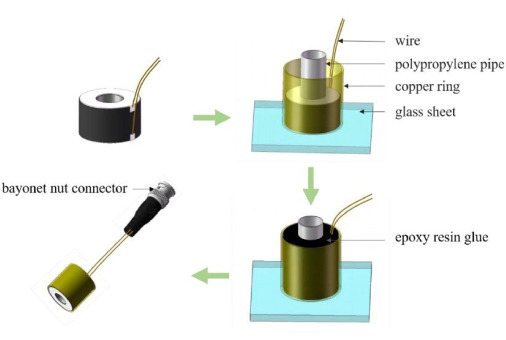
Figure 1: Production process of the transducer. This involves, in turn, connecting a piezoelectric sheet to a wire and then packaging it. Please click here to view a larger version of this figure.

Figure 2: Ultrasound field measurement setup and characterization for ultrasonic transducer. (A) The setup for ultrasound field measurement includes a hydrophone, motor system, control software, signal generator, and oscilloscope. (B, D) Schematic diagram of ultrasonic transducer measurements in free and transcranial acoustic fields and the results of transverse and longitudinal sound field measurements. (C, E) Diagram of the transverse sound field at the transducer focal position, with the red line indicating the sound field at the -3 dB position. (F, G) Waveform diagram of the output measured by the hydrophone for the transducer. The area within the red dashed box and the area within the blue dashed box represent the periods before the waveform reaches a stable amplitude and the ringing period of the transducer at the end, respectively. The area within the orange dashed box represents the stable part of the waveform, which is used for calculating the pressure amplitude, noted as p. Please click here to view a larger version of this figure.
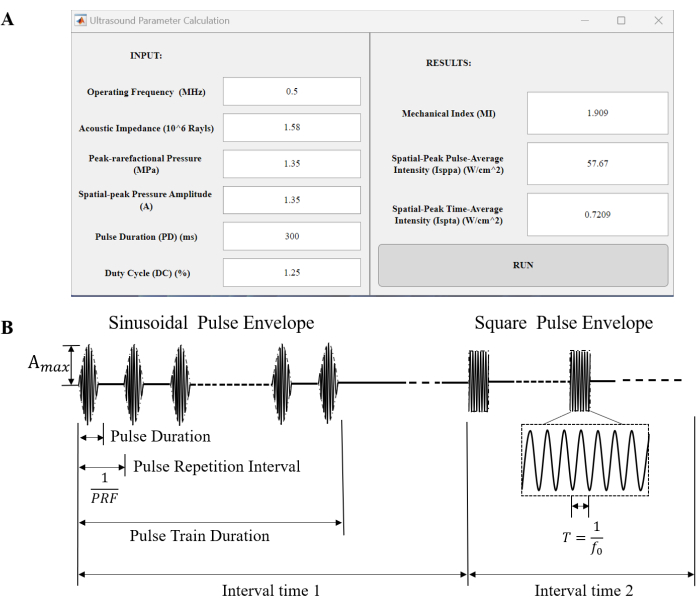
Figure 3: Calculation software and ultrasound parameter. (A) A homemade ultrasound parameter calculation interface. MI, Isppa, and Ispta were calculated. The interface could be obtained from https://github.com/HQArrayLab/Ultrasound_Parameter_Caculation. (B) Schematics of ultrasound pressure waveforms. A sinusoidal Pulse Envelope and a rectangular Pulse Envelope are used. The period (T) represents the duration of a single cycle of the operating frequency. A pulse, known as a single continuous sonication, lasts for a specified duration called the pulse duration (PD). Typically, pulses are repeated in a sequence known as a pulse train. The time interval between two consecutive pulses in a pulse train is referred to as the pulse repetition interval (PRI), calculated as the reciprocal of the pulse repetition frequency (PRF). The entire sequence of pulses, known as the pulse train, has a specific duration known as the pulse train duration. The interval time means the duration of a single trial. Please click here to view a larger version of this figure.
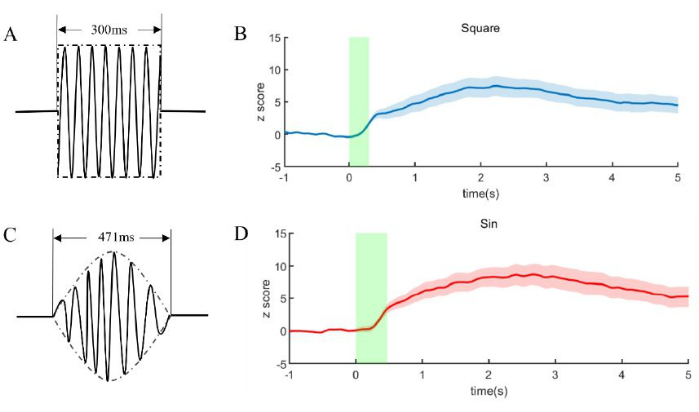
Figure 4: Signal of fiber photometry during FUN. (A, C) Ultrasound parameters enveloped by square (B) and sinusoidal (D). (B, D) The fiber photometry signal respectively during FUN of (A) and (C). The green shadow is the duration of FUN. The solid line is the mean, and the shades of blue and red are the mean and standard deviation of recorded signals. Five male mice were used in the experiment. Please click here to view a larger version of this figure.
Discussion
This approach combines FUN with optical photometry recording, enabling the investigation of mouse brain function and in vivo FUN mechanism. The complete operational process, from transducer fabrication to surgical procedures, is outlined, allowing researchers to independently perform FUN from outside the field.
One crucial aspect of the protocol is ensuring that the optical implant is smoothly inserted into the transducer, the dental cement across the skull is thin enough for ultrasound penetration into the brain, the optical implant is securely connected to the skull to prevent dislodging during the experiment, and the energy output of the transducer is sufficient for effective neuromodulation. The thickness of dental cement surrounding the implant should be equal to or less than the diameter of the transducer hole. Therefore, it is advisable to use the same polypropylene pipe for both the transducer fabrication process and surgery. Since polypropylene pipe does not adhere to dental cement, it is selected to mold the dental cement around the implant, with a side cut, to facilitate easy removal of the polypropylene pipe.
Electrophysiological recording and optical photometry recording are commonly utilized technologies for monitoring brain activity in vivo, offering high temporal-spatial resolution. However, electrophysiological recording captures the firing activity signal from neurons attached to the electrodes directly. The ultrasound waves could directly vibrate the electrodes, inducing unnecessary confounding effects. Fortunately, the fiber photometry technology, which is less invasive, captures the activity of neurons beneath it, which could reduce the confounding effect of ultrasound vibration on the implant7,19,26. As a result, the technology of simultaneous focused ultrasound neuromodulation and fiber photometry recording in free-moving mice allows for the study of in vivo mechanisms of ultrasonic neuromodulation and enables the observation of the mice's behavioral responses without the interference of anesthesia.
However, fiber photometry's spatial resolution is restricted as it is unable to monitor the activity of subcellular and microcircuits24. Moreover, it provides an indirect representation of neuronal activity since it does not directly record the electrical signals produced by neuronal activity.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work is supported in part by the National Natural Science Foundation of China (32371151), Guangdong High Level Innovation Research Institute(2021B0909050004), the Hong Kong Research Grants Council Collaborative Research Fund (C5053-22GF), General Research Fund (15224323 and 15104520), Hong Kong Innovation Technology Fund (MHP/014/19), internal funding from the Hong Kong Polytechnic University (G-SACD and 1-CDJM), and the Natural Science Foundation of Liaoning Province- Joint Open Fund of State Key Laboratory of Robotics (2022-KF-22-03). The authors would like to thank the facility and technical support from the University Research Facility in Life Sciences (ULS) and University Research Facility in Behavioral and Systems Neuroscience (UBSN) of The Hong Kong Polytechnic University.
Materials
| 1ml disposable syringe | DOUBLE-DOVE | 1ml | Injection needles |
| 26-gauge needle | Jin mao | JM-J02 | Preparation needles |
| 70% ethanol | Dong de alcohol | 0.7 | Disinfect |
| alcohol | Dong de alcohol | 0.75 | Clean the transducer surface |
| Bayonet Nut Connector | Risym | 75-5 | The other end of the connecting wire is connected to the ultrasonic excitation device |
| copper ring | Guowei Metal Materials | Outer diameter, wall thickness, height (8mm, 0.2mm, 8mm) | The outer protective case of the transducer |
| disposable syringe | DOUBLE-DOVE | 1ml | The inhalation of epoxy resin allows precise small amounts to be injected into the copper pipe |
| double-sided tape | 3M | 3M55236 | It is used to fix the transducer and the wire to ensure that the epoxy silver glue does not move before drying |
| electronic soldering iron | Victor | 868A+ | The soldered wires are connected to the BNC |
| epoxy resin glue | Kraft | K 9741 | Seal the rear of the transducer |
| epoxy silver paste | Vonroll | CB-052 | The wire is attached to the positive and negative poles of the piezoelectric ceramic sheet and the resistance is kept low |
| fader | JOQO | YP-7021 | Remove the head hair of the mouse |
| gas anesthesia machine | RWD | R500 | It is used for anesthesia in mice |
| glass sheet | Square glass | 80mm*80mm | A temporary operating surface for placing piezoelectric ceramics and wires can be used to coat the surface of the glass plate with double-sided tape |
| ketamine/xylazine | Shutai/shengxin | Zoletil 50/2ml*10 | Anesthetize the mouse |
| medical coupling agent | Bestman | 120g | The couplant acts as a medium to conduct the ultrasound signal |
| mouse | Bai shi tong | GCaMp6 | Test subject |
| ophthalmic ointment | Yun Zhi | 0.5% x 2.5 g x1 | Moistens the eye area to prevent blindness |
| piezoelectric plate | Jiaming Electronics Factory | Diameter, pore, thickness (7mm, 3mm, 3.56mm) | The electrical energy is emitted in the form of ultrasound |
| polypropylene pipe | Baihao Pipe Factory | Outer diameter, inner diameter, length (3mm, 2mm, 500mm) | Prevent the epoxy resin from plugging the holes and leaving the holes |
| povidone-iodine | lefeke | 500ml | Disinfect |
| signal record of fiber | Thinker Tech Nanjing Biotech | Three-color single-channel fiber optic recording system | Record fiber photometry signals |
| stereotaxic frame | RWD | 68805 | Fix the head of the mouse and localize the brain region |
| sterile saline | Shijiazhuang si yao | 500ML,4.5g | As a solvent, dissolves the drug |
| stimulation of ultrasound | Deep Brain Technology | DB-USNM | Provides stable input to the transducer |
| weighing machine | Qin bo shi | 1718 | Weigh the mouse |
| wire | Jinpeng Cable Factory | 0.3mm2 | Voltage is supplied to the transducer |
Referências
- Wang, J. B., et al. Focused ultrasound for noninvasive, focal pharmacologic neurointervention. Front Neurosci. 14, 514541 (2020).
- Bystritsky, A., et al. A review of low-intensity focused ultrasound pulsation. Brain stimulation. 4 (3), 125-136 (2011).
- Di Ianni, T., et al. High-throughput ultrasound neuromodulation in awake and freely behaving rats. Brain stimulation. 16 (6), 1743-1752 (2023).
- Legon, W., et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 17 (2), 322-329 (2014).
- Badran, B. W., et al. Sonication of the anterior thalamus with mri-guided transcranial focused ultrasound (tfus) alters pain thresholds in healthy adults: A double-blind, sham-controlled study. Brain stimulation. 13 (6), 1805-1812 (2020).
- Yaakub, S. N., et al. Transcranial focused ultrasound-mediated neurochemical and functional connectivity changes in deep cortical regions in humans. Nat Comm. 14 (1), 5318 (2023).
- Murphy, K. R., et al. A tool for monitoring cell type-specific focused ultrasound neuromodulation and control of chronic epilepsy. Proc Natl Acad Sci. 119 (46), e2206828119 (2022).
- Niu, X., Yu, K., He, B. Transcranial focused ultrasound induces sustained synaptic plasticity in rat hippocampus. Brain Stimulation. 15 (2), 352-359 (2022).
- Tufail, Y., et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 66 (5), 681-694 (2010).
- Yang, Y., et al. Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound. Nat Metabol. 5 (5), 789-803 (2023).
- Kubanek, J., et al. Remote, brain region-specific control of choice behavior with ultrasonic waves. Sci Adv. 6 (21), eaaz4193 (2020).
- Deffieux, T., et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr Biol. 23 (23), 2430-2433 (2013).
- Gaur, P., et al. Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep. Brain stimulation. 13 (3), 804-814 (2020).
- Fouragnan, E. F., et al. The macaque anterior cingulate cortex translates counterfactual choice value into actual behavioral change. Nat Neurosci. 22 (5), 797-808 (2019).
- Folloni, D. Ultrasound neuromodulation of the deep brain. Science. 377 (6606), 589-589 (2022).
- Verhagen, L., et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife. 8, e40541 (2019).
- Yang, P. -. F., et al. Neuromodulation of sensory networks in monkey brain by focused ultrasound with mri guidance and detection. Sci Rep. 8 (1), 7993 (2018).
- Yu, K., Niu, X., Krook-Magnuson, E., He, B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Comm. 12 (1), 2519 (2021).
- Zhu, J., et al. The mechanosensitive ion channel piezo1 contributes to ultrasound neuromodulation. Proc Natl Acad Sci. 120 (18), e2300291120 (2023).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Shen, W., et al. M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of l-dopa-induced dyskinesia. Neuron. 88 (4), 762-773 (2015).
- Dana, H., et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Meth. 16 (7), 649-657 (2019).
- Inoue, M., et al. Rational engineering of xcamps, a multicolor geci suite for in vivo imaging of complex brain circuit dynamics. Cell. 177 (5), 1346-1360.e24 (2019).
- Legaria, A. A., et al. Fiber photometry in striatum reflects primarily nonsomatic changes in calcium. Nat Neurosci. 25 (9), 1124-1128 (2022).
- Kamimura, H. A., et al. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 mhz. Med Phys. 43 (10), 5730-5735 (2016).
- Xian, Q., et al. Modulation of deep neural circuits with sonogenetics. Proc Natl Acad Sci. 120 (22), e2220575120 (2023).
- Mohammadjavadi, M., et al. Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain stimulation. 12 (4), 901-910 (2019).
- Harris, G. R., et al. Hydrophone measurements for biomedical ultrasound applications: A review. IEEE Trans Ultrasonics Ferroelect Freq Cont. 70 (2), 85-100 (2022).
Tags

.
