Induction of Ocular Surface Inflammation and Collection of Involved Tissues
Summary
Ocular surface inflammation harms the ocular surface tissues and compromises vital functions of the eye. The present protocol describes a method to induce ocular inflammation and collect compromised tissues in a mouse model of Meibomian gland dysfunction (MGD).
Abstract
Ocular surface diseases include a range of disorders that disturb the functions and structures of the cornea, conjunctiva, and the associated ocular surface gland network. Meibomian glands (MG) secrete lipids that create a covering layer that prevents the evaporation of the aqueous part of the tear film. Neutrophils and extracellular DNA traps populate MG and the ocular surface in a mouse model of allergic eye disease. Aggregated neutrophil extracellular traps (aggNETs) formulate a mesh-like matrix composed of extracellular chromatin that occludes MG outlets and conditions MG dysfunction. Here, a method for inducing ocular surface inflammation and MG dysfunction is presented. The procedures for collecting organs related to the ocular surface, such as the cornea, conjunctiva, and eyelids, are described in detail. Using established techniques for processing each organ, the major morphological and histopathological features of MG dysfunction are also shown. Ocular exudates offer the opportunity to assess the inflammatory state of the ocular surface. These procedures enable the investigation of topical and systemic anti-inflammatory interventions at the preclinical level.
Introduction
Every blink of an eye replenishes the smooth tear film dispersed over the cornea. The ocular surface epithelia facilitate the distribution and correct orientation of the tear film on the ocular surface. Mucins are provided by the cornea and conjunctiva epithelial cells to help position the aqueous part of the tear film coming from the lacrimal glands on the eyes' surface. Finally, MG secretes lipids that create a covering layer that prevents the evaporation of the aqueous part of the tear film1,2,3. In this fashion, the coordinated functions of all the ocular organs protect the ocular surface from invading pathogens or injury and support crystal clear vision without any pain or discomfort.
In a healthy ocular surface, the ocular flowing discharge or eye rheum sweeps away dust, dead epithelial cells, bacteria, mucus, and immune cells. Aggregated neutrophil extracellular traps (aggNETs) formulate a mesh-like matrix composed of extracellular chromatin and incorporate these components in the eye rheum. AggNETs resolve inflammation by the proteolytic degradation of pro-inflammatory cytokines and chemokines4. However, when they become dysfunctional, these aberrant aggNETs drive the pathogenesis of diseases such as vascular occlusions in COVID-195, gallstones6, and sialolithiasis7. Similarly, aggNETs on the ocular surface play a protective role and contribute to resolving inflammation of the highly exposed surface8. Either an exaggerated formation or lack of aggNETs in the ocular surface can impair the tear film stability and/or cause corneal wounds, cicatrizing conjunctivitis, and dry eye disease. For example, the obstruction of MG is a leading cause of dry eye disease9. AggNETs are also known to plug the flow of lipid secretion from the ducts of MG and cause Meibomian gland dysfunction (MGD). The congestion of MG orifices by aggNETs causes a lack of fatty fluid enveloping the ocular surface and retrograde bottled-up fluid, resulting in dysfunction of the gland function and acinar damage. This dysfunction can result in tear film evaporation, fibrosis of the margins on the eyelids, eye inflammation, and detrimental damage to the MG10,11.
Several animal models have been developed over the years to imitate the pathological process of MGD in humans. For example, C57BL/6 mice aged 1 year have helped study age-related effects on dry eye disease (DED) and MGD, reflecting the ocular disease pathology in patients aged 50 years and older12,13,14. Furthermore, rabbits are appropriate models for investigating the effects of pharmacological interventions. Therefore, inducing MGD in rabbits has been reported by either the topical administration of epinephrine or the systemic introduction of 13-cis-retinoic acid (isotretinoin)15,16,17,18,19.
Although these animal models were adequate for determining the different factors contributing to the pathophysiology of MGD, they were restricted in their utilization. For instance, the murine model of age-related MGD was ideal for deciphering elements in older adults only, and hence, rabbits appeared to be the most suitable animal model to study ocular surface diseases, as they enable the investigation of multiple pathophysiological mechanisms. However, due to the lack of comprehensive analytical tools to detect proteins at the ocular surface and because many parts of the rabbit genome are unannotated, they are limited for investigations20,21.
In addition, these animal models used to investigate the pathogenesis of dry eye disease did not provide adequate details to analyze the immunological arm of the disorder that instigates the inflammation of the ocular surface. Accordingly, the murine model of MGD developed by Reyes et al. showed an association between allergic eye disease in mice and MGD in humans and highlighted the immune etiology responsible for obstructive MGD21. This model associates allergic eye disease with a TH17 response that recruits neutrophils to the conjunctiva and eyelid, causing MGD and chronic ocular inflammation21. The induction of MGD and ocular inflammation in this murine model is a valuable tool for investigating upstream events during the development of local inflammation driven by an ongoing immune response21. The current protocol describes the ocular surface inflammation accompanied by obstructive MGD. In this method, mice are immunized and, after 2 weeks, challenged on the ocular surface with the immunogen for 7 days. Furthermore, the steps to isolate ocular exudate and the associated ocular organs during acute inflammation and the dissection of the cornea, conjunctiva, and eyelids are described.
Protocol
All procedures involving animals were conducted according to the institutional guidelines on animal welfare and approved by the animal welfare commission of the Friedrich-Alexander-University Erlangen-Nuremberg (FAU) (permit number: 55.2.2-2532-2-1217). Female C57Bl/6 mice, aged 7-9 weeks were used for the present study. The mice were obtained from commercial sources (see Table of Materials) and kept in specific pathogen-free conditions with 12 h day/night cycles.
1. Induction of murine ocular surface inflammation
- Perform immunogen preparation for immunization.
- Prepare the immunogen freshly on the day of immunization, mixing ovalbumin (OVA, 50 mg/mL) and pertussis toxin (100 µg/mL) in saline and the adjuvant aluminum hydroxide (40 mg/mL) (see Table of Materials) in a 1:1 proportion.
CAUTION: Perform the opening, reconstitution, and immunogen preparation of pertussis toxin in a laminar safety cabinet. Wear protective clothing and avoid any contact with the skin. - Incubate the immunogen and adjuvant mixture at room temperature for 30 min and load 100 µL into a 1 mL syringe.
- Perform intraperitoneal injection of the immunogen solution in a non-anesthetized mouse.
- Hold the mouse softly by the tail while grasping the cage grid. Hold firmly the skin of the back and the neck region between the thumb and index finger and fix the tail and lower limbs between the ring and little finger against the palm of the hand.
- Keep the fixed mouse with its head downward.
- Inject 100 µL of the prepared immunogen solution in the right or left quadrant of the lower abdominal cavity.
- Prepare the immunogen freshly on the day of immunization, mixing ovalbumin (OVA, 50 mg/mL) and pertussis toxin (100 µg/mL) in saline and the adjuvant aluminum hydroxide (40 mg/mL) (see Table of Materials) in a 1:1 proportion.
- Perform ocular surface challenge 2 weeks after immunization.
- Anesthetize the mouse with isoflurane (2.5%).
- Apply 5 µL of OVA (50 mg/mL) or saline (0.9 % NaCl) per eye on both eyes and wait until the drop gets absorbed by the eye. This takes ~5 min.
- Repeat the procedure 1x daily for 7 days.
NOTE: The topical administration of medications can be done in the same way.
2. Collection of ocular exudates
- Recover the ocular exudates formed during the challenge phase by applying 50 µL of sterile saline to the eye immediately after the challenge.
- To obtain a single-cell suspension, treat the collected ocular discharge with recombinant MNase (2 x 106 gel U/mL, see Table of Materials) containing the cofactor calcium (5 mM) at 37 °C for 20 min.
- Centrifuge at 400 x g for 7 min at room temperature.
- Isolate the supernatant and measure the cytokines and chemokines using multiplexed ELISA according to the manufacturer's instructions (see Table of Materials).
NOTE: The obtained supernatant can be used for protein analysis and the pellet for functional assays such as immunophenotyping, phagocytosis, degranulation, and gene expression.
3. Excision of ocular surface tissues
- Dissect the eyelids and eye globe following the steps below.
- Euthanize the mouse by CO2 asphyxiation and cervical dislocation.
- Place the mouse on an even surface.
- Disinfect the orbital area around the eye with a swab impregnated with 70% ethanol.
- Make an incision between the ear and the retro-orbital sinus and along the surface above the squamous bone vertically, extending the incision horizontally below the lower eyelid along the maxillary bone and above the upper eyelid along the frontal bone. This forms an incision around the eye (Figure 1).
- Carefully hold the dissected tissue around the eye using curved forceps and pull the tissue and eyeball out.
- Place the excised organs in sterile PBS.
- Trim excess facial muscle tissue around the excised upper eyelids using a scalpel and under the stereomicroscope.
- Collect the conjunctiva following the steps below.
- Place the upper eyelid on a dry Petri dish under the stereomicroscope.
- Using fine tweezers and a scalpel, peel the whitish-mucous layer very gently out of the inner surface of the eyelid.
- Next, dissect the cornea following the steps below.
- Place the eye globe on a new dry Petri dish on top of dry ice for 3 min.
- Take the Petri dish onto the bench surface in a stable position.
- Make a small incision at the border of the cornea next to the limbus using fine sharp scissors.
- Prolong the incision with the scalpel around the eye globe, separating the sclera from the cornea.
- Remove remnants of the iris and the lens from the backside of the cornea by generously flushing with saline solution.
4. Documentation of Meibomian gland (MG) obstruction
- To assess the MG and its orifices, place excised eyelids in an upright position under the stereomicroscope (Figure 2).
- Capture images with white light epi-illumination according to the camera's exposure time and ISO settings.
NOTE: Morphometric analysis of these images provides reliable quantification of the plug size at the outlet of the glands. The quantification can be performed by outlining with the wand tool the eye plugs and executing the "Analyze Particles" command of the Image J software (see Table of Materials).
5. Transillumination of eyelids (Meibomian gland morphology)
- To assess the MG area, place the excised eyelids in a horizontal position and turn on the backlight of the stereomicroscope equipped with an infrared camera (Figure 3).
- Capture images by adjusting the exposure time according to the camera's (see Table of Materials) ISO.
NOTE: Infrared imaging of these transilluminated eyelids under the stereomicroscope can help measure the shape and size of each acini. Morphometric analysis of these images provides reliable quantification of the size and number of MG. The quantification can be performed by outlining the MG with the wand tool and executing the "Analyze Particles" command of the Image J software.
Representative Results
The present protocol describes the sequential steps for establishing a murine model of ocular surface inflammation. The protocols aim to show how to apply therapeutics locally, obtain ocular exudates, and excise associated accessory organs such as healthy and inflamed eyelids (Figure 2), the cornea, and the conjunctiva. Attention must be paid when the upper eyelids are dissected for the isolation of the conjunctiva, and it must be stored in 1x PBS during the dissection of the cornea. This will prevent the drying of the conjunctiva, which can be used for histological, pharmacokinetic, and gene expression studies.
OVA and saline were applied topically for 7 consecutive days following the abovementioned protocol. Mice challenged topically with saline showed a healthy ocular surface with wide-open eyes and a regular blinking pattern. However, ocular inflammation was instigated in immunized C57BL/6J mice challenged with OVA. The instillation of OVA solution on the ocular surface caused itching, and not pain, for the first 2 hours after instillation. Exudate and eyelid eczema was observed only during the last 3 days of the challenge phase. No pain was evident, judging the behavior of the animals. Therefore, a moderate level of stress for the mice over a short period of time was assumed. The daily OVA challenge triggered clinical manifestations like abundant ocular discharge, chemosis, and narrow opening of the eyes. In addition, the upper and lower eyelids frequently adhered to each other, impairing the essential function of blinking (Figure 2). Strict interruption criteria (Supplementary File) were followed for this model and were approved by the local ethical board for animal experimentation. The immediate interruption was carried out if one mouse reached 15 points at any given time.
Supplementary File. Please click here to download this File.
After euthanasia, the ocular organs were excised and were observed at higher magnification. The excised eyelids under a microscope showed large occlusions plugging the orifices of the MG and edema, in stark contrast to the healthy eyelids showing small plugs of the gland lining the eyelid (Figure 3).
Further examination of the gland's infrared transillumination reveals the racemic appearance of the acini of the MG. This allows for quantifying the visible Meibomian glands (indicated in red). The eyelids from saline-challenged mice showed the round acini forming the MG. In comparison, the application of OVA induced the destruction and loss of some MG in mice with allergic eye disease (AED, Figure 4). The histological analysis of the eyelids displayed dilated MG compared to naïve mice administered with only saline for 7 days (Figure 5), allowing the accumulation of neutrophils producing aggNETs that finally obstruct the orifices of the gland.
Separating the cornea from the eye's sclera can be cumbersome due to the slippery mucous surface of the eye globe. Incubating the eyeball on dry ice in a Petri dish for 3 min allows for fixing the eye, making a small incision at the limbus, and dissecting the cornea (Figure 6).
The steps enlisted in the protocol section facilitated the collection of the cornea and conjunctiva. In addition, the local administration of OVA inflicted severe inflammation to the conjunctiva, with a hyperaemic appearance (Figure 7).
The analysis of ocular exudates reveals molecular mechanisms in the development of MGD. Cytokine and chemokine quantification as described showed elevated levels of the major chemokine facilitating neutrophil extravasation, phagocytosis, and degranulation (CXCL-1) in the supernatants from mice with AED8. In addition, the prime mediator of the acute phase response and neutrophil production, IL-6, was also significantly elevated in mice with AED. On the other hand, the concentration of IL-10, an anti-inflammatory cytokine, showed no significant changes in both naïve and AED mice (Figure 8).

Figure 1: Excision of the facial tissue surrounding the orbital region. The incision trail is indicated in red. This allows for excising the ocular organs and investigating the influence and effects of various topically administered therapeutics on accessory ocular organs such as the conjunctiva and cornea. Please click here to view a larger version of this figure.
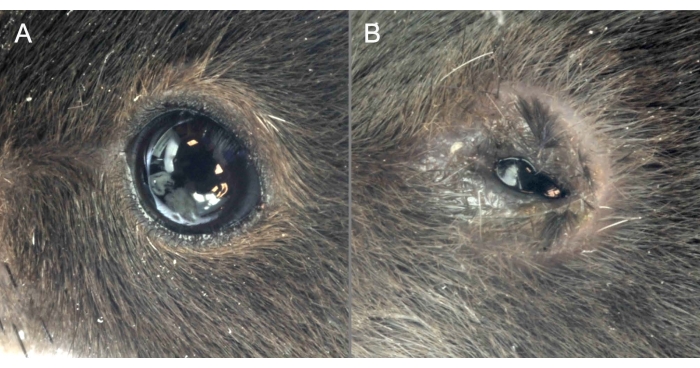
Figure 2: OVA administration causes clinical manifestations of MGD. (A) Mice administered saline show a healthy ocular surface with eyes wide open. (B) OVA application induces severe ocular surface inflammation, the narrow opening of the eyes, and signs of chemosis. Please click here to view a larger version of this figure.
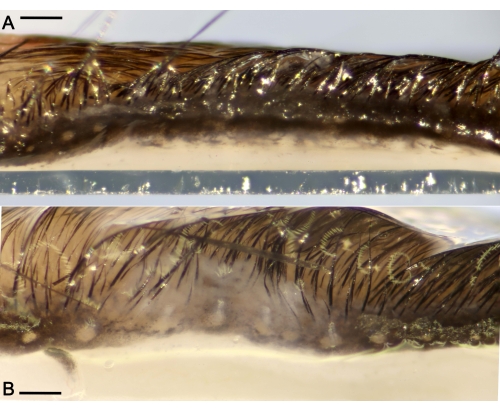
Figure 3: Ductal plugs obstructing the Meibomian glands (MG) located along the eyelids. (A) Representative macro photograph of a healthy eyelid without excessive ocular discharge from a mouse administered saline for 7 days only. (B) Eyelid showing large ductal occlusions of the MG and edema after the challenge period. Scale bar = 300 µm. Please click here to view a larger version of this figure.
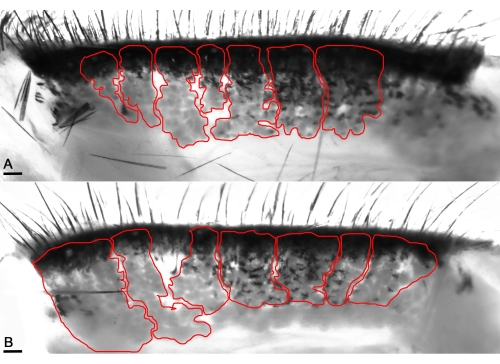
Figure 4: Transilluminating macrophotography enables the visualization of the MG's acini (red lines). (A) Enhancing infrared levels visualizes the healthy acini of naïve mice and unveils the distinct pocket-shaped acini in the healthy eyelids. (B) The acini appear thick in the mice's affected eyelids with OVA application. Scale bar = 100 µm. Please click here to view a larger version of this figure.
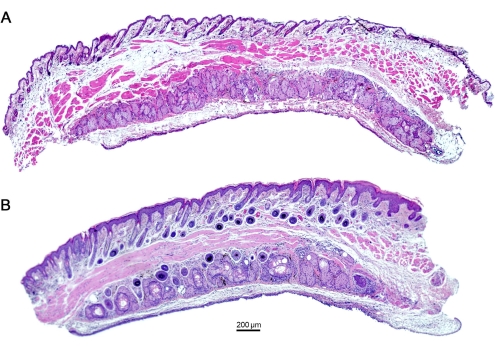
Figure 5: Histological analysis of the eyelids shows dilated ducts of MG in mice with repeated OVA insult for 7 days. (A) Naïve mouse eyelids show the absence of any dilated ducts, depicting a healthy functioning ocular organ (B) in contrast to mice with OVA challenge. Scale bar = 200 µm. Please click here to view a larger version of this figure.
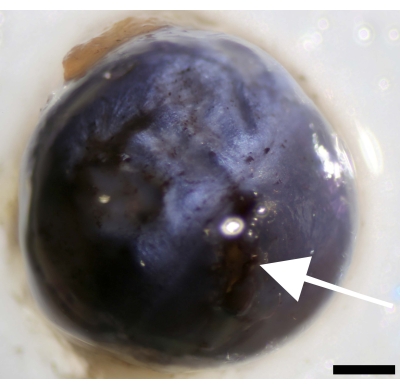
Figure 6: Incision at the cornea next to the limbus and separation of the cornea from the sclera. Image of the eyeball showing the point of incision (indicated by white arrow). Scale bar = 500 µm. Please click here to view a larger version of this figure.
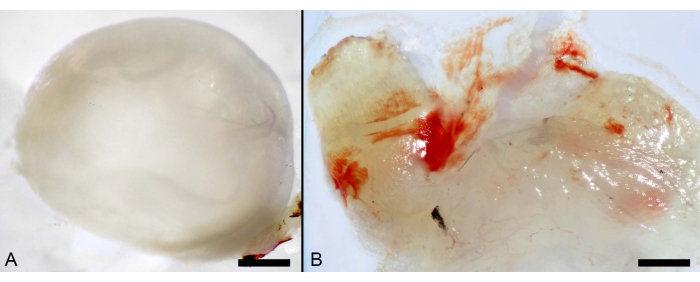
Figure 7: Local administration of OVA. (A) Macro photographs of murine ocular organs showing the cornea. Scale bar: 600 µm. (B) Inflamed conjunctiva from mice challenged with OVA. Scale bar: 100 µm. Please click here to view a larger version of this figure.
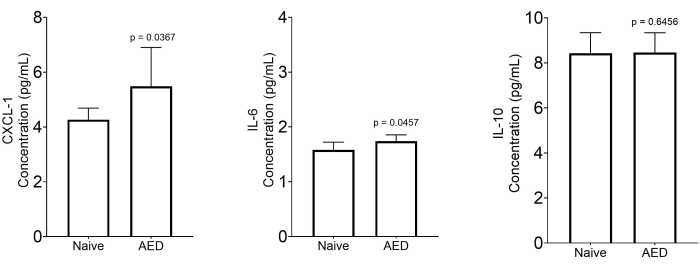
Figure 8: Measurement of inflammatory markers in the collected ocular exudates. Quantitative analysis of cytokines and chemokines in the supernatant of centrifuged ocular exudate of naïve mice (n = 7) and mice with MGD (n = 8). Data are expressed as median with a 5%-95% range. Statistical significance was calculated by using the two-tailed Mann-Whitney U-test. Please click here to view a larger version of this figure.
Discussion
The oily secretion of the Meibomian glands is of great importance for a healthy eye22. However, the obstruction of these sebaceous glands by aggregated neutrophil extracellular traps (aggNETs) that line up as parallel strands located on the tarsal plates of both eyelids can disrupt the tear film23. This disruption results in Meibomian gland dysfunction (MGD)1 and accelerated tear evaporation and conditions the damage of the ocular surface2. This protocol describes establishing immune-mediated ocular surface inflammation that leads to MG obstruction.
Studies from mouse models have confirmed the underlying immune-mediated mechanisms causing MG obstruction associated with ocular surface inflammation. A robust TH17 response recruiting neutrophils in the mouse model of AED and an increase in neutrophils and other myeloid cells in the tear fluid of patients with MGD indicates the role of neutrophils obstructing the MG21. The presence of aggNETs occluding the MG orifices was confirmed with peptidyl arginine deiminase 4 in mice with AED. In this model, neutrophils showed a diminished capacity to aggregate and formed aggNETs that obstructed the ducts and orifices of the MG glands10. Moreover, tear analysis from patients with MGD showed an increase in anaphylatoxin C5a and chemokine IL-8, demonstrating increased activity of the complement system and a high influx of neutrophil infiltration. Finally, an increase in the level of cytokines associated with several neutrophil functions, such as IL-6, IL-18, MCP-1/CCL-2, and MIG/CXCL9, underscores the prime position of neutrophils in the pathogenesis of MGD11.
The occurrence of MGD is a significant contributor to the development of DED, and examining the role of the numerous elements involved in the pathogenesis of ocular manifestations is complicated24. Therefore, the selection of animals as models is heavily influenced by the question under investigation. For instance, rabbits serve as conventional models for pharmaceutical testing of formulations; feline, porcine, and canine models are suitable for investigating pathological characteristics similar to human patients, and mice are suitable primarily for genetic manipulation25.
Animal models are valuable tools for investigating disease pathogenesis and examining the therapeutic efficacy of various interventions in vivo. For example, conventional treatments include the administration of eye drop instillations to treat clinical ocular manifestations; however, due to tear flow and the lacrimal drainage system, such ocular interventions are swiftly removed from the ocular surface. This causes only temporary action and requires repeated administrations and high doses. To solve this issue, the rabbit model of dry eye disease (DED) served as the ideal medium to examine thermogels and carbonized nanogels (CNGs) as alternative topical treatments. Thermogels obtained by conjugating different degrees of sulfation of hyaluronic acid and an amine-terminated poly (N-isopropyl acrylamide), when administered to the eye, transform into a gel. In the rabbit model of DED, this gel was retained for a longer time, and a single drop repaired the damaged corneal epithelia, halted apoptosis, and repressed ocular inflammation during a follow-up of 7 days, suggesting an interruption in the leukocyte infiltration due to the inhibition of selectin-mediated leukocyte interaction26.
Furthermore, in the rabbit model of DED, carbonized nanogels (CNGs) as eye drops showed free radical scavenging treating tear deficiency and excessive tear evaporation caused by an exacerbated inflammatory response and oxidative stress. CNGs were obtained via the pyrolysis of lysine hydrochloride (Lys-CNGs) and administered, and a single dose mitigated the DED symptoms within 4 days. A similar therapeutic effect was only achievable with several treatments of a 10-fold higher amount of cyclosporine A eye drop instillations. These novel biocompatible pharmaceutical interventions exhibited superior perspectives as single-dose interventions and more extended ocular retention in preclinical animal models27.
The knockout of several genes in mouse models has unveiled the role of multiple proteins involved in the pathogenesis of MGD. For instance, the dysregulated gene expression of the peroxisome proliferator-activated receptor-gamma (PPAR γ)24 and alterations in its signaling pathway induce changes in cell cycle entry/proliferation, lipid synthesis, and Meibomian gland atrophy during aging13. In addition, the absence of the βENaC (β – epithelial sodium channel) in a mouse model indicated that the MG dysfunction phenotype prevalent in females was associated with MG atrophy and orifice obstruction as in humans with pseudohypoaldosteronism 1(PHA)28. The essential role of CD147 was also elucidated in mice, and it is believed to maintain the rich lipid content of meibocytes29. Mice deficient for the enzyme superoxide dismutase 1 showed elevated oxidative lipid and DNA damage, which was linked to an increase in MG inflammation30. Finally, a single mutation in the ELOVL4 encoding the enzyme required to synthesize extremely long-chain fatty acid residues forming the meibum lipidome leads to functional changes in the anterior ocular surface31. Interestingly, MGD was also induced in mice with a special diet with incomplete lipid composition to evaluate the therapeutic efficacy of azithromycin as an ophthalmic formulation32.
While most of the studies employing mouse models have identified several genes involved in MGD, many lack an underlying immunological condition. The AED model provides a whole range of possible interventions from the induction of the immune response, the generation of IgE antibodies, and the effector phase, accompanied by several pathological changes resembling human evaporative MGD.
When investigating MGD utilizing the protocol mentioned, it is crucial to add immunogen (OVA and pertussis toxin) to alum in a 1:1 proportion. One should incubate the mix for 30 min at room temperature for good adsorption of the ovalbumin-pertussis toxin to the alum. These interactions are vital for triggering an adequate immune response. In addition, during immunization, attention must be paid while injecting, as this may result in perforation of the underlying organs of the peritoneal cavity. This may result in inadequate immunization and systemic inflammation in the mouse, with profound effects on the immune response.
Ocular exudates are large web-like extracellular chromatin structures that cage infiltrating immune cells. Therefore, MNase treatment of collected ocular exudates from this model is vital to obtaining single-cell suspensions11. In addition, these exudates are easy to collect at the ocular surface and are a source of viable neutrophils that have transmigrated from the circulation. The main limitation of this technique is the amount of exudate to be collected from the ocular surfaces. Adding 50 µL of saline to each eye allows the recovery of up to 100 µL of exudate from one mouse. This is barely enough for 1-2 flow cytometry (FACS) stainings and supernatants for biochemical studies.
Dissecting with a scalpel to later pluck the eye and surrounding tissues can often cause injury to the superficial temporal vein, inferior palpebral vein, or ocular angle vein. It is advised to create an incision in the skin with less depth around the eye as the bleeding can interfere with further histological preparations. During the dissection of one tissue, storing other tissues in PBS is essential as drying or insufficient lubrication can result in loss of structural features.
During the dissection of the cornea from the eye globe, if the incubation of the eye globe on dry ice does not enable a steady control, one can extend the time to up to 5 min so that the incision at the limbus and separation of the cornea is possible.
Potential applications
Ocular surface inflammation associated with MGD is a multifactorial disease. The development and progression of dysfunction in these lipid secreting glands can be caused by ophthalmic, systemic, hormonal, and genetic factors, chemicals, drugs, mechanical noxious agents, and immunological responses33. Several studies have reported neutrophils causing occlusion of Meibomian glands and inflammation11,21,34,35,36,37,38. The development of topical and systemic anti-inflammatory interventions that interfere with these immunological pathways can offer relief to patients suffering from ocular discomfort due to MGD. The murine model of allergic eye disease can be utilized in a preclinical setting to investigate the pharmacokinetic and pharmacodynamic properties of these agents. This disease model enables the development of appropriate strategies to tackle MGD and helps determine the proper administration of topical or systemic anti-inflammatory agents that target vital components in disease-causing pathways.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was partially supported by the German Research Foundation (DFG) 2886 PANDORA Project-No.B3; SCHA 2040/1-1; MU 4240/2-1; CRC1181(C03); TRR241(B04), H2020-FETOPEN-2018-2020 Project 861878, and by the Volkswagen-Stiftung (Grant 97744) to MH.
Materials
| 1x PBS | Gibco | ||
| Aluminium Hydroxide | Imject alum Adjuvant | 77161 | 40 mg/ mL Final Concentration: in vivo: 1 mg/ 100 µL |
| C57Bl/6 mice, aged 7–9 weeks | Charles River Laboratories | ||
| Calcium | Carl roth | CN93.1 | 1 M Final Concentration: 5 mM |
| Curved forceps | FST by Dumont SWITZERLAND | 5/45 11251-35 | |
| Fine sharp scissor | FST Stainless steel, Germany | 15001-08 | |
| Laminar safety cabinet | Herasafe | ||
| Macrophotography Camera | Canon | EOS6D | |
| Macrophotography Camera (without IR filter) | Nikon | D5300 | |
| Mnase | New England biolabs | M0247S | 2 x 106 gel U/mL |
| Multi-analyte flow assay kit (Custom mouse 13-plex panel) | Biolegend | CLPX-200421AM-UERLAN | |
| NaCl 0,9% (Saline) | B.Braun | ||
| Ovalbumin (OVA) | Endofit, Invivogen | 9006-59-1 | 10 mg/200 µL in saline |
| Pertussis toxin | ThermoFisher Scientific | PHZ1174 | 50 µg/ 500 µL in saline Final Concentration: in vivo: 100 µg/ 100 µL |
| Petridish | Greiner bio-one | 628160 | |
| Scalpel | Feather disposable scalpel | No. 21 | Final Concentration: in vivo: 300 ng/ 100 µL |
| Stereomicroscope | Zaiss | Stemi508 | |
| Syringe (corneal/iris washing) | BD Microlane | 27 G x 3/4 – Nr.20 0,4 x 19 mm | |
| Syringe (i.p immunization) | BD Microlane | 24 G1"-Nr 17, 055* 25 mm |
Referências
- Gilbard, J. P., Rossi, S. R., Heyda, K. G. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 96 (8), 1180-1186 (1989).
- Mishima, S., Maurice, D. M. The oily layer of the tear film and evaporation from the corneal surface. Experimental Eye Research. 1, 39-45 (1961).
- Gipson, I. K. The ocular surface: The challenge to enable and protect vision: The Friedenwald lecture. Investigative Ophthalmology and Visual Science. 48 (10), 4391-4398 (2007).
- Hahn, J., et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. The FASEB Journal. 33 (1), 1401-1414 (2019).
- Leppkes, M., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 58, 102925 (2020).
- Munoz, L. E., et al. Neutrophil extracellular traps initiate gallstone formation. Immunity. 51 (3), 443-450 (2019).
- Schapher, M., et al. Neutrophil extracellular traps promote the development and growth of human salivary stones. Cells. 9 (9), 2139 (2020).
- Mahajan, A., et al. Frontline science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. Journal of Leukocyte Biology. 105 (6), 1087-1098 (2019).
- DEWS Definition and Classification Subcommittee. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. The Ocular Surface. 5 (2), 75-92 (2007).
- Nichols, K. K., et al. The international workshop on meibomian gland dysfunction: Executive summary. Investigative Ophthalmology and Visual Science. 52 (4), 1922-1929 (2011).
- Mahajan, A., et al. Aggregated neutrophil extracellular traps occlude Meibomian glands during ocular surface inflammation. The Ocular Surface. 20, 1-12 (2021).
- Jester, B. E., Nien, C. J., Winkler, M., Brown, D. J., Jester, J. V. Volumetric reconstruction of the mouse meibomian gland using high-resolution nonlinear optical imaging. The Anatomical Record. 294 (2), 185-192 (2011).
- Nien, C. J., et al. Age-related changes in the meibomian gland. Experimental Eye Research. 89 (6), 1021-1027 (2009).
- Parfitt, G. J., Xie, Y., Geyfman, M., Brown, D. J., Jester, J. V. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging. 5 (11), 825-834 (2013).
- Lambert, R. W., Smith, R. E. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Investigative Ophthalmology and Visual Science. 29 (10), 1559-1564 (1988).
- Jester, J. V., Nicolaides, N., Kiss-Palvolgyi, I., Smith, R. E. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Investigative Ophthalmology and Visual Science. 30 (5), 936-945 (1989).
- Jester, J. V., et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Investigative Ophthalmology and Visual Science. 22 (5), 660-667 (1982).
- Lambert, R., Smith, R. E. Hyperkeratinization in a rabbit model of meibomian gland dysfunction. American Journal of Ophthalmology. 105 (6), 703-705 (1988).
- Knop, E., Knop, N., Millar, T., Obata, H., Sullivan, D. A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative Ophthalmology and Visual Science. 52 (4), 1938-1978 (2011).
- Huang, W., Tourmouzis, K., Perry, H., Honkanen, R. A., Rigas, B. Animal models of dry eye disease: Useful, varied and evolving (Review). Experimental and Therapeutic Medicine. 22 (6), 1394 (2021).
- Reyes, N. J., et al. Neutrophils cause obstruction of eyelid sebaceous glands in inflammatory eye disease in mice. Science Translational Medicine. 10 (451), (2018).
- Knop, E., Korb, D. R., Blackie, C. A., Knop, N. The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Developments in Ophthalmology. 45, 108-122 (2010).
- Knop, N., Knop, E. Meibomian glands. Part I: anatomy, embryology and histology of the Meibomian glands. Ophthalmologe. 106 (10), 872-883 (2009).
- Nien, C. J., et al. Effects of age and dysfunction on human meibomian glands. Archives of Ophthalmology. 129 (4), 462-469 (2011).
- Lio, C. T., Dhanda, S. K., Bose, T. Cluster analysis of dry eye disease models based on immune cell parameters – New insight into therapeutic perspective. Frontiers in Immunology. 11, 1930 (2020).
- Nguyen, D. D., Luo, L. J., Lai, J. Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Materials Today Bio. 13, 100183 (2022).
- Lin, P. H., et al. Alleviation of dry eye syndrome with one dose of antioxidant, anti-inflammatory, and mucoadhesive lysine-carbonized nanogels. Acta Biomaterialia. 141, 140-150 (2022).
- Yu, D., et al. Loss of beta epithelial sodium channel function in meibomian glands produces pseudohypoaldosteronism 1-like ocular disease in mice. American Journal of Pathology. 188 (1), 95-110 (2018).
- Mauris, J., et al. Loss of CD147 results in impaired epithelial cell differentiation and malformation of the meibomian gland. Cell Death & Disease. 6 (4), 1726 (2015).
- Ibrahim, O. M., et al. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PloS One. 9 (7), 99328 (2014).
- McMahon, A., Lu, H., Butovich, I. A. A role for ELOVL4 in the mouse meibomian gland and sebocyte cell biology. Investigative Ophthalmology and Visual Science. 55 (5), 2832-2840 (2014).
- Miyake, H., Oda, T., Katsuta, O., Seno, M., Nakamura, M. Meibomian gland dysfunction model in hairless mice fed a special diet with limited lipid content. Investigative Ophthalmology and Visual Science. 57 (7), 3268-3275 (2016).
- Schaumberg, D. A., et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investigative Ophthalmology and Visual Science. 52 (4), 1994-2005 (2011).
- Lee, S. Y., et al. Analysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patients. American Journal of Ophthalmology. 156 (2), 247-253 (2013).
- Nakae, S., et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17 (3), 375-387 (2002).
- von Vietinghoff, S., Ley, K. IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. Journal of Immunology. 183 (2), 865-873 (2009).
- Langrish, C. L., et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. Journal of Experimental Medicine. 201 (2), 233-240 (2005).
- Chen, Y., et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. Journal of Clinical Investigation. 116 (5), 1317-1326 (2006).

