Automatically Generated
Metal Oxide Heterojunction for Photocatalytic Activities
Summary
The development of a heterojunction boosts the photocatalytic activities of solution combustion synthesis, which is a time-/energy-efficient process. Advanced analytical characterization techniques were used in this protocol to evaluate the materials' characteristics, and nanocomposites demonstrated improved acid orange-8 dye degradation.
Abstract
There is a significant global demand for improvements in synthesis techniques and their optimal characteristics, especially for industrial-scale applications. Sol-gel-based solution combustion synthesis (SG-SCS) is a simple method to produce ordered porous materials. In this regard, Pearson's hard and soft acids and bases theory assists in selecting host-dopant reactivity to form a proper heterojunction.
The formation of a heterojunction also changes the essential properties of the materials, improving photocatalysis via charge transfer or synergistic activities. A calcination temperature of 500 °C is ideal for this process based on the results of the stability assessment via a differential thermogravimetry ratio analysis (DTG).
The nanoscale dimensions of the nanoparticles (NPs) and nanocomposites (NCs) generated were validated using X-ray diffraction and high-resolution transmission electron microscopy (HRTEM). Furthermore, the scanning electron microscopy micrographs and BET analyses confirmed the porosity nature of the materials. HRTEM, X-ray photoelectron spectroscopy, and energy-dispersive X-ray investigations established the materials composition. The study found that NCs degraded the acid orange 8 (AO8) color more efficiently than bare ZnO.
Introduction
Environmental protection has become a major concern with the rapid rise in companies worldwide. Consequently, nanotechnology-based nanomaterials (NMs) and their synthesis have attracted researchers' attention over bulk materials in the modern scientific world1. Several physicochemical approaches have been adapted to treat organic and inorganic contaminants2,3. In this regard, due to its simplicity and capability of dissolving toxins without creating secondary contamination, heterogeneous photocatalysis is regarded as an adaptive remediation technique4. Studies have designed a heterojunction or doping between suitable bandgap semiconductors, which helps to reduce the constituent's electron-hole recombination, surface area, and volume. This condition subsequently increased the photocatalytic degradation of dyes5,6,7. Recent works have also reported a synergistic and charger transfer improvement role through heterojunctions/hybrids8,9, and semiconductor metal oxides demonstrate unique physical and chemical properties for multifunctional applications10. As a result, TiO2 and zinc oxide NPs (ZnO NPs) have received significant attention11,12 among researchers.
Compared to single materials, the formation of a heterojunction has become one of the unique preferences for increasing the surface area and volume ratio of materials and improving a material's photocatalytic and antibacterial performance. Furthermore, the synergistic impact of binary heterojunctions improves the separation of photogenerated electron/hole pairs compared to binary heterojunctions13,14. Studies have shown that a heterojunction between Mn2O3 and ZnO NPs15 improves the stability and substrate adsorption capacity and reduces charge transfer resistance in synthesized NPs. Moreover, several studies have used host-dopant reactivity based on Pearson's hard and soft acids and bases (HSAB) theory to test heterojunction or dopant formation. It was found that hard Lewis acids (such as Mn(III)) cannot diffuse into the borderline of the Zn (II) host lattice in the presence of a hard base solvent like water16,17. They are adsorbed onto the host surface and oxidized to form a hybrid upon calcination.
Due to its potential, the present global focus for industrially scalable applications of material synthesis is on improving the approach and its critical outlooks13. Solution combustion synthesis (SCS) is a simple, time-/energy-efficient method to create regularly ordered porous materials18, which play a significant role in the ion-/mass-transport phenomenon19. SCS comprises a decent dopant-host distribution or heterojunction based on Pearson's hard and soft acids and bases (HSAB) theory. The doping/heterojunction can tune the materials' optical, magnetic, and electrical properties, subsequently boosting the application of materials through effective charge transfer and/or synergistic roles20. The architecture-directing agent (ADA)-assisted SCS can also produce ordered colloidal nanocrystal frameworks (CNFs) used for mass-/ion-transport in energy-converting devices21,22.
This study produced a poly-vinyl alcohol (PVA) surfactant and complexing agent to synthesize ZnO NPs and ZnO-based binary nanocomposite (NCs) heterojunction through an environmentally friendly SG-SCS approach. The heterojunction between the oxides, which plays a vital role in charge transfer was estimated based on the HSAB theory. Characterization techniques were utilized to understand the materials' structural, optical, and morphological properties. The material's degradation efficiency was tested on both stable and toxic AO8 dyes.
Protocol
1. Nanomaterial synthesis
- ZnO-Mn2O3 nanocomposite synthesis
- Synthesize nanocomposites using poly-vinyl alcohol as surfactant and a complexing agent-assisted SG-SCS approach. For a graphical illustration of the SG-SCS approach, see Supplementary Figure S1.
- Dissolve 1.5 g of PVA polymer in 100 mL of distilled water with continuous stirring on a magnetic stirrer for about 15 min at 115 °C23.
- Pour the salt precursor solutions, zinc nitrate hexahydrate at a concentration of 90% v/v, and manganese sulfate at a concentration of 10%v/v into the above-dissolved PVA solution with continuous stirring for about 10 min and decrease the temperature to 70 °C.
NOTE: Salt precursors were mixed simultaneously to balance the nanocomposite precursor reactivity to follow the nucleation-doping approach16,24. The temperature was reduced to 70 °C to control the nanoparticles' accelerated growth and aggregation, following the La Mar model25,26. - Age the metal hydroxide's developed sol (colloidal particles) by keeping it in a closed and dark area for 2 days. Then, dehydrate the solution by heating at 110 °C (in the air) to form a gel.
NOTE: The PVA polymer acts as an architecture, directing templates and complexing agents, which assist in the homogeneous dispersion of metal cations, initiating the combustion process and preventing aggregation/agglomeration properties. - Subject the gel to combustion in air by heating the oven to an ignition temperature of ~150-250 °C (approximate temperature checked using a simple thermometer). The ignition temperature is the minimum temperature required to start combustion. During combustion, use hoods to collect all the toxic gas by-products that affect human health.
NOTE: The combustion process was activated by forming complexes between PVA polymer and nitrate precursors, which act as fuel to facilitate the combustion process. - Calcine the combusted materials for 3 h at 500 °C in a muffle furnace, optimized using the differential thermogravimetry (DTG) analytical technique. DTG decomposes the non-burnt impurities and improves the crystallinity of the materials27.
- Bare ZnO and Mn2O3 NPs synthesis
- Synthesize bare metal oxides using the sol-gel approach. Synthesize bare ZnO and Mn2O3 without PVA using all steps mentioned earlier, steps 1.1.1.-1.1.6., except for step1.1.2. Due to the absence of the metal nitrate and PVA polymer complexes, no self-propagation process occurs during the final drying step.
2. NP characterization
- Determine the thermogravimetry ratio, specifically the thermal thermogravimetric/differential thermal (DT/DTA analysis), in a nitrogen atmosphere at a 20.0 mL/min flow rate and a 50 °C/min ramp time to study the thermal stability and degradation behavior of the NPs and NCs.
- Perform the Fourier transform-infrared spectroscopy (FTIR) using KBr pellets in the range of 400-4000 cm−1 to study the surface functional group behavior of NPs and NCs.
- Perform X-ray diffraction (XRD) to study the crystallographic structure of PVA, NPs, and NCs.
- Use the Brunauer-Emmett-Teller (BET; N2 adsorption-desorption isotherms) method to calculate the specific surface area of the samples in the relative pressure (P/Po) range of 0.05-0.35. Determine the samples' pore-size distributions using the Barrett-Joyner-Halenda (BJH) method. Lastly, measure the N2 sorption of all the NPs and NCs at −196.15 °C.
- Use scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX) and high-resolution transmission electron microscopy (HRTEM) to study the morphologies and perform compositional studies of the NPs and NCs.
- Perform an X-ray photoelectron spectroscopy (XPS) analysis on a system integrated with a Kratos patented magnetic immersion lens, charge neutralization system, and spherical mirror analyzer. Calibrate the peak energies based on the energy of external carbon.
NOTE: The researcher adopted all standard procedures and protocols during the characterization process.
3. Batch degradation studies
- Perform the photocatalytic experiment by dissolving 20 ppm of AO8 dye in 250 mL of aqueous solution (water solvent) with 0.06 g of ZnO NPs and NCs photocatalysts.
- Use the degradation experiment as a conductor in a 176.6 cm2 circular glass reactor. For this experiment, use a medium-pressure mercury vapor lamp (Hg lamp) as a light source (λmax = 365 nm, 125 W)28. Before illumination, stir the reaction suspension continuously in the dark for 30 min to create the adsorption/desorption equilibrium of AO8/CR on the NPs/NCs.
- Irradiate the samples directly by focusing light on the reaction mixture from a distance of 20 cm. Use a magnetic stirrer at 110 rpm to continuously mix the solution. Control the temperature of the overall reactor during the experiment using water circulation.
- Draw 5 mL of the dye solution every 15 min to measure their concentrations at time t by UV-vis spectrophotometer. Calculate the percentage of photocatalytic degradation efficiency using the equation:
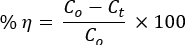
where Co and Ct are the initial and after time t irradiation concentrations, respectively, of the AO8 and CR dyes solution; and η is the photo decolorization efficiency, - Use the pseudo-first-order kinetic equation to study reaction dynamics:
Pseudo – first – order kinetics: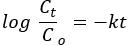
where Co and Ct are the initial and equilibrium concentrations of AO8 dye (mg/L), respectively, k is the rate constant, and t is the time in minutes.
Representative Results
Figure 1A depicts the thermal stabilities of binary NCs before a DTG instrument analyzes calcination in the N2 atmosphere. A sequence of vaporization of adsorbed H2O molecules, intramolecular decay, metal hydroxides or/and PVA side-chain decomposition, intermolecular/PVA main chain decomposition, and finally, the crystalline part took place to give carbon, hydrocarbons, and ash29,30.
The NCs showed stability loss above 720 °C. The XRD pattern's angles of diffraction with their corresponding crystal planes were compliant with the hexagonal structure of ZnO NPs (ICSD: 00-036-1451; Figure 1B). The ZnO NPs exhibited sharp peaks compared to the composites, indicating the less crystalline properties of NCs31. The crystal structures of ZnO (Figure 1C) and Mn2O3 (Figure 1C) were formed using the VESTA 3D imaging program software.
The approximate average crystallite sizes were then calculated using Debye-Scherrer's formula:
D = Kλ/(β cos θ)
where λ is the wavelength of X-ray radiation (for Cu Kα radiation, λ = 0.15418 nm), K is the constant, β is the line width at the maximum half-height, and θ is the diffracting angle32. The approximate sizes for ZnO NPs and NCs are 59 nm and 23 nm, respectively.
The absence of peak shifts for NCs relative to ZnO indicates the formation of only local heterojunctions between oxides, fulfilling Pearson's HSAB theory16,17,33. The XRD peaks of PVA were also not observed in the XRD patterns of ZnO and NCs. This showed a complete decomposition of PVA at 500 °C, as validated in the DTG analysis.
Among the basic pore shape models, the BET plots of ZnO NPs and NCs appeared to have cylindrical shapes (Figure 2B)34. Among the six types of adsorption isotherms and four types of hysteresis loops of IUPAC classification, NPs and NCs were matched with the type IV adsorption isotherm and H3 hysteresis loop35. The sharp increase at the relative pressure of 0.8 P/P0 shows the co-occurrence of mesoporous and macroporous pore size distributions36.
The Barrett-Joyner-Halenda (BJH) pore size distribution plots show the domination of mesoporous pore size distribution (Figure 2 inset)34. The absorption bands of FTIR spectra for both ZnO NPs and NCs at ~3650 cm−1 and ~1650 cm−1 can be assigned to the vibrations of hydroxyl groups and water molecules, respectively (Figure 2B). The morphological, compositional, and structural features of the NPs and NCs possibly influence the number and position of the peaks. The morphological modification from the spherical dimension to one-, two-, or three-dimensional particles is believed to cause broadness and splitting of the bands37,38. For ZnO NPs, the absorption peaks were divided into two parts, while for the NCs, only one peak was observed at 450 cm−1. The wavenumber shift is dependent on the strength and weakness of the metal-oxygen bond39. The peak shift toward lower wavenumber/frequency for NCs (3560 cm−1) compared to ZnO (3655 cm−1) corroborates the weakening of the metal-oxygen bond due to the introduction of the Mn2O3 phase40. The appearance of other peaks could be due to the transitional impurities produced during synthesis 41.
Figure 3A,B shows the SEM images of ZnO and NC materials. The SEM images revealed a higher porosity for NCs than ZnO. This result complies with the BET interpretation. The more porous the materials, the greater surface defects/active sites, charge transfer, and visible light absorption efficiency. Furthermore, the compositional analysis by the EDX technique verified the reality of the predictable Zn, Mn, and O elemental compositions (Figure 3C), with the observation of the respective major peaks at 1 keV, 0.5 keV, and 0.45 keV. The elements C and S were detected as impurities.
The XPS spectrum of ZnO NPs and NCs (Supplementary Figure S1) confirmed the existence of Zn 2p, Mn 2p, O 1s, and C 1s chemical states. The high-resolution Mn 2p orbital region on the NCs corroborates that the chemical states of Mn 2 p3/2 and Mn 2 p1/2 are present at the binding energies of 641.1 eV and 653.2 eV, respectively42. The approximate splitting energy of 12.1 eV between Mn 2p3/2 and Mn 2p1/2 represents a typical value for Mn3+ 43. Lastly, the binding energy of Zn 2p in the NCs (1022.7 eV) shows a positive shift compared to that of pure ZnO (1022.0 eV). This shift is due to the electron transfer from the Fermi level of ZnO to the Fermi level of Fe2O3 or/and Mn2O344,45,46.
The TEM images (Figure 3D) indicate that the sizes of the synthesized NCs were in the nanometer range (~20 – 50 nm), consistent with the XRD analysis. The TEM images also visualize the presence of twin crystallites (two different-sized crystallites) separated by a boundary known as an oriented attachment47. This attachment allows the particles to share a common crystallographic orientation47,48 and assists in the occurrence of continuous charge transfer capability49. The diffraction spots present exactly on the SAED ring indicate the crystallinity of ZnO NPs. The spots outside the ring represent the presence of Mn2O3 (Figure 3E)50.
The measured interplanar spacing values from SAED for NCs comply with the hexagonal wurtzite ZnO structure (Figure 3E inset). The d-spacing value of 0.34 nm from the HRTEM image matches the 221 planes of α-Mn2O3 (Figure 3F)51,52. The detected stacking faults on the IFFT image of HRTEM revealed the porous properties of the NCs (IFFT images of Figure 3E inset). On the other hand, the lattice fringe for ZnO was not seen for NCs. This is possibly due to the random selection of the crystallite during resolution.
Figure 4A,B shows the photodegradation activities of ZnO NPs and NCs to degrade AO8. A small percentage of adsorption occurred in the dark during the adsorption/desorption equilibration. Both ZnO and NCs showed good photocatalytic activities on AO8 dye.
The time-dependent data (Ct/Co vs. t and log (Ct/Co) vs. t) are given in Figure 4C and inset, respectively. The rate constant, k, values for ZnO and ZnO-Mn2O3 NCs obtained were deduced to be 0.0058 min−1 and 0.0087 min−1, respectively. The good photocatalytic activities of ZnO NPs are associated with some defects (vacancy) preventing the e−/h+ recombination. The redox potentials for zinc and manganese oxides before and after heterojunction are shown in Figure 4D (left).
The study observed that the bandgaps of Mn2O3 (Fermi level near the VB) and ZnO (Fermi level near the CB) move up and down, respectively to attain their Fermi level stability and, finally, attain equilibrium, as seen in Figure 4C (right). The photocatalytic activity of binary NCs on AO8 dye is probably due to the formation of a suitable broken gap (type III) band alignment. Moreover, the broken gap type of heterojunction transfers the electrons from ZnO's more negative CB potential to the more negative VB potential of Mn2O353,54. Consequently, the e− and h+ separation was increased to enhance the degradation efficiency55.
Even though their band edge position depends on the surface charge, semiconductor metal oxides have typical bandgap energy, which absorbs a specific frequency of light56. For semiconductors, having more negative CB than H+/H2 reduction potential and more positive VB than O2/H2O reduction potential is crucial for pollutant degradation57. The presence of a good charge transfer property shows the formation of heterojunctions/local contact between metal oxides, which has a noticeable influence on diminishing e−/h+ recombination. The study results and reviews suggest a possible proposed degradation mechanism of the NCs after the heterojunction33,46, as shown in Figure 4D.
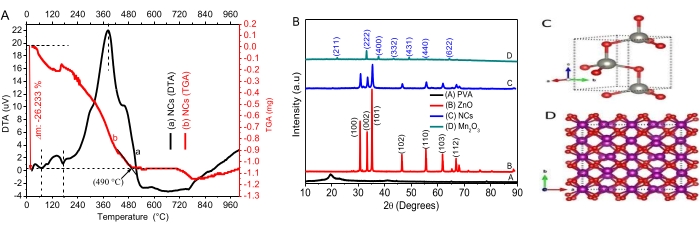
Figure 1. Materials stability analysis. (A) TGA/DTA plots of PVA-ZnO/Mn2O3 before calcination. (B) XRD patterns of PVA, ZnO, PVA-ZnO/Mn2O3, and Mn2O3; the ball-and-stick style crystal structures of (C) ZnO and (D) Mn2O3 created using the VESTA 3D imaging program software (red is for O atom) calcined at 500 °C. This figure has been modified from28. Please click here to view a larger version of this figure.
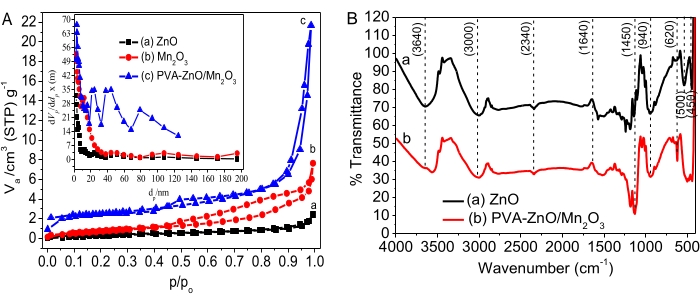
Figure 2. Textural properties and chemical bonding analysis. (A) The BET plots of ZnO, Mn2O3, and PVA-ZnO/Mn2O3 calcined at 500 °C. The inset figure shows the BJH plots. (B) FT-IR spectra of ZnO NPs and PVA-ZnO/Mn2O3 samples calcined at 500 °C. This figure has been modified from28. Please click here to view a larger version of this figure.
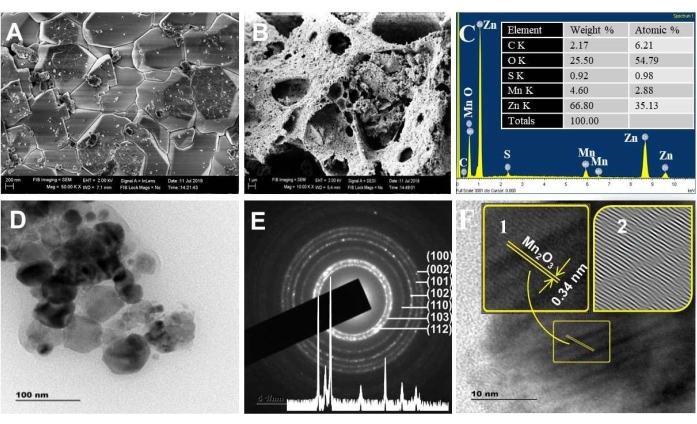
Figure 3. Morphological analysis. SEM image of (A) ZnO and (B) ZnO/Mn2O3, (C) EDXS spectra calcined at 500 °C. Inset in C is the elemental weight % and atomic % result. (D) TEM, (E) SAED, and (F) HRTEM images calcined at 500 °C. The inset in E is the XRD pattern; the inset in F is the magnified lattice fringes (1) and the IFFT pattern (2). This figure has been modified from28. Please click here to view a larger version of this figure.
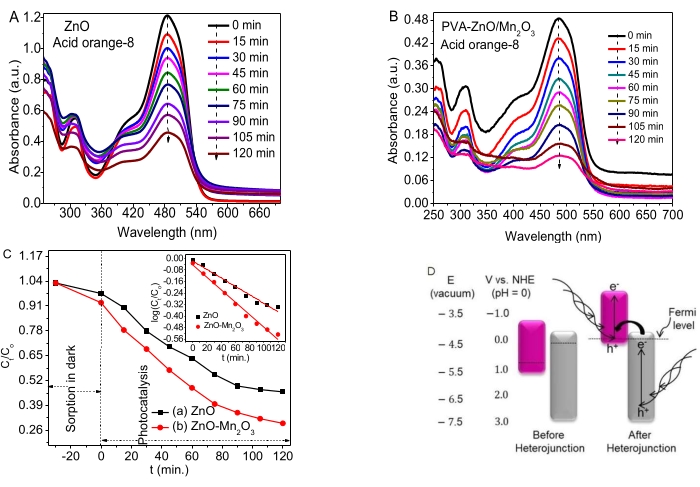
Figure 4. Photocatalytic activities of ZnO NPs and ZnO-Mn2O3 NCs (A, B) Absorbance vs. wavelength plots of ZnO and ZnO/Mn2O3, respectively. (C) The Ct/Co vs. t and log (Ct/Co) vs. t time-dependent kinetics data plot. (D) A broken gap type of a possible proposed mechanism (left, before heterojunction and right, after heterojunction). This figure has been modified from28. Please click here to view a larger version of this figure.
Supplementary Figure S1. The schematic diagram illustrates the architecture-directing agent-assisted solution combustion synthesis that produces a noble mass-/ion-transport active porous nanoscale material. (A) precursor solution; (B) the formed gel upon dehydration at 110 °C; (C) explosion of gaseous by-producton further heating to ignition the temperature; (D) the stable porous by-product produced upon calcination at 500 °C.This figure has been modified from28. Please click here to download this File.
Discussion
The present protocol describes the synthesis of nanocrystals using a bottom-up strategy with precise shape, size, and structure. The study observed that the nucleation and growth of nanocrystals were significant before forming the nanocrystals. Here, the ZnO and manganese oxides were synthesized based on LaMer's group theory25, which postulates the process of nanocrystal formation after reducing precursors into atoms and nuclei, leading to seed formation to produce nanocrystals. In this regard, the overall shape and size of the nanocrystals are reliant on the growth of the seeds, the properties of the seeds, and the reactivation of the surfactants/capping agent. Polyvinyl alcohol can act as a reducing and capping/stabilization agent58,59. Meanwhile, unlike the conventional SG-SCS approach, which uses fuel as an oxidant, only PVA polymer was used as a complexing and capping agent without using any fuel. In this case, water was used as a solvent, which is not common as other studies have used toxic, cancer-causing, and mutagenic solvents.
This study modified the reactivity of zinc and manganese based on the hard and soft acids and bases (HSAB) theory16,24. The less reactive and soluble manganese sulfate dopant was used to form a local heterojunction with the more reactive zinc nitrate precursor. It started the nucleation of zinc, and the manganese atoms were diffused and attached to the suitable surface sites (steps and kinks)26.
The reducers, neutral/chloride-based, or oxidant/nitrate-based salt oxidizers can be used as SG-SCS. As information, the reducer- and neutral/chlorine-based precursors need additional oxidants and release HCl, resulting in contamination of the final products. In general, the nitrate precursor is the best oxidizer, with crucial properties such as adequate oxidizing potential and stable decomposition temperature, which assist in forming pure product60, and noble solubility61. The sulfate precursor used to control the reactivity in this work produced impurities requiring a high decomposition temperature (see the EDX analysis, Figure 3C). As the nitrate salt has a suitable oxidizing and complex forming potential61, it is suggested that nitrate precursor and other conditions are used for balancing the host-dopant reactivity in place of surface salt.
The SG-SCS in this study followed a sequence of steps, including the colloidal/sol formation, dehydration (gel-formation), and self-sustained combustion reaction1,62. This resulted in the evolution of gasses that improve the porosity/textural properties of the product63 and, finally, quenching of the reaction by the gases evolution (see Supplementary Figure 1). During the SG-SCS process, the combustion processes may form a spongy/foam-like structure by combusting at many points or a long wire structure through combusting at a spot/point. Furthermore, the calcination of the combusted materials assists in decomposing the non-burnt impurities and improves the crystallinity of the materials27.
This study presented the solution combustion synthesis (SCS) approach as a novel time and energy-efficient methodology to produce highly stable and porous nanomaterials that can be applied effectively in an industrially scalable setting. SG-SCS procedures successfully synthesized the porous ZnO-based binary NCs. The study observed that the sol-gel method improved SAS for the synthesized NCs. The porosity of the NCs was verified through the SEM image, SAED ring, and BET analysis, while the optimized PVA degradation temperature for NCs was identified as 500 °C from DTG analysis. The XRD and TEM image analysis confirmed that the crystallite sizes of the NPs and NCs were at the nano level. The EDX, XPS, and HRTEM analyses were applied for the compositional and actuality investigation. Ultimately, the binary NCs showed good AO8 dye degradation, proving their efficiency. In general, any material could be synthesized reasonably by addressing the mentioned drawbacks, and SCS could provide a less costly, effortless, and time-/energy-efficient method to power energy devices in the future.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge Adama Science and Technology University for their support in this work. Funding was provided from Taif University Researchers Supporting Project number (TURSP-2020/44), Taif University, Taif, Saudi Arabia.
Materials
| Acid orange 8 | Sigma-Aldrich | 65%, | |
| Chlorine | Sigma-Aldrich | 7782-50-5 | |
| Dithienogermole | Sigma-Aldrich | 773881-43-9 | |
| HCl | Sigma-Aldrich | 7647-01-0 | |
| Manganese nitrate (10%) salt | Sigma-Aldrich | 15710-66-4 | 10% |
| Manganese sulfate monohydrate | Sigma-Aldrich | Density: 2.95 g/cm³; solubility in water: 70 g/100 mL (70 °C); 99.95%, MnSO4.H2O | |
| Poly (vinyl alcohol) | Sigma-Aldrich | 9002-89-5 | Density: 1.19–1.31 g/cm³ @20 °C, soluble in water only @ > 80 °C |
| Zinc nitrate hexahydrate (90%) | Sigma-Aldrich | 10196-18-6 | 98%; Density: 2.065 g/cm³ @20 °C; solubility in water: 184.3 g/100 mL @20 °C |
| Instruments used | |||
| Materials name | Model | Analysis | |
| BET (N2 adsorption-desorption isotherms) | Quanta chrome instrument. | Textural properties | |
| DT/DTA | Shimadzu DTG-60H | Measure thermal stability | |
| FTIR | Perkin Elmer FT-IR, Spectrum 65 | Chemical bonding information | |
| HRTEM | JEOL TEM 2100 HRTEM | Morphological, size, and composition analysis | |
| SEM-EDX | SEM-EDX-EVO 18 with low vacuum facility and ALTO 1000 cryo attachment | Morphological analysis | |
| XPS | AXIS ULTRA from AXIS 165 | ||
| XRD | Shimadzu, XRD-7000 | Crystallinity, structure, and approximate average crystallite size | |
| Common software used | |||
| Name | Company | Use | |
| Mendeley | Mendeley-Desktop-1.19.8-win32 | For citing references | |
| Origin | OriginPro 8 | XRD, BET, UV-vis-DRS data analysis |
References
- Khort, A., et al. Corrosion and transformation of solution combustion synthesized Co, Ni and CoNi nanoparticles in synthetic freshwater with and without natural organic matter. Scientific Reports. 11 (1), 7860 (2021).
- Pype, M., Lawrence, M. G., Keller, J., Gernjak, W. Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal – A review. Water Research. 98, 384-395 (2016).
- Adeleye, A. S., et al. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chemical Engineering Journal. 286, 640-662 (2016).
- Gómez-Pastora, J., et al. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chemical Engineering Journal. 310 (2), 407-427 (2017).
- Nadeem, M. S., et al. Enhancement in the photocatalytic and antimicrobial properties of ZnO nanoparticles by structural variations and energy bandgap tuning through Fe and Co co-doping. Ceramics International. 47 (8), 11109-11121 (2021).
- Nadeem, M. S., et al. Energy-levels well-matched direct Z-scheme ZnNiNdO/CdS heterojunction for elimination of diverse pollutants from wastewater and microbial disinfection. Environmental Science and Pollution Research International. , (2022).
- Munawar, T., Iqbal, F., Yasmeen, S., Mahmood, K., Hussain, A. Multi metal oxide NiO-CdO-ZnO nanocomposite-Synthesis, structural, optical, electrical properties and enhanced sunlight driven photocatalytic activity. Ceramics International. 46 (2), 2421-2437 (2020).
- Srinivasa, N., et al. Facile synthesis of Ni/NiO nanocomposites: The effect of Ni content in NiO upon the oxygen evolution reaction within alkaline media. RSC Advances. 11 (24), 14654-14664 (2021).
- Chen, P., et al. Solution combustion synthesis of ternary Ni/WC/C composites with efficient electrocatalytic oxygen reduction performance. RSC Advances. 11 (61), 38718-38726 (2021).
- Nagvenkar, A. P., Perelshtein, I., Piunno, Y., Mantecca, P., Gedanken, A. Sonochemical one-step synthesis of polymer-capped metal oxide nanocolloids: Antibacterial activity and cytotoxicity. ACS Omega. 4 (9), 13631-13639 (2019).
- Janotti, A., Van de Walle, C. G. Fundamentals of zinc oxide as a semiconductor. Reports on Progress in Physics. 72 (12), 126501 (2009).
- Abebe, B., Murthy, H. C. A., Amare, E. Enhancing the photocatalytic efficiency of ZnO: Defects, heterojunction, and optimization. Environmental Nanotechnology, Monitoring. & Management. 14, 100336 (2020).
- Abebe, B., Murthy, H. C. A., Zereffa, E. A. Multifunctional application of PVA-aided Zn-Fe-Mn coupled oxide nanocomposite. Nanoscale Research Letters. 16, 1 (2021).
- Shekofteh-Gohari, M., Habibi-Yangjeh, A. Fe3O4/ZnO/CoWO4 nanocomposites: Novel magnetically separable visible-light-driven photocatalysts with enhanced activity in degradation of different dye pollutants. Ceramics International. 43 (3), 3063-3071 (2017).
- Saravanan, R., Gupta, V. K. K., Narayanan, V., Stephen, A. Visible light degradation of textile effluent using novel catalyst ZnO/γ-Mn2O3. Journal of the Taiwan Institute of Chemical Engineers. 45 (4), 1910-1917 (2014).
- Buonsanti, R., Milliron, D. J. Chemistry of doped colloidal nanocrystals. Chemistry of Materials. 25 (8), 1305-1317 (2013).
- Hu, H., He, H., Zhang, J., Hou, X., Wu, P. Optical sensing at the nanobiointerface of metal ion-optically-active nanocrystals. Nanoscale. 10 (11), 5035-5046 (2018).
- Deganello, F., Tyagi, A. K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Progress in Crystal Growth and Characterization of Materials. 64 (2), 23-61 (2018).
- Buonsanti, R., et al. Assembly of ligand-stripped nanocrystals into precisely controlled mesoporous architectures. Nano Letters. 12 (7), 3872-3877 (2012).
- Li, F., Ran, J., Jaroniec, M., Qiao, S. Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale. 7 (42), 17590-17610 (2015).
- Williams, T. E., et al. Nearest-neighbour nanocrystal bonding dictates framework stability or collapse in colloidal nanocrystal frameworks. Chemical Communications. 53 (35), 4853-4856 (2017).
- Helms, B. A., Williams, T. E., Buonsanti, R., Milliron, D. J. Colloidal nanocrystal frameworks. Advanced Materials. 27 (38), 5820-5829 (2015).
- Liu, B., et al. Synthesis of ZnO nano-powders via a novel PVA-assisted freeze-drying process. RSC Advances. 6 (111), 110349-110355 (2016).
- Abebe, B., Murthy, H. C. A. Insights into ZnO-based doped porous nanocrystal frameworks. RSC Advances. 12 (10), 5816-5833 (2022).
- LaMer, V. K., Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. Journal of the American Chemical Society. 72 (11), 4847-4854 (1950).
- Jun, Y. -. S., et al. Classical and nonclassical nucleation and growth mechanisms for nanoparticle formation. Annual Review of Physical Chemistry. 73, 453-477 (2022).
- Gao, Y., Meng, F., Li, X., Wen, J. Z., Li, Z. Factors controlling nanosized Ni-Al 2 O 3 catalysts synthesized by solution combustion for slurry-phase CO methanation: the ratio of reducing valences to oxidizing valences in redox systems. Catalysis Science & Technology. 6 (21), 7800-7811 (2016).
- Abebe, B., Zereffa, E. A., Murthy, H. C. A. Synthesis of poly(vinyl alcohol)-aided ZnO/Mn 2 O 3 nanocomposites for acid orange-8 dye degradation: Mechanism and antibacterial activity. ACS Omega. 6 (1), 954-964 (2021).
- Kumar, S., Krishnakumar, B., Sobral, A. J. F. N., Koh, J. Bio-based ( chitosan / PVA / ZnO ) nanocomposites fi lm Thermally stable and photoluminescence material for removal of organic dye. Carbohydrate Polymers. 205, 559-564 (2019).
- Dai, Y., et al. Enhanced mechanical, thermal, and UV-shielding properties of poly(vinyl alcohol)/metal-organic framework nanocomposites. RSC Advances. 8 (67), 38681-38688 (2018).
- Munawar, T., et al. Novel tri-phase heterostructured ZnO-Yb2O3-Pr2O3 nanocomposite; structural, optical, photocatalytic and antibacterial studies. Ceramics International. 46 (8), 11101-11114 (2020).
- Mukhtar, F., et al. Enhancement in carrier separation of ZnO-Ho2O3-Sm2O3 hetrostuctured nanocomposite with rGO and PANI supported direct dual Z-scheme for antimicrobial inactivation and sunlight driven photocatalysis. Advanced Powder Technology. 32 (10), 3770-3787 (2021).
- Lachheb, H., et al. Electron transfer in ZnO-Fe 2 O 3 aqueous slurry systems and its effects on visible light photocatalytic activity. Catalysis Science & Technology. 7 (18), 4041-4047 (2017).
- Thommes, M., et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry. 87 (9-10), 1051-1069 (2015).
- Kumar, P., Kim, K. -. H., Kwon, E. E., Szulejko, J. E. Metal-organic frameworks for the control and management of air quality: advances and future direction. Journal of Materials Chemistry A. 4 (2), 345-361 (2016).
- Liu, J., et al. NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil. Scientific Reports. 6, 23667 (2016).
- Fatehah, M. O., Aziz, H. A., Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. Journal of Colloid Science and Biotechnology. 3 (1), 75-84 (2014).
- Sigoli, F. A., Davolos, M. R., Jafelicci, M. Morphological evolution of zinc oxide originating from zinc hydroxide carbonate. Journal of Alloys and Compounds. 262-263, 292-295 (1997).
- Wachs, I. E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catalysis Today. 27 (3-4), 437-455 (1996).
- Parler, C. M., Ritter, J. A., Amiridis, M. D. Infrared spectroscopic study of sol-gel derived mixed-metal oxides. Journal of Non-Crystalline Solids. 279 (2-3), 119-125 (2001).
- Anžlovar, A., Kogej, K., Crnjak Orel, Z., Žigon, M. Polyol mediated nano size zinc oxide and nanocomposites with poly(methyl methacrylate). Express Polymer Letters. 5 (7), 604-619 (2011).
- Saravanan, R., et al. ZnO/Ag/Mn 2 O 3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activity. RSC Advances. 5 (44), 34645-34651 (2015).
- Yang, G., Yan, W., Wang, J., Yang, H. Fabrication and formation mechanism of Mn 2 O 3 hollow nanofibers by single-spinneret electrospinning. CrystEngComm. 16 (30), 6907-6913 (2014).
- Liu, Y., et al. A magnetically separable photocatalyst based on nest-like γ-Fe 2 O 3 /ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale. 4 (1), 183-187 (2012).
- Hu, Y., et al. A microwave-assisted rapid route to synthesize ZnO/ZnS core-shell nanostructures via controllable surface sulfidation of ZnO nanorods. CrystEngComm. 13 (10), 3438-3443 (2011).
- Zhang, J., et al. Synthesis and gas sensing properties of α-Fe 2 O 3 @ ZnO core-shell nanospindles. Nanotechnology. 22 (18), 185501 (2011).
- Penn, R. L. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science. 281 (5379), 969-971 (1998).
- Zhang, J., Huang, F., Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale. 2 (1), 18-34 (2009).
- Zeng, Z., et al. A fluorescence-electrochemical study of carbon nanodots (CNDs) in bio- and photoelectronic applications and energy gap investigation. Physical Chemistry Chemical Physics. 19 (30), 20101-20109 (2017).
- Zhai, T., et al. Controllable synthesis of hierarchical ZnO nanodisks for highly photocatalytic activity. CrystEngComm. 14 (5), 1850-1855 (2012).
- Li, N., et al. Efficient removal of chromium from water by Mn3O4 @ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Applied Catalysis B: Environmental. 214, 126-136 (2017).
- Abebe, B. Polymer assisted colloidal nanocrystal framework synthesis: Sol-gel approach. Materials Research Express. 8 (12), 125005 (2021).
- Jiamprasertboon, A., et al. Heterojunction α-Fe2O3/ZnO films with enhanced photocatalytic properties grown by aerosol-assisted chemical vapour deposition. Chemistry – A European Journal. 25 (48), 11337-11345 (2019).
- Mukhtar, F., et al. Dual S-scheme heterojunction ZnO-V2O5-WO3 nanocomposite with enhanced photocatalytic and antimicrobial activity. Materials Chemistry and Physics. 263, 124372 (2021).
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Advanced Functional Materials. 24 (17), 2421-2440 (2013).
- Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO 2-based nanomaterials. Advances in Physical Chemistry. 2011, 786759 (2011).
- Hoffmann, M. R., Martin, S. T., Choi, W., Bahnemann, D. W. Environmental applications of semiconductor photocatalysis. Chemical Reviews. 95 (1), 69-96 (1995).
- Wu, Y., Wang, D., Li, Y. Understanding of the major reactions in solution synthesis of functional nanomaterials. Science China Materials. 59, 938-996 (2016).
- Xia, Y., Xiong, Y., Lim, B., Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics. Angewandte Chemie. 48 (1), 60-103 (2008).
- Kim, S. J., Yoon, S., Kim, H. J. Review of solution-processed oxide thin-film transistors. Japanese Journal of Applied Physics. 53, (2014).
- Zhang, J., Guo, Q., Liu, Y., Cheng, Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Industrial & Engineering Chemistry Research. 51 (39), 12773-12781 (2012).
- Novitskaya, E., Kelly, J. P., Bhaduri, S., Graeve, O. A. A review of solution combustion synthesis: an analysis of parameters controlling powder characteristics. International Materials Reviews. 66 (3), 188-214 (2021).
- González-Cortés, S. L., Imbert, F. E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Applied Catalysis A: General. 452, 117-131 (2013).
Tags

.
