Combining Raman Imaging and Multivariate Analysis to Visualize Lignin, Cellulose, and Hemicellulose in the Plant Cell Wall
Summary
This protocol aims to present a general method to visualize lignin, cellulose, and hemicellulose in plant cell walls using Raman imaging and multivariate analysis.
Abstract
The application of Raman imaging to plant biomass is increasing because it can offer spatial and compositional information on aqueous solutions. The analysis does not usually require extensive sample preparation; structural and chemical information can be obtained without labeling. However, each Raman image contains thousands of spectra; this raises difficulties when extracting hidden information, especially for components with similar chemical structures. This work introduces a multivariate analysis to address this issue. The protocol establishes a general method to visualize the main components, including lignin, cellulose, and hemicellulose within the plant cell wall. In this protocol, procedures for sample preparation, spectral acquisition, and data processing are described. It is highly dependent upon operator skill at sample preparation and data analysis. By using this approach, a Raman investigation can be performed by a non-specialist user to acquire high-quality data and meaningful results for plant cell wall analysis.
Introduction
Plant biomass is the most abundant renewable resource on Earth; is mainly composed of lignin, cellulose, and hemicellulose; and is considered an attractive source of bioenergy and bio-based chemicals1. Unfortunately, it can resist degradation and confer hydrolytic stability or structural robustness to the plant cell wall. Such resistance is attributable to the accessible surface area, biomass particle size, degree of polymerization, cellulose crystallinity, and protective lignin2. A comprehensive understanding of the structural and chemical nature of the plant cell wall is thus significant from the viewpoint of plant biology and chemistry, as well as from that of commercial utilization. Commonly used wet chemistry analyses, such as chromatography, mass spectrometry, and nuclear magnetic resonance spectroscopy, only provide average compositional data of the measured sample. Furthermore, these methods are invasive and destroy the original structure of the plant tissue3.
The Raman imaging technique is a powerful tool for the nondestructive visualization of spatially resolved chemical information4. It uses a laser light to cause inelastic scattering with a photon and relies on changes in polarizability arising from the molecular vibrations. In this case, water causes weak Raman scattering, which makes this approach suitable for in situ investigations of biological samples5. The application of the Raman imaging technique to the plant cell wall can elucidate the structure and composition of plant cell walls in their native state, with the resolution on the scale of the single cell and even of the cell wall layers6. A typical Raman imaging analysis of a plant cell wall generally consists of three steps: 1) sample preparation, 2) spectral acquisition, and 3) data processing.
Although one of the major advantages of Raman imaging is the ability to achieve label-free and non-destructive spectra with minimal sample preparation, physical sample sectioning is still necessary to expose the surface of interest. This process should be performed carefully to obtain a flat surface, since the technique depends on maintaining optical focus7. Spectral acquisition requires a balance between image quality and extensive acquisition times8. Data processing aims to effectively extract the chemical information from the image data, especially for the components with similar chemical structures, such as cellulose and hemicellulose. Due to the strong spectral overlap, the exact spectra are difficult to discern. In this case, multivariate analysis is a straightforward approach to effectively uncover the hiding structural and chemical information9. This work presents a general protocol describing the use of Raman imaging to visualize the main components in plant cell walls, including lignin, cellulose, and hemicellulose.
Protocol
1. Sample Preparation
- Cut a small tissue block (about 3 mm x 3 mm x 5 mm) from the plant sample (e.g., a poplar stem).
- Immerse the tissue in boiling deionized water for 30 min. Immediately transfer it to deionized water at room temperature (RT) for 30 min. Repeat this step until the tissue sinks to the bottom of the container, indicating that the air in tissue has been removed and that the tissue has softened.
Note: For the samples that sink to the bottom prior to this step, generally repeat this cycle 3-5 times. - Prepare 20, 50, 70, 90% (v/v) aliquots of polyethylene glycol (PEG) in deionized water, as well as pure PEG, and keep the solutions at 65 °C.
- Incubate the tissue in a series of graded PEG baths to displace the water and allow the PEG to infiltrate.
Note: Typically, PEG with a molecular weight 2,000 (PEG2000) is used for embedding.- Process the tissue with graded PEG in a drying oven as follows: (a) 20% PEG for 1 h, (b) 50% PEG for 1.5 h, (c) 70% PEG for 2 h, (d) 90% PEG for 2 h, and (e) 100% PEG for 10 h.
- Pre-warm a cassette at 65 °C in an oven. Pour the PEG containing the block into the cassette and then place the tissue block in a desired position using pre-warmed tweezers or needles.
- Slowly cool down the cassette and store the tissue at RT until use.
- Dissect the PEG block containing the target tissue into a small block (about 1 cm x 1 cm x 2 cm) using a sharp razor blade and mount it on the microtome.
- Cut thin sections from the PEG block (typically 3-10 µm).
- Rinse the section with deionized water in a watch glass 10 times to remove the PEG from the tissue.
- Immerse the sections with toluene/ethanol (2:1, v/v) for 6 h to remove the extractives. Prepare the reaction liquid by mixing 65 mL of deionized water, 0.5 mL of acetic acid, and 0.6 g of sodium chlorite in a beaker. Add one section and 3 mL of reaction liquid to a 5 mL vial. Screw on the top of the vial.
- Heat the vial in water bath at 75 °C for 2 h to remove the lignin of the tissue. Rinse the section with deionized water in a watch glass 10 times.
- Transfer the untreated/delignified section to a glass microscope slide. Unfold the section carefully using brushes or needles. Remove the extra deionized water with tissue paper.
- Immerse the section into D2O. Cover the sample with a glass cover slip. Seal the cover slip with nail polish to prevent the evaporation of the D2O.
2. Spectral Acquisition
- Open the instrument operating software of the confocal Raman microscope. Turn on the laser (wavelength = 532 nm), focus on the surface of the crystalline silicon with a 100X microscope objective, and click the calibration button to calibrate the instrument.
- Switch the instrument to the optical microscope mode and turn on the microscope lamp. Mount the microscope slide on the stage, with the cover slip facing the objective.
- View the sample with a 20X microscope objective and locate the area of interest. Apply immersion oil to the coverslip and switch to the immersion microscope objective (60X, numerical aperture NA = 1.35). Focus on the surface of the sample.
- Switch the instrument to the Raman testing mode and turn off the microscope lamp.
- Choose a mapping area by using a rectangular tool. Alter the step size to determine the number of obtained spectra.
Note: Be aware of the step size (usually larger than the spot diameter calculated by the numerical aperture of the objective; theoretically 1.22λ/NA). Sizes below this will result in oversampling. - Set the optimum spectral parameters to obtain the best signal-to-noise ratio (SNR) and spectral quality in an appropriate acquisition time (generally < 8 h), depending on the sample suitability. Generally, input the imaging parameters in the instrument software as follows: laser (532 nm), filter (100%), hole (300), slit (100), spectrometer (1,840 cm-1), grooves (1200t), objective (60X oil), and acquisition time (2 s).
- Save the spectral data before data processing and convert them to a universal format (e.g., TXT files).
3. Data Analysis
- Load the spectral data (TXT files) into the data analysis software (e.g., Matlab). Apply a noise-reduction technique on the data set to improve the SNR (e.g., the Savitzsky-Golay algorithm or the wavelet algorithm)
- Use untreated samples to produce the images of lignin and polysaccharides. For lignin imaging, consider the spectral peak around 1,600 cm-1 due to the aromatic ring symmetric stretching vibration. For polysaccharide imaging (including cellulose and hemicellulose), use the spectral peak around 2,889 cm-1 because of the CH and CH2 stretches.
- Use delignified samples to generate the images of cellulose and hemicellulose. Perform self-modeling curve resolution (SMCR) on the acquired spectra to discriminate the spectra of cellulose and hemicellulose and to image their distributions.
- Perform principal component analysis (PCA) and clustering analysis on the acquired data to distinguish the Raman spectra from different cell wall layers.
Representative Results
Figure 1 presents an overview of a typical micro-Raman system for the Raman imaging of a plant cell wall. As an example, the original Raman spectra of poplar (Populus nigra L.) have significant baseline drifts and spikes (Figure 2a). After performing the automatic pre-processing method for Raman imaging data set (APRI), these two spectral contaminants are successfully removed (Figure 2b). A typical Raman spectrum of poplar is displayed in Figure 3, and its band assignments are listed in Table 1. The Raman image of lignin is generated by the integration of the spectral region from 1,550-1,650 cm-1, which is attributed to the aromatic ring structure (Figure 4a). Figure 4d displays the Raman image of polysaccharides, which is achieved by integrating the peak at 2,889 cm-1. To image cellulose and hemicellulose, SMCR is performed on the Raman imaging data of a delignified sample. The corresponding spectra and images are shown in Figure 5. Figure 6 presents the results achieved by PCA and clustering analysis.
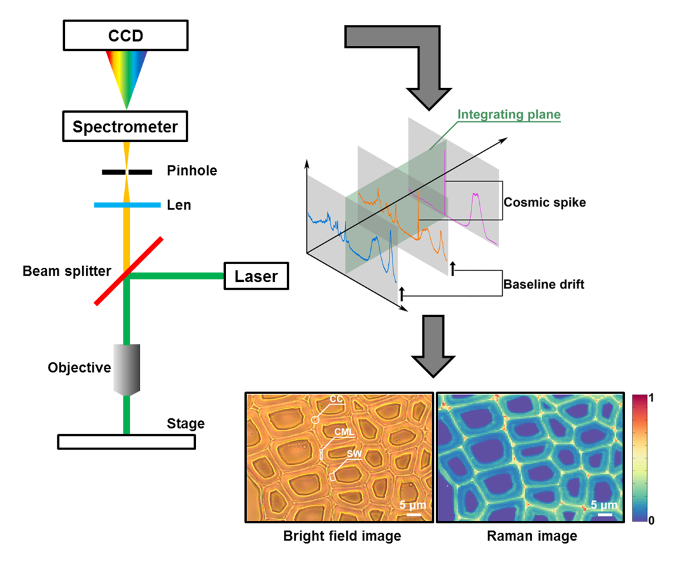
Figure 1: A Schematic of Raman Imaging for the Plant Cell Wall. The cross-section sample (poplar as an example) is measured by a micro-Raman system that couples an optical microscope to a Raman high-resolution spectrometer with a charge coupled device (CCD) detector. In the bright-field image, the plant cell wall is organized into the cell corner (CC), compound middle lamella (CML), and secondary wall (SW). Conventionally, a Raman image is generated by single-peak integration or intensity. Two spectral contaminants (i.e., baseline drifts and cosmic spikes) are found in the original data. Please click here to view a larger version of this figure.
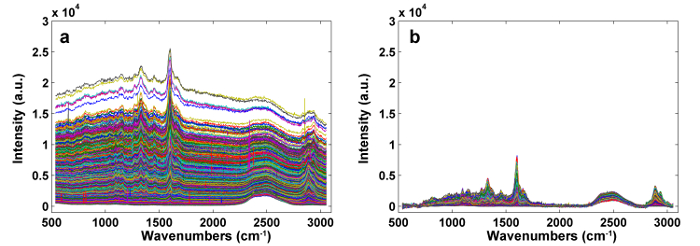
Figure 2: The Raman Spectra Before (a) and After (b) Pre-processing by APRI. Two spectral contaminants are successfully removed. Please click here to view a larger version of this figure.
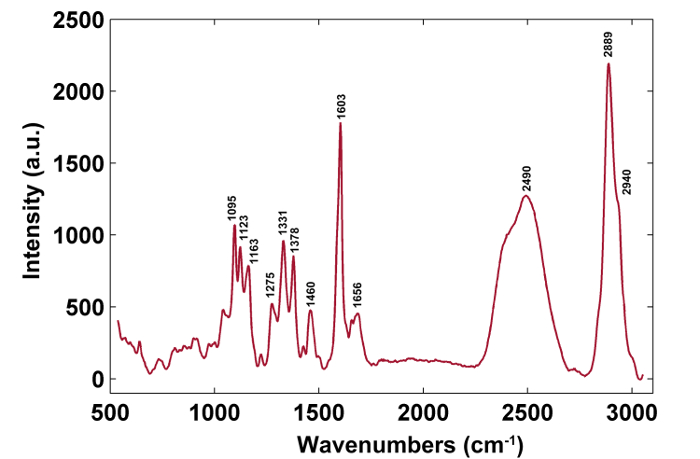
Figure 3: A Typical Raman Spectrum of the Poplar Cell Wall. The typical Raman spectrum is from the secondary wall. The band assignments are shown in Table 1. Please click here to view a larger version of this figure.
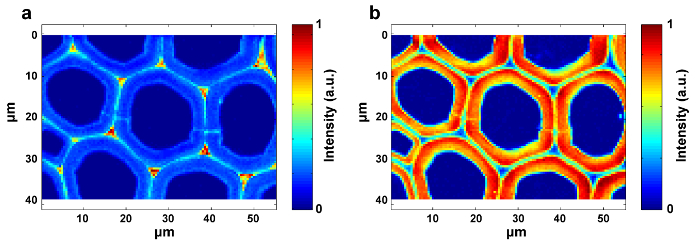
Figure 4: Raman Images of Lignin (a) and Polysaccharides (b) within the Poplar Cell Wall. The lignin image is generated by integrating the peak around 1,600 cm-1. The polysaccharide image is produced by integrating the peak around 2,889 cm-1. Please click here to view a larger version of this figure.
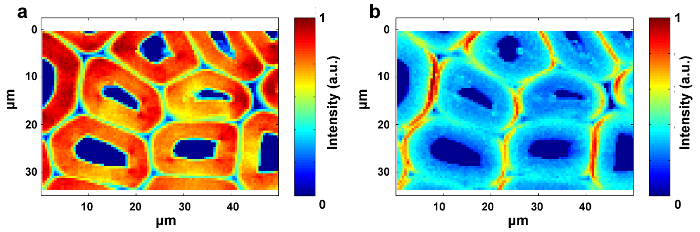
Figure 5: Raman Images of Cellulose (a) and Hemicellulose (b) within the Poplar Cell Wall. SMCR is performed on the Raman imaging data of a delignified sample. Delignification contributes to the exposure of the spectral characteristics of cellulose and hemicellulose. Cellulose is mostly concentrated in the SW, whilst the distribution of hemicellulose is almost uniform throughout the poplar cell wall. Please click here to view a larger version of this figure.
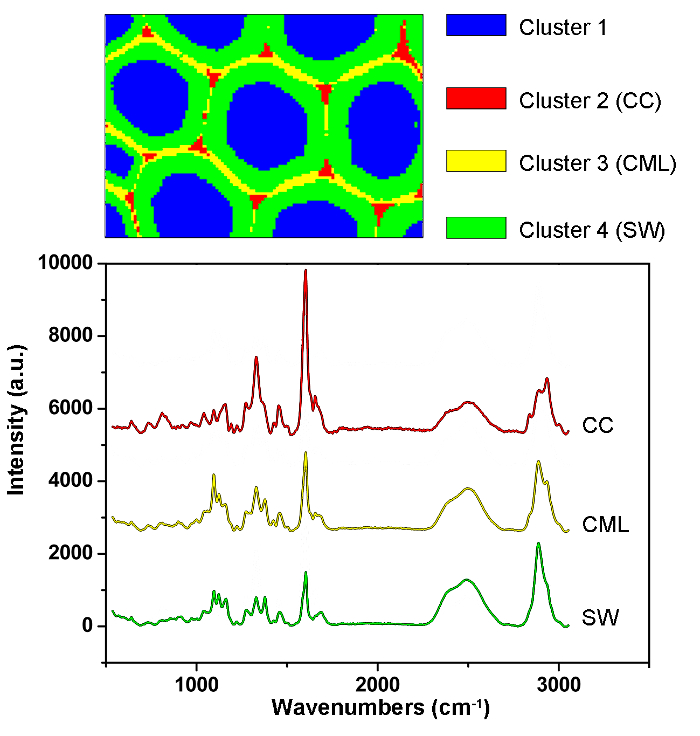
Figure 6: PCA and Clustering Analysis Results for Automatically Identifying Different Cell Wall Layers in the Poplar Cell Wall. By using PCA and clustering analysis, the spectra are divided into four parts corresponding to the cell lumen, CC, CML and SW. The average spectra of these layers are given below. The results revealed that the lignin is concentrated along the CML and in the CC, while the polysaccharides are mostly concentrated in the SW. Please click here to view a larger version of this figure.
| Wavenumbers (cm-1) | Components | Assignments |
| 1,095 | Cellulose, Hemicellulose | heavy atom CC and CO stretching vibration |
| 1,123 | Cellulose, Hemicellulose | heavy atom CC and CO stretching vibration |
| 1,163 | Cellulose, Hemicellulose | heavy atom CC and CO stretching vibration plus HCC and HCO bending vibration |
| 1,275 | Lignin | aryl-O of aryl OH and aryl O−CH3; guaiacyl ring with C=O group |
| 1,331 | Lignin, Cellulose, Hemicellulose | HCC and HCO bending vibration |
| 1,378 | Cellulose, Hemicellulose | HCC, HCO, and HOC bending vibration |
| 1,460 | Lignin, Cellulose, Hemicellulose | HCH and HOC bending vibration |
| 1,603 | Lignin | aryl ring stretching vibration, symmetrical vibration |
| 1,656 | Lignin | ring conjugated C=C stretching vibration of coniferyl alcohol; C=O stretching vibration of coniferaldehyde |
| 2,889 | C, H | CH and CH2 stretching vibration |
| 2,940 | L, C, H | CH stretching vibration in OCH3 asymmetric vibration |
Table 1: Raman Peak Positions and Band Assignments
Discussion
The plant cell wall is a composite that is organized into several layers, including cell corner (CC), secondary wall (SW, with the S1, S2, and S3 layers), and compound middle lamella (CML, middle lamella plus the adjacent primary wall), which makes it difficult to obtain a flat surface during sample preparation. Thus, plant samples, especially grass, which has a more complicated structure than wood, often need to be solidified to allow for fine sectioning. PEG is an ideal hard matrix for cutting and Raman investigation, since it is soluble in water. It can be easily removed by rinsing with deionized water. PEG used for embedding has different molecular weights, varying from 1,000-20,000. The higher the molecular weight of the PEG is, the lower the penetration ability10. D2O will help to reduce the fluorescence of lignin and has a marked peak at 2,490 cm-1, but it will not eliminate the fluorescence interference. Two major noise signals spill over into the channels, along with the actual signals: 1) sample and background fluorescence, as well as thermal fluctuations of CCD, can result in baseline drifts and 2) cosmic rays can markedly affect the sensitive detectors, which manifest in spectra as narrow-bandwidth spikes. The APRI method was developed to address these issues11. APRI includes the adaptive iteratively reweighted penalized least-squares (airPLS) and the principal component analysis (PCA) to eliminate the baseline drifts and cosmic spikes by using the spectral features themselves.
To achieve distribution images, peak intensity/integration and whole-spectra linear fitting by multivariate methods should be selected. The former is suitable to the components with specific Raman peaks (e.g., lignin), while the latter is appropriate to the components with strong spectral overlap (e.g., cellulose and hemicellulose). The distribution images of lignin and polysaccharides are available by integrating the specific peaks around 1,600 cm-1 and 2,889 cm-1, respectively (Figure 4). However, the images of cellulose and hemicellulose are difficult to directly generate by peak integration due to the strong spectral overlap. Multivariate analysis allows the convoluted information content to be sorted according to the hypothesis that the original data is reconstructed from a limited number of significant factors12. It thus can be applied to discriminate their spectra and to produce corresponding Raman images. For plant samples, the presence of extractives and lignin interferes with the ability to reconstruct the spectra of cellulose and hemicellulose. It is necessary to remove these interferences prior to the SMCR analysis of the imaging data. SMCR was first introduced by Lawton and Sylvester as a multivariate technique specifically developed for resolving a pure component from a set of spectra, without any recourse to a spectral library, to analyze the imaging data13. Spectral classification is important for further understanding the structural and chemical nature of the plant cell wall. Here, the imaging data have been subjected to principal component analysis (PCA) and clustering analysis to distinguish Raman spectra from different cell wall layers14.
However, this technique has two limitations. First, the Raman effect is weak—the typical total Raman scattering cross-section is ~10-29 cm2 per molecule—which makes it vulnerable to intense fluorescence15. A possible way to improve the defect is by preparing the sections as thinly as possible and applying a baseline correction algorithm, but fluorescent signals are difficult to remove. Second, chemical treatment is a significant procedure when achieving Raman images of cellulose and hemicellulose, and it may increase the risk of changing the original chemical content. Therefore, it is best to remove the extractives and lignin as much as possible, without the cell wall structure looking too different from the untreated one.
In conclusion, this protocol is suitable for studying the distribution of lignin, cellulose, and hemicellulose within plant cell wall. Using Raman imaging to obtain the desired information is highly dependent upon the operator skill at sample preparation and data analysis. Good sample preparation is essential to collect high-quality Raman spectra. Appropriate data analysis provides insights into the large-scale spectra and extracts the hidden information from the image. As a fundamental method, this protocol can be used to trace the dynamic changes of major components during chemical, physical, or biological treatment at the micro-level.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the China Ministry of Science and Technology (2016YDF0600803) for the financial support.
Materials
| Microtome | Thermo Scientific | Microm HM430 | |
| Confocal Raman microscope | Horiba Jobin Yvon | Xplora | |
| Oven | Shanghai ZHICHENG | ZXFD-A5040 |
References
- Gonzalo, G. D., et al. Bacterial Enzymes Involved in Lignin Degradation. J. Biotechnol. 236, 110-119 (2016).
- Rosatella, A. A., Afonso, C. A. M. Chapter 2. Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives. , 38-64 (2016).
- Sun, L., et al. Understanding tissue specific compositions of bioenergy feedstocks Through hyperspectral Raman imaging. Bio. 108 (2), 286-295 (2009).
- Tolstik, T., et al. Classification and prediction of HCC tissues by Raman imaging with identification of fatty acids as potential lipid biomarkers. J. Cancer. Res. Clin. Oncol. 141 (3), 407-418 (2015).
- Schrader, B. . Infrared and Raman spectroscopy: methods and applications. , (2008).
- Gierlinger, N., et al. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 7 (9), 1694-1708 (2012).
- Luca, A. C. D., et al. Online fluorescence suppression in modulated Raman spectroscopy. Anal. Chem. 82 (2), 738-745 (2009).
- Schlücker, S., et al. Raman microspectroscopy: a comparison of point, line, and wide-field imaging methodologies. Anal. Chem. 75 (16), 4312-4318 (2003).
- Cooper, J. B. Chemometric analysis of Raman spectroscopic data for process control applications. Chemometr. Intell. Lab. Syst. 46 (2), 231-247 (1999).
- Cheng, H. J., Hsiau, S. S. The study of granular agglomeration mechanism. Powder Technol. 199 (3), 272-283 (2010).
- Zhang, X., et al. Method for removing spectral contaminants to improve analysis of Raman imaging data. Sci. Rep. 6, 39891 (2016).
- Shinzawa, H., et al. Multivariate data analysis for Raman spectroscopic imaging. J. Raman Spectrosc. 40 (12), 1720-1725 (2009).
- Lawton, W. H., Sylvestre, E. A. Self modeling curve resolution. Technometrics. 13, 617-633 (1971).
- Zhang, X., et al. Method for automatically identifying spectra of different wood cell wall layers in Raman imaging data set. Anal. Chem. 87 (2), 1344-1350 (2015).
- Kudelski, A. Analytical application of Raman spectroscopy. Talanta. 76 (1), 1-8 (2008).

