A Minimally Invasive, Fast Spinal Cord Lateral Hemisection Technique for Modeling Open Spinal Cord Injuries in Rats
Summary
Here, we describe a new, fast technique modeling open spinal cord injury in rats that eliminates laminectomy. Lateral hemisection is performed while viewing through a microscope. The technique is versatile and can also be used in the cervical, thoracic, and lumbar regions of the spinal cord of other animals.
Abstract
Open spinal cord injury techniques modeling laceration-like injuries are time-consuming and invasive because they involve laminectomy. This new technique eliminates laminectomy by removing two spinous processes and lifting, then tilting the caudal vertebral arch. The surgical area opens up without the need for laminectomy. Lateral hemisection is then performed with direct visible control under a microscope. The trauma is minimized, requiring only a small bone wound.
This technique has several advantages: it is faster and, therefore, less of a burden for the animal, and the bone wound is smaller. Because the laminectomy is eliminated, there is less chance for unwanted injury to the spinal cord, and there are no bone splinters that can cause problems (bone splinters embedded in the spinal cord can cause swelling and secondary damage). The vertebral canal remains intact. The main limitation is that the hemisection can only be performed in the intervertebral spaces.
The results show that this technique can be performed much faster than the traditional surgical approach, using laminectomy (11 min vs. 35 min). This technique can be useful for researchers working with animal models of open spinal cord injury as it is widely adaptable and does not require any additional specialized instrumentation.
Introduction
Spinal cord injuries (SCIs) are unfortunately prevalent injuries in humans. SCIs can be complicated in different ways, for example, by infections, and it is clinically important to study these injuries1. Because there is no single, definite cure for SCIs, animal models are still needed to further the understanding of researchers and advance possible treatments2,3. Although closed injuries are most commonly modeled (compression and contusion), it is clinically important to understand lacerations, which can only be modeled in open injuries4. Open-wound models using transection or hemisection can be used to demonstrate a more precise localization of a wound compared to closed injury models, owing to the nature of the injury (contusion vs. surgical cut). Open-wound experiments can shed light on more specific neuronal injuries in a controlled, reliable, and replicable way5. The complete or partial transection of the spinal cord is a widely used open-wound technique and can be viewed in detail in the article by Brown and Martinez6.
When studying open spinal cord injury in rats, several animals presented problems that arose from the surgery: bone splinters from the laminectomy became embedded in the spinal cord and caused swelling; the larger bone wound needed a long time to heal; the surgery took too long. An alternate surgical technique was developed to eliminate these problems. The goal was to develop a faster technique that is gentler for the animal. This newly developed technique is much faster than traditional SCI techniques. The surgical approach is minimally invasive, resulting in a smaller bone wound while eliminating problems arising from the laminectomy.
All open-wound techniques involve opening the dura7. Several recent studies have examined different, newly developed techniques, aiming to improve the previous methods8,9. Even though the opening of the dura cannot be excluded using this new technique, it causes a smaller wound on the dura while offering a reliable, controlled injury of the spinal cord. Consulting the literature on spinal cord injury techniques, many authors tried to minimize the time of surgery by implementing minor changes to the original technique10. Laminectomy is always part of these surgical procedures, although it is time-consuming and requires a larger bone wound to be made6. This surgical technique can be appropriate for researchers using open wound spinal cord injury models, specifically complete transection or lateral hemisection performed in the intervertebral spaces (Figure 1).
Protocol
All animal procedures were carried out according to the EU Directive (2010/63/EU) and were approved by the animal ethics committee of the Hungarian National Food Chain Safety Office (PEI/001/2894-11/2014). All applicable institutional and governmental regulations concerning the ethical use of animals were followed during this study.
1. Preparation before surgery
- Sterilize all instruments used during the procedure (see the Table of Materials) and disinfect the surfaces where the work is to be performed prior to the procedure.
- Inject a single dose of subcutaneous antibiotics prophylactically.
NOTE: See the Table of Materials for antibiotic dosage details. - Leave the animals in the operating room for 1 h to acclimate them and decrease their stress prior to surgery.
- Anesthetize the rat via an intramuscular injection of a combination of ketamine and xylazine (ketamine 80 mg/kg body weight (bw) and xylazine 8 mg/kg bw).
NOTE: In addition to anesthesia, the ketamine-xylazine combination provides sufficient analgesia for this procedure. The analgesia regimen can be modified per the institutional animal use guidelines. - Keep the rat warm during the procedure using a heated table or infrared light and keep the eyes moist throughout the anesthesia using ophthalmic ointment (reapply as necessary).
- Fixate the animal on the operating table using surgical tape on its front and back paws and tail, and depending on the site of injury, on the neck as well. If necessary, place the rat in a stereotaxic frame to stabilize it during surgery.
- Using sterile surgical suture, place a loop around the upper front teeth of the rat and fixate this on the edge of the operating table.
- Pull out the tongue sideways for airway management.
- Shave the fur on the back, at least 2 cm in each direction of where the incision will be made.
- Disinfect the skin of the surgical area at least three times, using a povidone-iodine solution and sterile gauze. Take special care to soak the fur surrounding the area. Secure the surgical site with a sterile drape.
- Assess the adequacy of the anesthesia before placing the first incision by pinching the toes and the tail of the animal. Continue monitoring the adequacy of anesthesia during the entire procedure.
2. Surgery
- Place the skin incision using a scalpel blade 20. To open the surgical area, place a 2-2.5 cm long incision along the spine, cutting through all the layers of the skin. Position this incision parallel to the spinal column using the L1 vertebra as a midpoint, making it extend ~1 cm in both the cranial and caudal direction along the spinal column.
- Mobilize the sides of the wound by cutting through the connective tissue surrounding the muscles.
- Place two parallel incisions along the spine, penetrating the periosteum. Place the incisions right next to the spinal processes on both sides, spanning the distance between the Th13 and L1 vertebrae.
- Dissect the muscles attached to the vertebrae with the aid of a raspatorium until all the spinal ligaments are visible. Put in place a retractor.
- Remove the spinal processes of the 13th thoracic vertebra and the 1st lumbar vertebra using dental bone forceps to visualize the entire surgical area. Hereon, control the procedure by viewing an enlarged (4x-16x magnification) microscopic image.
- Use sterile gauze to control bleeding throughout the procedure, when necessary.
- Carefully lift the remainder of the L1 spinal processes, elevating the L1 vertebral arch. Sever the ligamentum flavum to access the spinal cord. Raise the caudal spinal processes further, allowing access to the spinal dura mater, which is also severed. Tip the caudal spinal processes in the cranial direction to visualize the pia mater.
- Look through the pia mater for the posterior median vein, showcasing the midline of the spine.
- Using the vein as a directional bisector, place an incision using a microsurgical scalpel while sparing the vein. Place the incision under the vein, in the transversal plane through the anteroposterior diameter of the spinal cord. Sever half of the spinal cord by moving the blade laterally away from the centerline.
NOTE: The incision is unilateral, on the right side, at the 4th lumbar segment. - Try to place the incision to avoid cutting the anterior spinal artery. Ensure excess pressure is not applied on the vertebral body when cutting the spinal cord to spare the anterior spinal artery on the ventral side of the spinal cord.
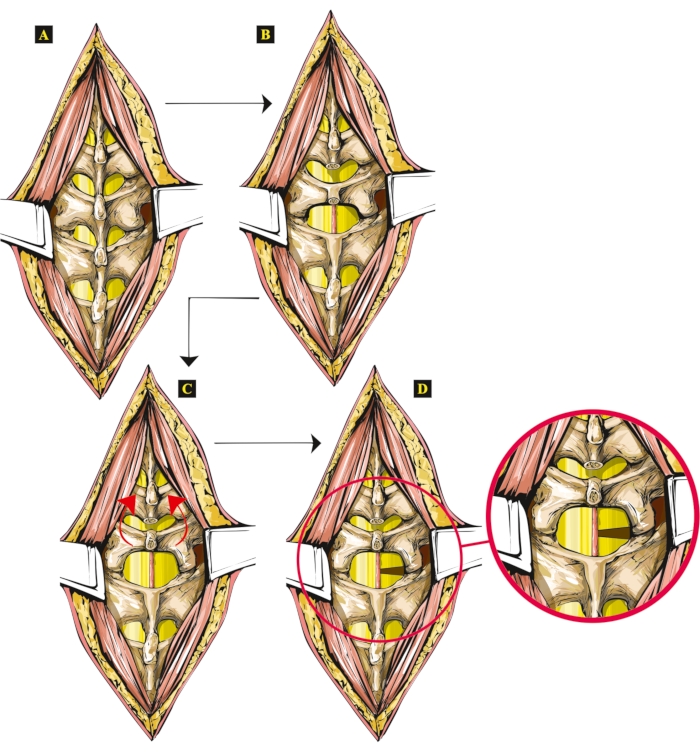
Figure 1: Artwork showing the steps of the new open SCI technique in rats. (A) The exposed vertebrae. (B) Spinal processes removed (Th13 and L1). (C) The lifted and tilted vertebral arch of the L1 vertebra. (D) Hemisection performed on the right side, with hemisected spinal cord shown separately, zoomed in. Please click here to view a larger version of this figure.
- Do not close the dura mater directly during wound closure. Tightly suture (suture size 4-0) the muscles along the spinal processes, indirectly closing the small wound on the dura mater.
- Close the dorsal connective tissue layer with sutures.
- Finally, suture the skin around the incision site.
3. Postsurgical care and follow-up
- Allow the animals to awaken in their cages. Keep the animal(s) warm using a heat lamp in addition to the temperature-controlled room. Do not leave the rats alone after they awaken after surgery, and do not put them together with other rats in the same cage.
- Monitor their respiratory rate at least every 10 min until they are fully awake. If necessary, apply gentle stimulation (e.g., rub the head) to aid their awakening from anesthesia.
- When the animals are alert and appropriately active, transport them safely back to the animal house.
- Keep the rats under close surveillance for the first 24 h post surgery. Following the first 24 h post surgery, check the animals at least twice a day until the end of the experiment, monitoring for signs of distress.
- Thoroughly assess them once a day for signs of distress using the relevant institutional animal welfare protocol and take special care to check their wounds for signs of infection and inflammation.
NOTE: Stress and infection affect the welfare of the animals and the outcome of experiments. - Administer antibiotics subcutaneously every day until the end of the experiments. Keep the animals in sterile cages, one animal per cage, and give food and water ad libitum, same as before. At the end of the experiments (or if any serious adverse reaction is observed during the timeframe of the experiment), euthanize the animals humanely, in accordance with the relevant institutional animal welfare protocol.
NOTE: Here, the animals were euthanized (in a deep sleep induced by the combination of ketamine-xylazine) by first administering a physiological saline perfusion (1/3 mL/g bw) followed by a 4% paraformaldehyde perfusion (1 mL/g bw).
Representative Results
Following the hemisection, the rats show paralysis in the ipsilateral hindlimb (in vivo proof of successful hemisection). Thorough specimen evaluation can only be done following the removal of the spinal cord (see Figure 2, where the removed spinal cord can be seen from both the ventral and dorsal sides).

Figure 2: Ventral and dorsal views of the removed spinal cord following hemisection. The entire removed spinal cord viewed from the ventral side (A) and the dorsal side (B) shown side-by-side. Please click here to view a larger version of this figure.
First, the removed spinal cord is analyzed in its entirety under a microscope using 4x-16x magnification (to evaluate the degree and precision of the injury). The specimen is then further analyzed using histology, where the site of the injury can be seen in greater detail. Hematoxylin and eosin (H&E) stain was used to prepare the samples (Figure 3).
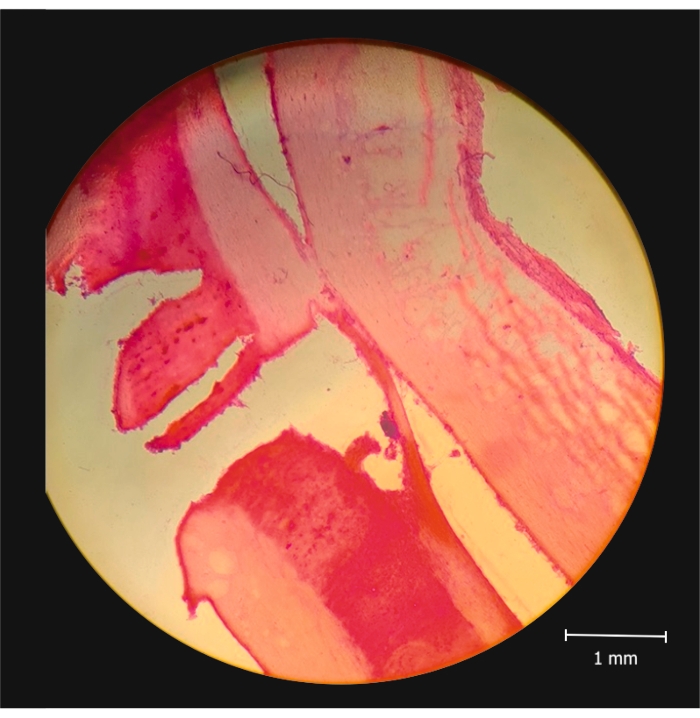
Figure 3: Histological sample showing hemisection. Histological sample stained using hematoxylin and eosin, showing the hemisection, viewed under a microscope (16x magnification). Scale bar = 1 mm. Please click here to view a larger version of this figure.
Figure 2 and Figure 3 show that the incision is perfectly acceptable in length and placement. The quality of the samples was at least as good as those obtained from animals whose spinal cords were hemisected using the traditional surgical approach with laminectomy (for a detailed description of the traditional surgical method, see 6). The images are not qualitatively different from the result of any other surgical approach, even though this technique is faster and there is no laminectomy.
The results show that this technique can be performed much faster than the traditional surgical approach using laminectomy (11 min vs. 35 min). The spinal cord is exposed for 10-15 s with this method, compared to a minimum of 3.5 min using laminectomy (until the closure of the dura). In conclusion, this new minimally invasive SCI method without laminectomy is much faster and does not require any additional specialized instrumentation.
Discussion
This minimally invasive spinal cord injury technique was developed when studying spinal cord-injured rats, and the team was faced with problems arising from the surgery itself (bone splinters from the laminectomy causing compression and damaging the spinal cord, surgery taking too long, slow healing of a large bone wound). By eliminating the laminectomy, the procedure became much faster (11 min vs. 35 min), the structure of the vertebral canal remained intact, the bone wound was much smaller, and there were no bone splinters that could damage the spinal cord.
The removal of the spinous processes cannot be eliminated because the removal of the upper (cranial) spinous process is necessary to tilt the lower (caudal) spinous process backward. The removal of the lower spinous process greatly improves the visibility of the spinal cord, facilitating hemisection.
The hemisection is the most critical part of the protocol. Here, the hemisection is performed freehand, although this is not a prerequisite. A stereotaxic instrument can be used instead. The rat can also be placed in a stereotaxic frame to stabilize the animal during surgery6. This step will require only a slight modification in the technique outlined here. This can also be helpful if someone with little experience is performing the procedure.
This new technique is extremely versatile. Here, the procedure was performed at the L4 lumbar segment (L1 vertebra); however, it can be used in other segments of the spinal cord tailored to the specific needs of the actual experiment (this technique has been used in the thoracic and the cervical regions as well). It could also be easily adjusted to implement a complete transection of the spinal cord instead of a hemisection. Lifting the vertebral arch allows direct inspection of the given part of the spinal cord. Thus, a small disc of spinal cord tissue can also be removed to ensure complete transection.
The use of this new technique is not limited to rats but can also be applied to other species used to model spinal cord injuries (e.g., mice, pigs, dogs). The main limitation of this technique is that because the hemisection (or transection) can only be performed in intervertebral spaces, it is not suitable for those who specifically need the cut to be placed in the vertebral spaces. Moreover, because it is an open-wound technique, it is not optimal for modeling contusions or compression injuries.
However, this technique can be the ideal choice for studying open SCI, as the hemisection (or transection) is executed precisely and is easily reproducible. Spinal pathways can also be studied with fewer artifacts as the vertebral canal remains intact. It can be especially useful when studying minimally invasive therapeutic approaches. Using this technique, the focus of attention can be solely on the treatment instead of on possible side effects of the surgery11.
In conclusion, this new, minimally invasive technique requires neither new equipment nor expensive settings as only equipment readily available in laboratories working with animals is utilized. It can easily be adapted to the specific needs of a given study (site of injury; hemi- or transection; type of animal). It is also easy to learn. Therefore, this modification could be of interest to researchers working with open SCI animal models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Gergely Ángyán for the original artwork. This research work was funded by Semmelweis University, Budapest, Hungary. This study was also supported by the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006). Additional support was received from the Thematic Excellence Programme (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapy thematic program of Semmelweis University.
Materials
| Augmentin (1,000 mg/200 mg powder) | GlaxoSmithKline, UK | One-time dose of s.c. antibiotics prophylactically (10 mg of amoxicillin and 2 mg clavulanic acid; Augmentin 1,000 mg/200 mg powder). Every day following surgery, 10 mg of amoxicillin and 2 mg of clavulanic acid (Augmentin 1,000 mg/200 mg powder) per day per animal | |
| Betadine | EGIS, Hungary | Disinfect the skin of the surgical area using a povidone-iodine solution | |
| Calypsol (50 mg/mL) | Richter Gedeon, Hungary | Anesthesia: combination of ketamine 80 mg/kg and xylazine 8 mg/kg intramuscularly | |
| CP XYLAZIN 2% (20 mg/mL) | Produlab Pharma B.V., the Netherlands | Anesthesia: combination of ketamine 80 mg/kg and xylazine 8 mg/kg intramuscularly | |
| Dental bone forceps | Dentech, Hungary | BS 0127 | Remove the spinous processes of the 13th thoracic vertebra and the 1st lumbar vertebra using dental bone forceps |
| dental surgical micromotor | W&H, Austria | MF-TECTORQUE | Using a dental surgical micromotor, a laminectomy is performed at the L1 vertebra |
| optical microscope | Zeiss, Germany | OPMI19-FC | Control the procedure by viewing an enlarged (16x magnification) microscopic image |
| physiological saline solution (0.9% NaCl) | Fresenius Kabi, Germany | Keep the rat's eyes moist throughout the entire anesthesia using physiological saline solution drops (reapply as necessary) | |
| raspatorium | Dentech, Hungary | FK 1164 | Dissect the muscles attached to the vertebrae with the aid of a raspatorium, until all the spinal ligaments are visible. |
| retractor | Dentech, Hungary | RT 1253 | |
| scalpel | Dentech, Hungary | BB 173 | |
| scalpel | Dentech, Hungary | BB 184 | |
| scalpel blade 12 | B. Braun, Germany | 12 | |
| scalpel blade 20 | B. Braun, Germany | 20 | |
| sterile cut gauze 10 x 10 cm | Sterilux, Hartmann, Germany | ||
| sutures (monofilament, synthetic; absorbable and nonabsorbable), size: 4-0 | B. Braun, Germany | ||
| tweezer (13 cm) | Dentech, Hungary | BD 1555 | |
| tweezer (delicate tissue forceps) | Dentech, Hungary | BD 1670 |
References
- Failli, V., et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain. 135, 3238-3250 (2012).
- Guan, B., Chen, R., Zhong, M., Liu, N., Chen, Q. Protective effect of Oxymatrine against acute spinal cord injury in rats via modulating oxidative stress, inflammation and apoptosis. Metabolic Brain Disease. 35 (1), 149-157 (2020).
- Kjell, J., Olson, L. Rat models of spinal cord injury: from pathology to potential therapies. Disease Models & Mechanisms. 9 (10), 1125-1137 (2016).
- Minakov, A. N., Chernov, A. S., Asutin, D. S., Konovalov, N. A., Telegin, G. B. Experimental models of spinal cord injury in laboratory rats. Acta Naturae. 10 (3), 4-10 (2018).
- Borbély, Z., et al. Effect of rat spinal cord injury (hemisection) on the ex vivo uptake and release of [3H]noradrenaline from a slice preparation. Brain Research Bulletin. 131, 150-155 (2017).
- Brown, A. R., Martinez, M. Thoracic spinal cord hemisection surgery and open-field locomotor assessment in the rat. Journal of Visualized Experiments: JoVE. (148), e59738 (2019).
- Taoka, Y., Okajima, K. Spinal cord injury in the rat. Progress in Neurobiology. 56 (3), 341-358 (1998).
- Hou, S., Saltos, T. M., Iredia, I. W., Tom, V. J. Surgical techniques influence local environment of injured spinal cord and cause various grafted cell survival and integration. Journal of Neuroscience Methods. 293, 144-150 (2018).
- Mattucci, S., et al. Development of a traumatic cervical dislocation spinal cord injury model with residual compression in the rat. Journal of Neuroscience Methods. 322, 58-70 (2019).
- Ahmed, R. U., Alam, M., Zheng, Y. P. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 5 (3), 01324 (2019).
- Ashammakhi, N., et al. Regenerative therapies for spinal cord injury. Tissue Engineering. Part B, Reviews. 25 (6), 471-491 (2019).

