Inducement and Evaluation of a Murine Model of Experimental Myopia
Summary
In this protocol, we describe the full process of experimental myopia inducement in mice using newly designed eyeglasses and the technic needed for achieving stable and reproducible results in ocular parameter measurements.
Abstract
Murine model of myopia can be a powerful tool for myopia research because of the comparatively easy genetic manipulation. One way to induce myopia in animals is to put clear minus lenses in front of eyes for weeks (lens-induced myopia, LIM). However, extant protocols for inducement and evaluation vary from laboratory to laboratory. Here, we described a highly practical and reproducible method to induce LIM in mice using newly designed eyeglasses. The method fixes the lens stably in front of the mouse eye while allows the lens to be taken off for cleaning or topical drug administration. The phenotype is robust and efficient, and the variance is small. The method described here can be applied to mice right after weaning which extends the possible duration for experiments. We also gave technical advises for achieving reproducible results in refraction and axial length measurements. We hope the step-by-step protocol described here and the detailed article can help researchers perform myopia experiments with myopia more smoothly and make the data comparable across laboratories.
Introduction
The prevalence of myopia has increased dramatically recently, while the mechanism of its onset and progression are still largely unknow1. The most characteristic phenotype of myopia is the elongation of axial length (AL), which increases risk for retinal complications or even blindness2. To better understand the pathogenesis of myopia and develop effective treatments, robust myopic animal models and stable phenotype evaluation are necessary.
Briefly, two methods exist for inducing myopic states in animals: form-deprivation myopia (FDM) and lens-induced myopia (LIM)3. The former places diffusers in front of the eye or sutures the eyelid to obscure the image, which influences the normal development of the eyeball, resulting in a myopia phenotype. The latter places minus lenses in front of the eye to move the focal point behind the retina. The retina detects the shift of the focus and elongates the eyeball to realign the retina and focal point. For FDM, after the eyelid is closed or the diffuser has been fixed in front of the eye, almost no further maintenance is needed. For LIM, the lens needs to be taken off for cleaning in order to keep it transparent. Thus, FDM is relatively easy to be induced technically. However, the mechanisms of FDM and LIM are different, and which method mimics the myopia in human better is still under debate3. One of the strengths of LIM is the stronger phenotype compared with FDM, at least in the case of mice4.
Animals that have been used for inducing myopia include chicks5, monkeys6, tree shrews7, guinea pigs8, and mice4. Considering the possibility of genetic manipulation, abundant available antibodies, and low cost for breeding, mice could have been the first choice as the animal model of myopia. However, compared with other larger animals, fixing lenses or diffusers in front of the mouse eye is relatively difficult especially for young mice such as right after weaning. For the experiments that need topical drug administration or multiple interim eye measurements, it is also necessary for the frame to be removable. Another challenge is the small morphological change of mouse eyeball, which needs sophisticated technics and devices to evaluate. To date, different inducing and measuring protocols used in different research teams make it hard to compare and repeat the results across laboratories. A standard protocol with details is needed.
Previous works described multiple methods to fix lenses or diffusers in front of the mouse eye, such as gluing9, stitching10 and head-mounted goggle frame11,12. We combined the exist head-mounted goggle technics11,12,13 with our newly designed frame to develop an ameliorated protocol for inducing robust and efficient experimental myopia in mice. The protocol can be applied to young mice soon after weaning at postnatal day 21 (p21). We also optimized the processes for stable and precise evaluation of phenotypes including the refraction and AL. We hope this standardized protocol can help to make myopic mice a more easily accessible model for myopia research.
Protocol
All procedures were approved by the Ethics Committee on Animal Research of the Keio University School of Medicine adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the Institutional Guidelines on Animal Experimentation at Keio University, and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for the use of animals in research.
1. Assembling the Eyeglasses for Mice
- Prepare parts needed for assembling the eyeglasses (Figure 1a). For each mouse, prepare all followings: one head-mounted nylon stick, one higher and one lower titanium frame, two lenses with proper powers according to the purpose of the experiment, one M1.4 size screw with the length of 10 mm, one nut for M1.4 size screw, one plain washer for M1.4 size screw with the thickness of 0.3 mm, and two artificial fingernail tips (Table of Materials).
NOTE: Two kinds of frames exist with different heights. For one mouse, one higher frame and one lower frame are used as a set. Higher frames should be put above the lower ones in order to make the vision fields symmetrical. Frames and lenses are specially designed and manufactured by authors’ research group. These are available by contacting the corresponding authors of this article. Other parts can be found in retail tool shops. - Assemble the frame for the right and left eye separately.
- Arrange the fingernail tip to face the outside direction to achieve the best protective effect. Adjust the angle between the fingernail tip and the frame to be about 130° to avoid masking the mouse vision field (Figure 1b). Adhere the artificial fingernail tips to the frame using cyanoacrylate adhesive.
NOTE: Fingernail tips can help to protect the lens from being scratched by the mouse. The direction of the fingernail tips must be opposite because the higher frame and the lower frame are used for the right eye and the left eye, respectively. - Wait for at least 12 h to let the adhesive dry completely, then use a nail clipper to adjust the shape of the fingernail tips to be about 1 cm × 1 cm by cutting and trimming.
- Adhere the lens to the frame using cyanoacrylate adhesive. Put the lens onto the frame by hand and hold the frame horizontally. Inject the cyanoacrylate adhesive from the edge of the lens and let the adhesive spread by surface tension.
NOTE: The adhesive will erode the lens and influence its transparency, so make sure the adhesive only touches the peripheral part of the lens. Use 0 diopter (D) plano lens as control and minus lens to induce myopia. The effect of different powers of minus lenses (–10 D to –50 D) has been described elsewhere4. To maximize the myopia inducement, –30 D lens is recommended for inducing myopia. FDM can also be induced with the same device simply by changing the lens into the cap of 1.5 mL microtube as the diffuser.
- Arrange the fingernail tip to face the outside direction to achieve the best protective effect. Adjust the angle between the fingernail tip and the frame to be about 130° to avoid masking the mouse vision field (Figure 1b). Adhere the artificial fingernail tips to the frame using cyanoacrylate adhesive.
- Tighten the screw into the nylon stick together with the washer from the flat side of the stick. Prepare the eye glasses and sticks before the experiment respectively and stock for further use (Figure 1c).
NOTE: The protocol can be paused here.
2. Measurements of the Refraction and AL Baseline.
- Apply one drop of mydriatic agent containing 5 mg/mL tropicamide and 5 mg/mL phenylephrine hydrochloride on each side of the eyeball to dilate the pupil. Wait for at least 5 min before general anesthesia.
NOTE: Dilate the pupil under general anesthesia will cause reversible cataract which influences the measurement afterwards. Always dilate the pupil before general anesthesia and wait for at least 5 min. One drop (approximately 0.05 mL) of the mydriatic agent is enough for dilating the pupil of the mouse eye, while more agent will do no harm to the following steps. - Put the mouse under general anesthesia. Grasp the back skin of the mouse softly to keep the mouse from bumping its eye until it is completely anaesthetized. Judge the depth of general anesthesia to be enough if the mouse does not move around when it is put on the table freely.
NOTE: A combination of midazolam (40 μg/100 μL), medetomidine (7.5 μg/100 μL) and butorphanol tartrate (50 μg/100 μL) is used here for general anesthesia for mice with a volume of 0.01 mL/g intraperitoneal injection. Generally, less than 5 min are needed for mice to fall asleep. Long time of general anesthesia causes low body temperature and slow heartbeat. To avoid the potential influence of general anesthesia to the measurement, finishing all the measurements within 10 min after the injection of anesthetic is recommended. The butorphanol provided in the anesthetic cocktail provide approximately 4 hours of analgesia. Proper recipe for general anesthesia may different by institutions. - Measure the refraction of the mouse eye using an infrared photorefractor4,9,14. Adjust the direction of one of the mouse eyes to keep the first Purkinje image in the center of the pupil and measure the refraction along the optical axis. After the measurement, measure the opposite eye with the same procedures (Figure 2a).
NOTE: The value measured for the refraction is strongly influenced by the position of the mouse eye along the optic axis. Ensure the first Purkinje image is aligned within ±3 degrees from the center of the pupil. The workbench of the spectral domain optical coherence tomography (SD-OCT) system may be used for fine tuning of the direction of pupil in refraction measurements4. - Measure the AL of the mouse eye using a SD-OCT system4,15,16. Define the AL as the distance from the corneal vertex to the brightest boundary near the optical nerve (Figure 2b).
NOTE: Similar with the measurement of the refraction, the AL is strongly influenced by the direction of the mouse eye. The corneal vertex can be confirmed by the point-like reflection on the surface of the cornea. The boundary of the retina side will be very hazy at the point of optical nerve. Adjust the direction a little to let the boundary be clear enough for measuring but not too far from the optical nerve.
3. Fixation of the Frame onto the Mouse Cranium.
- Apply one drop of 0.1% purified sodium hyaluronate eye drop to prevent dryness on each eye.
NOTE: Eye drop on the eye surface will influence the values of refraction and axial length measurements. Do not use the eye drop after the anesthesia until all the measurements are done. - Put the chin of the anesthetized mouse onto a slope made of clay or anything can be used as a pillow to make the upfront plane of the cranium be horizontal.
- Sterilize the hair and scalp between ears and eyes using cotton swab with 70% ethanol.
- Cut the skin between ears and eyes to expose the skull for about 0.8 cm2 using surgical scissors and tweezers. Use dental etching liquid and cotton swab to remove the periosteum. Sterilize the surgical scissors and tweezers with 70% ethanol before the surgery of each mouse.
NOTE: Clipping the hair and wearing sterile gloves may be required. Check the provisions of relevant animal care committee before this procedure. In most cases, the dental etching liquid can be found as one of the attachments in the self-cure dental adhesive system. If the dental etching liquid is not available, use 70% ethanol instead. - Assemble a set of frames without lens to help fix the stick onto the right position. Put the frames onto the mouse head. Carefully adjust the position of the frames to make sure both eyes are in the middle of the empty frame.
- Use a self-cure dental adhesive system to attach the stick onto the mouse head. Be careful not to let the liquid of the adhere system flow into the mouse eye.
NOTE: The method for using the self-cure dental adhesive system is different between products. Refer to the instruction carefully.
CAUTION: Some of the reagents in the dental adhesive system may be toxic. - Wait for about 5 min and take off the frame and the nut carefully without changing the position of the stick (Figure 3a).
- Inject 0.75 mg/kg atipamezole hydrochloride intraperitoneally to help the mouse to recovery from the anesthesia more quickly. Put the mouse into an individual cage until fully recovered. Do not leave the mouse unattended until it has regained sufficient consciousness to maintain sternal recumbency. The protocol can be paused here.
4. Initiation of the Myopia Induction and the Maintenance Afterwards
- Wait for at least 24 h after the surgery to let the mouse recover from anesthesia completely. Grasp the stick and put on the frames with lenses. The inducement begins at this time point (Figure 3b).
NOTE: To minimize the discomfort during the recovery from anesthesia and give enough time for the dental adhesive system to coagulate, it is highly recommended to put on the frame after the mice fully recovered from anesthesia. The resin cement of the adhesive system will fully cover the cut of the scalp. Therefore, no specific post-surgical treatment is needed for preventing infection and ameliorating post-surgical pain. Right after the first time of putting on the frame, mice will be over conscious to the existence of the lens and scratch it a lot. The gross behavior will be back to normal after a few hours. The mice with lenses can be kept with the same density to the normal mice. - Remove the frame and clean the lenses and fingernail tips with cotton swabs at least twice a week to maintain the transparency of the lens.
NOTE: It is not necessary to put the mouse under anesthesia for removing and putting on the frame. Provide a support for the mice to sit on, rather than having them hang while working with them could reduce distress.- If an interim measurement is necessary, put the mouse under anesthesia and measure the mouse as previously described. The inducement can be continued after the mouse recovering from the anesthesia.
- Clean the cage at least twice a week.
NOTE: This help to maintain lens clarity. Putting a net between the mouse and the cage bottom to filtrate the food scraps may also be helpful. - Monitor the body weight and gross behavior. Compare them with the same age untouched mice during the whole experimental process.
NOTE: Except for some scratching behaviors, no significant change including the body weight is found between experiment mice with untreated mice.
Representative Results
At first, check if all the necessary parts are prepared (Figure 1a). An example of a piece of assembled eyeglasses is shown in Figure 1b. Except for the main body of the frames and the nut, all other parts are disposable for each mouse. A set of completed eyeglasses is shown in Figure 1c. Change the angle between the two frames to fit the mouse with different ages.
An example of the refraction measurement is shown in Figure 2a. This is a mouse eye induced by a -30 D lens for 3 weeks starting from p21. Note that the gaze shall be controlled close to 0 degree in both x and y axis which means that the mouse is seeing right in front of the camera9. Figure 2b shows an example of a whole eye image taken by a SD-OCT system tuned for mice and the definition of each part of the eye. Generally, SD-OCT systems present actual measured value directly.
An image of the stick adhered to the mouse head is shown in Figure 3a. The border of the cut should be at least 3 mm away from the eye to avoid influencing the function of the eyelid. The stick stays on the mouse head until the end of the inducement and provides the foundation to fix the eyeglasses. The adhesive strength of the dental adhesive system is supposed to be enough for the fixation of eyeglasses during the whole experiment for about 3 to 4 weeks. Assemble the eyeglasses as shown in Figure 3b. Two pieces of eyeglasses should be fairly symmetrical.
Presentative results are shown in Figure 4. In this experiment, 0 D plano lenses were fixed in front of the left eyes as internal controls and -30 D lenses were fixed in front of the right eye to induce myopia (C57B6/J mice, inducement started from p21, n = 4). Refractions and ALs were measured once a week after the beginning of the inducement for three weeks. Compared with 0 D plano lenses, -30 D induced a strong myopic shift in both refraction and AL. The change of refraction reached to the peak in the first week and stayed flat for the following two weeks. The difference between the change of myopic eyes and control eyes in AL was significant from the first week and became larger and larger in the following two weeks.

Figure 1: The design of the skull-mounted eyeglasses. (a) All parts needed for assembling the mouse glasses. (b) An example of the position of the frame and the nail tip for the right side of the eye. (c) An example of one set of assembled glasses. Adjust the joint part to change the angle of the frames to fit for mice in different ages. This figure has been modified from Jiang, X. et al4, available under a Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/. Please click here to view a larger version of this figure.
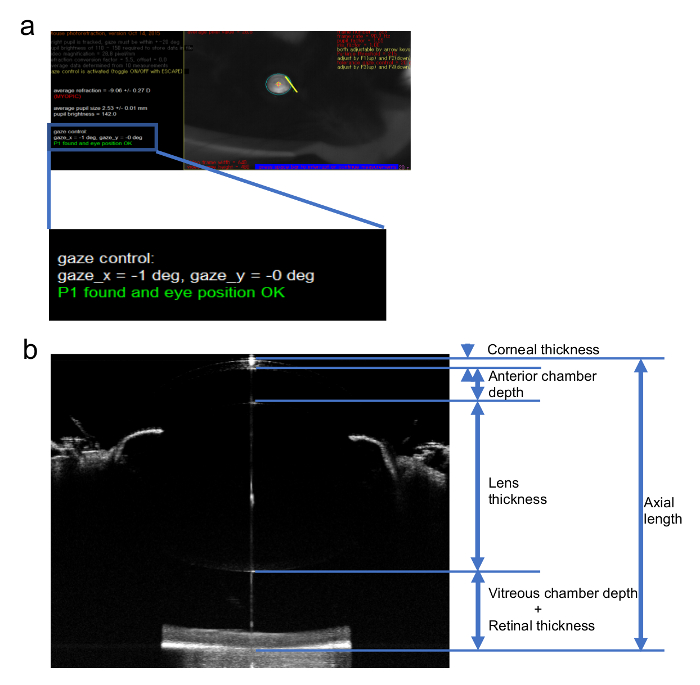
Figure 2: Sample images of the measurement of the refraction and AL. (a) An image of the refraction measurement for a myopic eye using an infrared photorefractor. (b) A sample image of an AL measurement of the mouse eye using a SD-OCT system and the definition of each part of the eye ball. This figure has been modified from Jiang, X. et al4, available under a Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/. Please click here to view a larger version of this figure.
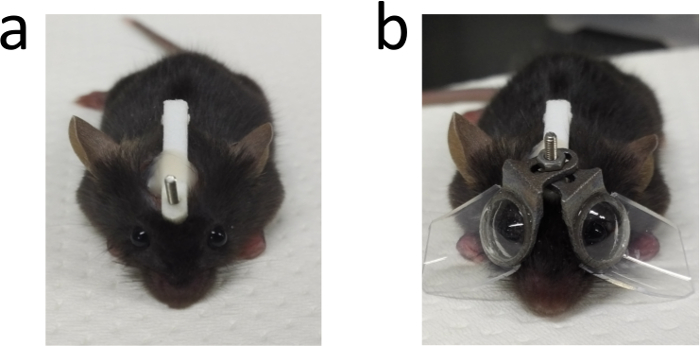
Figure 3: An image of one mouse after the surgery. (a) An example of one mouse after the surgery for adhering the stick. (b) One mouse with eyeglasses in both sides. This figure has been modified from Jiang, X. et al4, available under a Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/. Please click here to view a larger version of this figure.
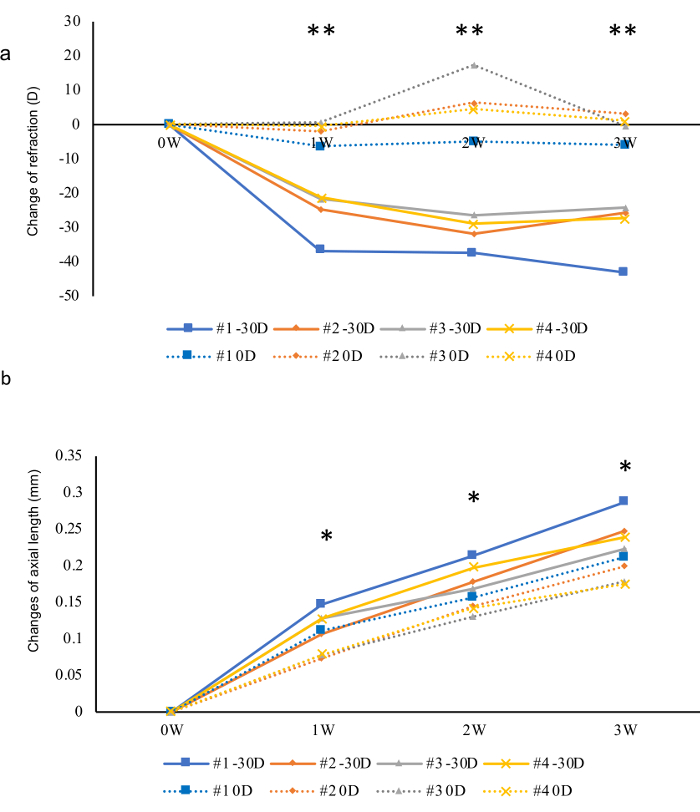
Figure 4: Presentative results of a three-week follow-up of myopia inducement in four mice using -30 D lenses and 0 D lenses as internal controls. (a) Changes of refractions for three weeks. A sudden change can be observed one week after the inducement. (b) In contrast, changes in AL were relatively mild. Asterisk marks showed the statistical significances between the eyes wearing 0 D and -30 D lenses in each week, respectively. *p < 0.05, **p < 0.01. Student t-test. Please click here to view a larger version of this figure.
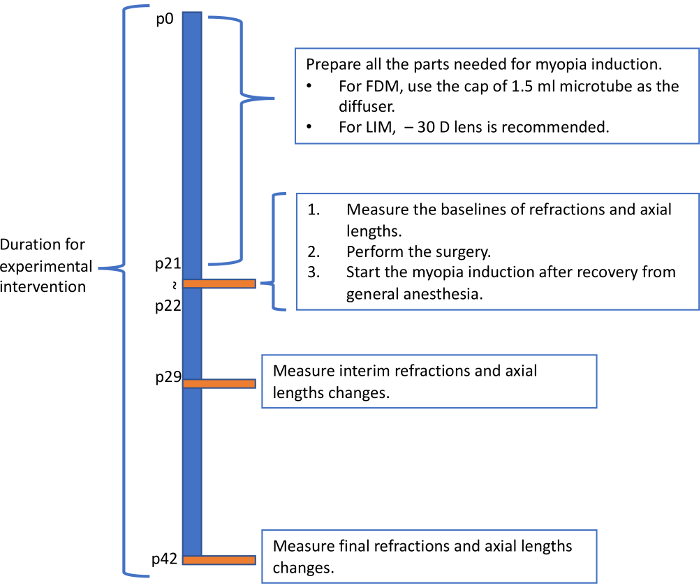
Figure 5: Recommended flowchart for inducing myopia in mice. The flowchart showed here represent one possible pattern for inducing myopia in mice. Please click here to view a larger version of this figure.
Discussion
To make sure the eyeglasses to be fixed stably on the mouse head, several steps in this protocol need to be paid great attention. The periosteum must be removed completely before using the dental adhesive system. The blood on the skull also need to be cleaned up with care. While a little fine tuning is acceptable right after the application of the adhesive, do not move the stick frequently before the adhesive system dry up. Follow the instruction of the adhesive system carefully, especially the ratio of each component of the final mixture. When grasp the mouse during the maintenance of the eyeglasses or the follow-up measurements after the surgery, do not grasp the stick with the body of the mouse together. The relative movement between the skull and the stick is the most frequent cause of the dropping down of the stick. With proper surgery and grasping skill, the number of individuals that drop down the stick would be less than one in a 3-week experiment including 20 individuals.
Hairs and food scraps may slip into the interval between the eye and the lens. This will not only influence the transparency of the lens, but also potentially damage the cornea surface physically. To keep the transparency of the lens, at least two times of cleaning are necessary each week. This will also give mice the chance to groom face to reduce the incidence of complications to the cornea. Leave a space for about 1 mm between the frame and the skin to allow the mouse to blink. The frame may make it hard for mice to reach the food outside the cage through the grid above. Therefore, it is recommended to put food on the floor of the cage directly. Change the cage into clean one for about twice a week will also help to keep the lenses clean. Dirty lenses become diffusers inducing myopia through form-deprivation mechanism. This may skew the data and make the result to be hard to interpret since evidences showed that FDM and LIM are different model systems in etiology3.
Due to the small size of the mouse eye, the measured eye parameters tend to be unstable. We recommend the infrared photorefractor developed by Dr. Schaeffel for the refraction measurement9 and a SDOCT system for AL4 (see Table of Materials). Both systems present enough information for judging the reliability of the measured value to ensure the repeatability of the measurement. For refraction, as argued in previous reports, it is extremely important to maintain the measurement on-axis4,14. Keeping the gaze control within ±3 degrees could receive stable results. To achieve this precise measurement, the tube of the SDOCT system might be helpful when tweaking the direction of the mouse eye. For AL measurement, the reflected light on the corneal vertex and the brightest boundary near the optical nerve are two reliable anatomical marks that ensure the repeatability of this measurement.
The protocol described here induced significant myopic states in mice within one week. Although the mechanism is still unknown, the change in refraction and AL are not necessarily parallel. Since the AL kept growing myopic after one week, we recommend the measurement to be done three times in total: before, one week and three weeks after the inducement. One of the example flowcharts for myopia induction is showed in Figure 5. Based on previous report4, starting from p21 and using – 30 D lenses are recommended for LIM. Experiments last for over 4 weeks may have high incidence of severe cornea complications that influence the measured value and accelerate the consumption of lenses. The most frequent complication is corneal scarring. Mice cannot clean their faces and eyelids wearing the frame, which might be the reason for the complication.
The device described in this protocol can be applied to mice right after weaning (p21). This may partially contribute to the strong phenotype observed4. The frame is removable without general anesthesia. This allows not only the researcher to clean up the lenses easily, but also make the topical drug delivery possible4. Another strength is that the lenses can be easily changed into diffusers or lenses with different diopters (e.g., plus lenses), which extends the possibilities of this method. One limitation of this method is that the surgery needs some practice before the frames can be adhered stably for the whole experiment. The measurements also need patience to collect reliable data.
Mice are irreplaceable animal model with its possibility of genetic manipulation and the low cost for breeding. We hope the protocol described here could make the experimental myopia in mice a more practical model for myopia research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank M.T. Pardue for advice on the SDOCT, F. Schaeffel for advice on measurements of refraction and corneal curvature, Mr. Sanshouo for recreating the three-dimensional frame data, M. Miyauchi; K. Tsubota; Y. Tanaka; S. Kondo; C. Shoda; M. Ibuki; Y. Miwa; Y. Hagiwara; A. Ishida; Y. Tomita; Y. Katada; E. Yotsukura; K. Takahashi; and Y. Wang for critical discussions. This work was supported by Grants inAid for Scientific Research (KAKENHI, number 15K10881) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to TK. This work is also supported by the grant for myopia research from Tsubota Laboratory, Inc. (Tokyo Japan).
Materials
| screw | NBK | SNZS-M1.4-10 | |
| washer | MonotaRO | 42166397 | |
| nut | MonotaRO | 42214243 | |
| stick | DMM Make | none | designed by authers and output by the 3D printer rented from DMM Make. |
| frame | DMM Make | none | designed by authers and output by the 3D printer rented from DMM Make. |
| lenses | RAINBOW CONTACT LENS | none | customized for mice use by the company |
| cyanoacrylate glue | OK MODEL | MP 20g | |
| dental adhesive resin cement | SUN MEDICAL | super bond | contains the etching liquid used for removing the periosteum of the mouse skull |
| infrared photorefractor | Steinbeis Transfer Center | none | designed and offered by Dr. Frank Schaeffel from university of Tübingen |
| Spectral domain OCT | Leica | R4310 | |
| Tropicamide, Penylephrine Hydrochloride solution | Santen | Mydrin-P | |
| midazolam | Sandoz K.K. | SANDOZ | components for the anesthetic |
| medetomidine | Orion Corporation | Domitor | components for the anesthetic |
| butorphanol tartrate | Meiji Seika Pharma | Vetorphale | components for the anesthetic |
| 0.1 % purified sodium hyaluronate | Santen | Hyalein | |
| atipamezole hydrochloride | Zenoaq | antisedan |
References
- Dolgin, E. The myopia boom. Nature. 519 (7543), 276-278 (2015).
- Ohno-Matsui, K. Pathologic Myopia. Asia-Pacific Journal of Ophthalmology (Philadelphia, Pa). 5 (6), 415-423 (2016).
- Morgan, I. G., Ashby, R. S., Nickla, D. L. Form deprivation and lens-induced myopia: are they different. Ophthalmic & Physiological Optics. 33 (3), 355-361 (2013).
- Jiang, X., et al. A highly efficient murine model of experimental myopia. Scientific Reports. 8 (1), 2026 (2018).
- Torii, H., et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine. 15, 210-219 (2017).
- Smith, E. L., et al. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Investigative Ophthalmology & Visual Science. 56 (11), 6490-6500 (2015).
- Gawne, T. J., Siegwart, J. T., Ward, A. H., Norton, T. T. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Experimental Eye Research. 155, 75-84 (2017).
- Wu, Y., et al. Early quantitative profiling of differential retinal protein expression in lens-induced myopia in guinea pig using fluorescence difference two-dimensional gel electrophoresis. Molecular Medicine Reports. , (2018).
- Schaeffel, F., Burkhardt, E., Howland, H. C., Williams, R. W. Measurement of refractive state and deprivation myopia in two strains of mice. Optometry and Vision Science. 81 (2), 99-110 (2004).
- Tkatchenko, T. V., Shen, Y., Tkatchenko, A. V. Mouse experimental myopia has features of primate myopia. Investigative Ophthalmology & Visual Science. 51 (3), 1297-1303 (2010).
- Faulkner, A. E., Kim, M. K., Iuvone, P. M., Pardue, M. T. Head-mounted goggles for murine form deprivation myopia. Journal of Neuroscience Methods. 161 (1), 96-100 (2007).
- Gu, Y., et al. A Head-Mounted Spectacle Frame for the Study of Mouse Lens-Induced Myopia. Journal of Ophthalmology. 2016, 8497278 (2016).
- Siegwart, J. T., Norton, T. T. Goggles for controlling the visual environment of small animals. Laboratory Animal Science. 44 (3), 292-294 (1994).
- Tkatchenko, T. V., Tkatchenko, A. V. Ketamine-xylazine anesthesia causes hyperopic refractive shift in mice. Journal of Neuroscience Methods. 193 (1), 67-71 (2010).
- Chou, T. H., et al. Postnatal elongation of eye size in DBA/2J mice compared with C57BL/6J mice: in vivo analysis with whole-eye OCT. Investigative Ophthalmology & Visual Science. 52 (6), 3604-3612 (2011).
- Park, H., et al. Assessment of axial length measurements in mouse eyes. Optometry and Vision Science. 89 (3), 296-303 (2012).

