Establishing a Co-Culture of Interneurons with Periventricular Endothelial Cells
Abstract
Source: Datta, D., et al. Migration, Chemo-Attraction, and Co-Culture Assays for Human Stem Cell-Derived Endothelial Cells and GABAergic Neurons. J. Vis. Exp. (2020)
This video demonstrates a technique for establishing a co-culture of interneurons with perivascular endothelial cells. The cells are added to a one-well insert placed inside a culture dish. After the cells adhere to the dish, the insert is removed, allowing the cells to proliferate and interact and form a co-culture.
Protocol
1. Culture and Storage of Human Periventricular Endothelial Cells
- Maintain human periventricular endothelial cells on basement membrane matrix-coated (see Table of Materials) 6-well plates in periventricular endothelial cell medium (E6 medium containing 50 ng/mL vascular endothelial growth factor A [VEGF-A], 100 ng/mL fibroblast growth factor 2 [FGF2] and 5 µM gamma-aminobutyric acid [GABA]) at 37 °C and 5% CO2. Change medium every alternate day.
- Thaw basement membrane matrix in 4 °C, and make a 1:100 solution by diluting it in cold Dulbecco's modified Eagle medium/nutrient mixture F-12 (DMEM/F12) medium. Coat each well of a 6-well plate with 1 mL of matrix solution. Incubate plates at 37 °C for at least 1 h before use.
- Allow human periventricular endothelial cells to reach a confluency of 80%-90%. Aspirate medium from the well. Wash the wells once with 1 mL of sterile 1x phosphate-buffered saline (PBS) per well.
- Detach cells by adding 1 mL of cell dissociation solution (see Table of Materials) per well. Incubate at 37 °C for 5 min. After 5 min, add 1 mL of periventricular endothelial cell medium. Transfer the cell solution into a 15 mL conical tube.
NOTE: We use Accutase for cell dissociation here, as opposed to TrypLE in sections 3 and 4. - Centrifuge cells at 500 x g for 5 min at room temperature, aspirate the supernatant and resuspend the cell pellet in 1 mL of periventricular endothelial cell medium.
- Count live cells using the trypan blue exclusion method. Seed cells in fresh matrix-coated plates at a density of 1.2 x 105 cells/cm2. Incubate at 37 °C and 5% CO2.
- Store human periventricular endothelial cells by cryopreserving in freezing medium (90% periventricular endothelial cell medium and 10% dimethyl sulfoxide [DMSO]).
- Dissociate and collect cells following steps 1.3 and 1.4 above. Count cells in the solution by the trypan blue exclusion method.
- Centrifuge cells at 500 x g for 5 min at room temperature. Aspirate the supernatant and resuspend the cell pellet at 5 x 106 cells/ mL of freezing medium.
- Dispense 1 mL of freezing medium plus cells per cryovial. Place the vials in isopropanol-filled chamber and cool overnight in -80 °C at 1 °C/min. Transfer vials to a liquid nitrogen tank on the next day for long term storage.
2. Preparation of Human Periventricular Endothelial Cells for Assay
- Allow human periventricular endothelial cells to reach 70%-80% confluency.
- Dissociate cells following steps 1.3 to 1.5 as described above. Count cells using the trypan blue exclusion method.
3. Preparation of Human GABAergic Interneurons for Assay
NOTE: Human induced pluripotent stem cell (iPSC)-derived GABAergic interneurons and the neuronal medium were commercially purchased (see Table of Materials). The neurons are generated by differentiating a human fibroblast-derived iPSC line following a protocol developed by the manufacturer. The cells were thawed and cultured according to manufacturer's protocol.
- Thaw human GABAergic interneurons and culture them in 12-well plate for two weeks to a confluency of 70%-80%.
- On the day of the assay, warm cell dissociation solution (see Table of Materials) and an aliquot of neuronal medium at 37 °C for 10 min before use.
- Aspirate medium from each well containing the cells. Wash cells with 1 mL of sterile 1x PBS per well.
- Detach cells by adding 0.5 mL of pre-warmed dissociation solution per well and incubate at 37 °C for 5 min. Add 1 mL of neuronal medium per well. Transfer cell solution into a 15 mL conical tube. Gently triturate to dissociate cell clumps.
- Centrifuge cells at 380 x g for 5 min at room temperature, aspirate the supernatant and resuspend the cell pellet in 1 mL of neuronal medium. Count live cells using the trypan blue exclusion method.
4. Preparation of Control Human Endothelial Cells for Assay
NOTE: Control human iPSC-derived endothelial cells and endothelial cell medium were commercially purchased (Table of Materials). These endothelial cells are generated by differentiating a human fibroblast-derived iPSC line to endothelial fate following a protocol developed by the manufacturer. The cells were thawed and cultured on Fibronectin substrate according to manufacturer's protocol. Fibronectin-coated plates were prepared following manufacturer's protocol.
- Thaw control human endothelial cells and culture them in 6-well plate to a confluency of 80%-90%.
- On the day of the assay, warm cell dissociation solution (see Table of Materials) and an aliquot of endothelial medium at 37 °C for 10 min before use.
- Aspirate the medium from each well containing the cells. Wash cells with 1 mL of sterile 1x PBS per well.
- Detach cells by adding 0.5 mL of pre-warmed dissociation solution per well. Incubate at room temperature for 5 min. Add 1 mL of endothelial cell medium per well to neutralize the dissociation solution. Transfer cell solution into a 15 mL conical tube.
- Centrifuge cells at 200 x g for 5 min at room temperature. Aspirate supernatant and resuspend the cell pellet in 1 mL of endothelial cell medium. Count live cells using the trypan blue exclusion method.
5. Preparation of One-well Culture Inserts
- Thaw 1 mg/mL laminin solution at room temperature or overnight at 4 °C.
- Coat an appropriate number of 35 mm dishes with 0.01% poly-L-ornithine solution (1 mL per dish). Incubate the dishes in room temperature for at least 1 h.
- Dilute 1 mg/mL laminin solution 1:300 in sterile water to a final concentration of 3.3 µg/mL immediately before use.
- Completely aspirate poly-L-ornithine from each dish. Rinse each dish thoroughly 3x with sterile water and aspirate completely to avoid poly-L-ornithine-induced cell toxicity.
- Add 1 mL of 3.3 µg/mL laminin solution to each dish and incubate at 37 °C overnight or at least 1 h. Remove laminin solution from the dish immediately before use.
NOTE: Alternatively, store the laminin-containing dishes in 4 °C. Equilibrate the dishes in a 37 °C cell culture incubator before use. - Cut three sides of one well of a two-well silicone culture insert (Figure 1A) using a sterile blade to generate a one-well insert (Figure 1B).
NOTE: Keep the two-well insert firmly attached to the surface of the original packaging while cutting to ensure a smooth cut and protect the adhesiveness of the insert. - Aspirate laminin solution from the dishes.
NOTE: Do not wash the dishes with sterile PBS or water after laminin incubation. Wet surfaces will prevent tight adhesion of the culture insert. - Remove one-well insert with sterile tweezers and place it in the center of the poly-L-ornithine/laminin coated dish. Press along the edges of the insert to fix it to the surface of the dish.
- Carefully turn the dish upside down to verify that the insert is firmly adhered.
- Keep the dish upside down and mark the boundary of the insert compartment using a permanent black marker with an ultra-fine tip (Figure 1C).
6. Co-culture Migration Assay
- Co-suspend 3 x 104 GABAergic interneurons and 3 x 104 human periventricular endothelial cells in 70 µL of co-culture medium (50% periventricular maintenance medium without GABA and 50% neuronal medium). Seed this cell solution inside the one-well insert compartment. Prepare an appropriate number of such assay dishes.
NOTE: GABA was not added in the co-culture medium to exclude the effect of exogenous GABA on migration. - Check under a microscope to verify that cells are not leaking from the insert compartment.
- Slowly add 1 mL of co-culture medium along the side of the dish to prevent the coating from drying.
NOTE: Add medium slowly along the edge of the dish so that the insert is not disturbed. - As a first control, seed 3 x 104 human GABAergic interneurons only in 70 µL of co-culture medium per one-well insert. Prepare an appropriate number of such dishes.
- As a second control, co-seed 3 x 104 GABAergic human interneurons with 3 x 104 control human endothelial cells in 70 µL of co-culture medium per one-well insert. Prepare an appropriate number of dishes.
- Incubate the dishes for 24 h at 37 °C and 5% CO2. After 24 h incubation, check under a microscope to verify that cells have attached properly and there is no leak.
- After 48 h of seeding, gently remove the insert using a sterile tweezer. Check under the microscope to verify that the cell layer is not disturbed (day 0).
- Remove medium and add 1 mL of fresh co-culture medium.
NOTE: Set aside an appropriate number of dishes for acquiring day 0 images. - Incubate cells for 5 days at 37 °C and 5% CO2.
Representative Results
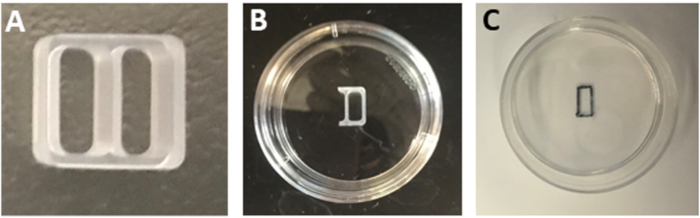
Figure 1: Preparation of the culture insert. (A) A two-well culture insert. (B) A one-well insert fixed in the center of a 35 mm dish. (C) The outline of the rectangular patch as observed after removing the insert.
Disclosures
The authors have nothing to disclose.
Materials
| Accutase dissociation solution | Millipore Sigma | SCR005 | Cell dissociation solution (for periventricular endothelial cells, step 1.4) |
| Control human endothelial cells | Cellular Dynamics | R1022 | |
| Control endothelial Cells Medium Supplement | Cellular Dynamics | M1019 | |
| Cryogenic vials | Fisher Scientific | 03-337-7Y | |
| DMEMF/12 medium | Thermofisher Scientific | 11320033 | |
| DMSO | Sigma-Aldrich | D2650 | |
| E6 medium | Thermofisher Scientific | A1516401 | |
| FGF2 | Thermofisher Scientific | PHG0261 | |
| Fibronectin | Thermofisher Scientific | 33016-015 | |
| Freezing Container | Thermofisher Scientific | 5100 | |
| GABA | Sigma-Aldrich | A2129 | |
| Hemacytometer | Sigma-Aldrich | Z359629 | |
| Human GABAergic neurons | Cellular Dynamics | R1013 | |
| Human GABAergic neurons base medium | Cellular Dynamics | M1010 | |
| Human GABAergic neuron Neural supplement | Cellular Dynamics | M1032 | |
| Laminin | Sigma | L2020 | |
| Matrigel | Corning | 356230 | Basement membrane matrix |
| Mounting Medium | Vector laboratories | H-1200 | |
| poly-L-ornithin | Sigma | p4957 | |
| PBS | Thermofisher Scientific | 14190 | |
| Trypan blue | Thermofisher Scientific | 15250061 | |
| TrypLE | Thermofisher Scientific | 12563011 | Cell dissociation solution (for GABAergic interneurons and endothelial cells, sections 3 and 4) |
| VEGF-A | Peprotech | 100-20 | |
| VascuLife VEGF Medium Complete Kit | Lifeline Cell Technologies | LL-0003 | Component of control human endothelial cell medium |
| 2-well silicone culture-Insert | ibidi | 80209 | |
| 3-well silicone culture-Insert | ibidi | 80369 | |
| 35 mm dish | Corning | 430165 | |
| 15-ml conical tube | Fisher Scientific | 07-200-886 | |
| 4% PFA solution | Fisher Scientific | AAJ19943K2 | |
| 6-well tissue culture plate | Fisher Scientific | 14-832-11 |

