A Technique for Human Skin Grafting in a Mouse Model
Abstract
Source: Moss, M. I. et al., Xenograft Skin Model to Manipulate Human Immune Responses In Vivo. J. Vis. Exp. (2022)
This video demonstrates a method for grafting human skin onto an immunodeficient mouse with a suppressed neutrophil response. The method involves applying human split-thickness skin to the mouse's graft bed, sealing it, and covering it for recovery. Eventually, the human skin adheres to the mouse's skin, establishing vascularization and circulation, resulting in a successful skin xenograft.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Processing of donor human skin sample
NOTE: The human skin sample used in this transplant was a large sample collected from the abdomen of a healthy patient. The sample must be at least 15 cm x 7.5 cm. Size limitations may affect the number of mice for which skin is available and the choice of graft size.
- Maintain the skin sample at cold temperatures (on ice; 4°C) before preparation and grafting. Keep the sample moist in a closed specimen collection cup with the gauze soaked in phosphate-buffered saline (PBS).
NOTE: Storing the skin sample at 4 °C for longer than 2 days is not recommended. However, reports exist where the skin samples are stored for a longer time.
CAUTION: Treat all human tissue with standard biohazard precautions. - Prepare to dermatome the human skin sample in a sterilized negative pressure tissue culture hood on a sterilized dissection board.
- Place the skin sample, epidermis side up, on the dissection board. Wipe the epidermis with a sterile alcohol prep pad and then with PBS.
- Pin the closer edge of the skin in place with a 1.5-inch dissecting T-pin (see Table of Materials).
- Dermatome the skin specimen at a 400 µm thickness, applying steady pressure while cutting forward at a 30°-45° angle. Follow all instrument-specific instructions and safety measures (see Table of Materials).
NOTE: For details on the dermatome technique, please see a previously published report. - Prepare a 100 mm x 20 mm Petri dish by placing a sterile gauze soaked in sterile PBS at the bottom of the dish. Place the skin, epidermis side up, onto the wet gauze.
- Seal and cover the plate edges with a semi-transparent sealing film (see Table of Materials) to ensure the sample is not contaminated. Store the sample at 4° C prior to grafting.
2. Pre-surgery conditioning and preparation
- Prepare the sterile instruments and a sterile surgical station for grafting. Use autoclaved paper towels as sterile surfaces for instrument and mouse placement.
NOTE: Mice may be grafted after weaning but are preferably grafted between 8-10 weeks of age. Mice of either sex may be grafted. - Perform the surgical preparation, such as hair removal, in an area physically separated from the surgical station.
- Prepare the anti-GR1 (see Table of Materials) by diluting it to 1 mg/mL in sterile saline. Dose each mouse with 100 µg/100 µL of the anti-GR1 solution intraperitoneally following anesthesia induction.
- Anesthetize the mice, one at a time, with isoflurane or other institutionally approved anesthetics.
NOTE: Isoflurane needs to be given at a 3%-5% concentration during induction. Once the mouse is immobile, lower the isoflurane concentration to 1%-3% to affect the duration of the surgery.- Monitor the mouse for appropriate depth of anesthesia by observing respiratory rate, absence of toe-pinch response, and appropriate pink coloration of ears and mouth.
CAUTION: Use appropriate anesthetic machinery and scavenging methods, and avoid exposure to isoflurane vapors.
- Monitor the mouse for appropriate depth of anesthesia by observing respiratory rate, absence of toe-pinch response, and appropriate pink coloration of ears and mouth.
- Transfer the mouse to a heating pad or another heat source (see Table of Materials).
- Administer the ophthalmic ointment by dabbing a small drop of ointment on the eye with a gloved finger.
- Administer analgesics Buprenorphine (0.08 mg/kg) and Carprofen (5 mg/kg) (see Table of Materials) subcutaneously by pinching the skin and injecting at an angle parallel to the body.
NOTE: Prepare the pre-treatment analgesia following institutional protocols. Follow institutional guidelines for the selection and administration of analgesics. The method of analgesia used in this study is outlined in step 2.7 and Figure 1. - Administer the anti-GR1 (prepared in step 2.4) intraperitoneally by slightly lifting the mouse by the tail, exposing the abdomen, and injecting at a 30° angle using an insulin 1 mL (12.7 mm) syringe.
- Use animal-safe electric clippers (see Table of Materials) to shave the middle and upper portions of the dorsal side of the mouse.
- Clear all the hair and apply a generous amount of hair removal ointment onto the shaved skin for 30 s to 1 min.
- Completely wipe away hair removal ointment with a paper towel and PBS.
3. Transplantation procedure
- Transfer the mouse to a secondary surgical location, away from the hair removal station.
- Sterilize the surgical site with the iodine swab stick in a circular motion, starting in the middle and working out toward the edge of the depilated area.
- Place a piece of sterile plastic wrap over the mouse and cut a window in the plastic slightly larger than the size of the area to be grafted.
- Cut a rectangle-shaped 10 mm x 10 mm portion of donor skin to be grafted with a scalpel. Do this by firmly holding the donor skin in place with the backside of the forceps and cutting alongside the forceps with the scalpel.
- Using the surgical scissors, snip a rectangular area of mouse skin matching the size of the donor skin piece, creating a graft bed. Use forceps to pull the skin away from the body, and cut the skin with the scissors angled away from the body to avoid cutting deeply into the facia.
- Place the donor skin piece, epidermis side up, onto the prepared graft bed.
- Using the back of the forceps, manipulate the skin, sliding back and forth until the donor skin lies completely flat against the graft bed.
- Add drops of surgical glue tissue adhesive (see Table of Materials) where the donor skin meets the mouse skin and hold the mouse and donor skin together with forceps for 1-2 s so that the glue adheres to the tissues. Completely seal the edge of the graft and allow for the glue to dry fully.
- Bandage the mice (Figure 2) following the steps below.
- Cut a piece of petrolatum gauze (see Table of Materials) large enough to cover the graft area completely.
- Cover the graft with the petrolatum gauze and lightly press the gauze against the skin using forceps.
- Cut a strip of a transparent film dressing lengthwise so that the width is large enough to cover the mouse's wound.
- Firmly press the transparent film dressing, adhesive side down, over the gauze. Quickly roll the mouse to wrap the dressing completely around the torso, ensuring it fits tightly without impeding respiration, and all limbs are free for movement.
- Place the mouse in a recovery cage and monitor it until it is alert and moving around. Provide a heat source on part of the cage for at least 15 min following recovery.
NOTE: The animals are expected to recover within 1-5 min after placing them in the recovery cage.
Representative Results
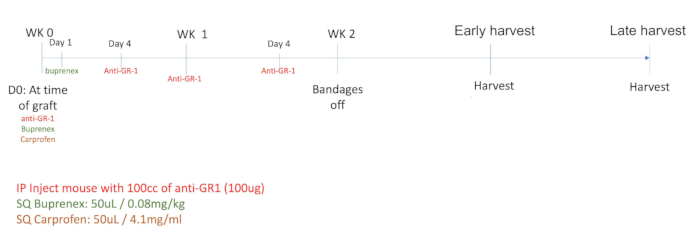
Figure 1: Timeline of the protocol adopted for the xenograft study.
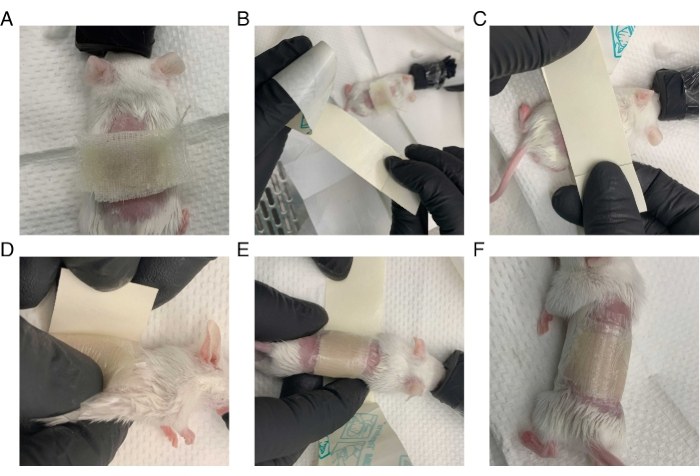
Figure 2: Method for bandaging mouse after surgery. The mouse is wrapped tightly in petrolatum gauze and transparent film dressing while under anesthesia. (A) Petrolatum gauze is placed over an area slightly larger than the surgical wound. (B) The transparent film dressing is prepared: a long strip is cut slightly wider than the petrolatum gauze. The backing covering the adhesive side of the transparent film dressing is partially removed. (C) The adhesive side of the film is firmly pressed against the back of the mouse, leaving an inch of space on the front end of the film. (D) The mouse is turned on its back. The overhanging front portion of the film is pressed onto the mouse, and the backing is removed. (E) The mouse is tightly wrapped in the film, and the remaining support is removed as the mouse is wrapped. (F) The mouse is successfully wrapped in the dressing, and its arms and legs are unconstrained.
Disclosures
The authors have nothing to disclose.
Materials
| 10% Neutral Buffered Formalin | Fisher | SF100-20 | Fixative for histology |
| 3M Vetbond Tissue Adhesive | 3M | 1469SB | Surgical glue |
| Anti-GR1 clone RB6-8C5 | BioXcell | BE0075 | Anti-rejection |
| Autoclave pouches | VWR | 89140-800 | For autoclaving tools and paper towels |
| Buprenex 0.3 mg/mL | Covetrus | 59122 | Analgesia |
| Carprofen 50 mg/mL | Zoetis | NADA # 141-199 | Analgesia |
| D42 Dermatome blade | Humeca | 5.D42BL10 | Dermatome (1 blade per sample) |
| Dermatome D42 | Humeca | 4.D42 | Dermatome |
| Disposable Scalpel | Bard-Parker | 371610 | skin preparation |
| Dissecting T-Pins; 1-1/2 inch, 1000/CS 1.5 | Cole-Parmer | UX-10915-03 | To pin skin specimen for dermatome |
| Dissection scissors | medicon | 02.04.10 | Sample preparation and mouse dissection |
| DNAse | Sigma-Aldrich | DN25-1G | Skin digestion |
| Electric clippers | Kent | CL8787-KIT | Hair removal |
| Forceps | medicon | 07.60.07 | Sample preparation and mouse dissection |
| Gauze | Fisherbrand | 22-362-178 | Sample preparation |
| Heating lamp | Morganville Scientific | HL0100 | Post-surgical care |
| Heating pads 4" x 10" | Pristech | 20415 | Surgical heat supply |
| Insulin 1cc 12.7 mm syringes | BD | 329410 | Drug administration |
| Isoflurane | United States Pharmacopeia (USP) | NDC 66794-013-25 | Anesthesia |
| Isoflurane machine | VetEquip | 911103 | Anesthesia |
| Nair for Men | Nair | 10022600588556 | Hair removal |
| Neomycin and Polymyxin Bisulfates and Bacitracin Zinc Ophthalmic ointment | Dechra | NDC 17478-235-35 | Eye ointment to prevent drying |
| NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice | The Jackson Laboratory | 5557 | Mice |
| Paper towels | Kleenex | 100848 | May be autoclaved for sterile surfaces |
| Parafilm | Fisher Scientific | 13-374-12 | Semitransparent sealing film |
| Petri Dish 150 mm | Corning | 430597 | Sample storage |
| Plastic Wrap | Fisherbrand | 22-305-654 | Site preparation |
| Providone-Iodine Swab stick | PDI | S41350 | Site sterilization |
| Soft-Feed and Oral Hydration (Napa Nectar) | Se Lab Group Inc | NC9066511 | For supplementing poorly recovering mice post-surgery |
| Specimen Collection Cups | Fisher Scientific | 22-150-266 | Sample storage |
| Sterile alcohol prep pad | Fisherbrand | 22-363-750 | Skin preparation |
| Sterile PBS | Gibco | 14190-144 | Media for sample storage |
| Sterile saline | Hospira | NDC 0409-4888-02 | For drug dilution |
| Tegaderm Film 4" x 43/4" | 3M | 1626 | Transparent film wound dressing |
| Vaseline Petrolatum Gauze 3" x 8" | Kendall | 414600 | Wound dressing |

