6.8:
Constant Pressure Calorimetry
77,396 Views
•
•
Calorimetry is a technique used to measure the amount of heat involved in a chemical or physical process or to measure the heat transferred to or from a substance. The heat is exchanged with a calibrated and insulated device called the calorimeter. Calorimetry experiments are based on the assumption that there is no heat exchange between the insulated calorimeter and the external environment. The well-insulated calorimeters prevent the transfer of heat between the calorimeter and its external environment, which effectively limits the “surroundings” to the nonsystem components within the calorimeter (and the calorimeter itself). This enables the accurate determination of the heat involved in chemical processes, such as the energy content of foods.
The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. In a calorimeter, a system is defined as the substance or substances undergoing the chemical or physical change, or in other words, the reaction, and the surroundings are all other matter, including the solution and any other components in the calorimeter that either provide heat to the system or absorb heat from the system.
Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose a hot piece of metal at a high-temperature is placed in a low-temperature substance, such as cool water. Heat will flow from the hot metal to the water. The temperature of the metal will decrease, and the temperature of the water will increase until the two substances have the same temperature—that is when they reach thermal equilibrium. If this occurs in a calorimeter, all of the heat is transferred between the two substances, with no heat gained or lost by its external environment. Under these ideal circumstances, the net heat change is zero:

This relationship can be rearranged to show that the heat gained by the metal is equal to the heat lost by substance the water:

The magnitude of the heat (change) is, therefore, the same for both substances. The negative sign merely shows that qmetal and qwater are opposite in direction of heat flow (gain or loss), but it does not indicate the arithmetic sign of either q value (that is determined by whether the matter in question gains or loses heat, per definition). In the specific situation described, qmetal is a negative value and qwater is a positive value since heat is transferred from the metal to the water.
When using calorimetry to determine the heat involved in a chemical reaction, the same principles apply. The amount of heat absorbed by the calorimeter is often small enough that it can most often be neglected, and the calorimeter minimizes energy exchange with the outside environment. When an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. When an endothermic reaction occurs, the heat required is absorbed from the thermal energy of the solution, which decreases its temperature. The temperature change (ΔT), along with the specific heat (csoln) and mass of the solution (msoln), can then be used to calculate the amount of heat (qsoln) involved in either case.
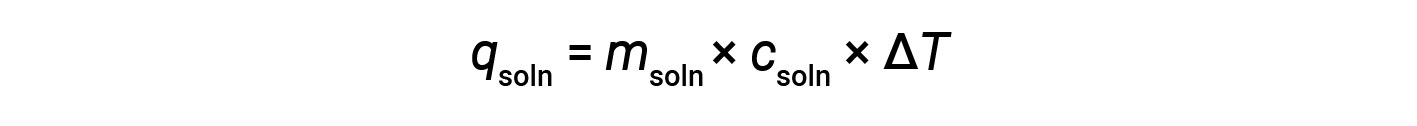
A simple calorimeter — called the coffee cup calorimeter — is constructed from two nested polystyrene cups closed with a loose-fitting lid. Coffee cup calorimeters are used to measure the heat of reactions that take place in solutions (mostly aqueous solutions) and involve no or very little volume change. Because energy is neither created nor destroyed during a chemical reaction, the heat produced or consumed in the reaction (the “system”), qrxn, plus the heat absorbed or lost by the solution (the “surroundings”), qsoln, must add up to zero:
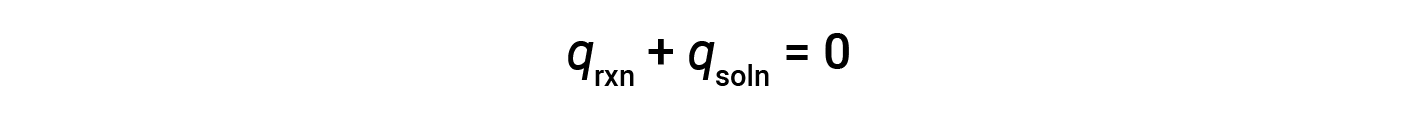
This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution:
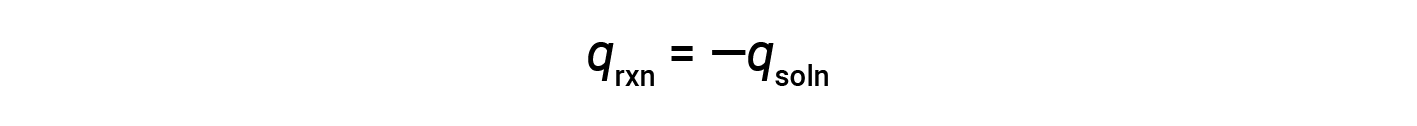
The coffee cup calorimeter is a constant-pressure calorimeter, and the measured heat of the reaction is equivalent to the change in enthalpy.

This text is adapted from Openstax, Chemistry 2e, Section 5.2: Calorimetry.
