4.10:
Oxidation-Reduction Reactions
57,704 Views
•
•
Oxidation–Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation–reduction (redox) reactions.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:

It is helpful to view the process with regard to each individual reactant, that is, to represent the fate of each reactant in the form of an equation called a half-reaction:

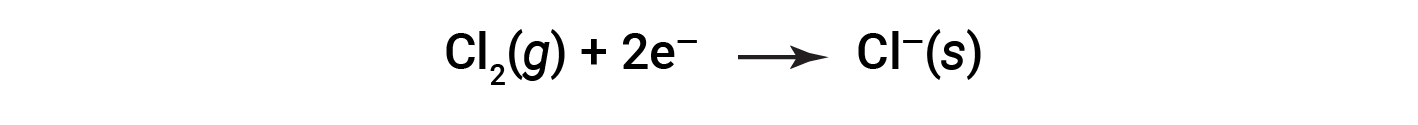
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “s” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
oxidation = loss of electrons
reduction = gain of electrons
In this reaction, sodium is oxidized and chlorine undergoes reduction. Viewed from a more active perspective, sodium functions as a reducing agent (reductant), since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant), as it effectively removes electrons from (oxidizes) sodium.
reducing agent = species that is oxidized
oxidizing agent = species that is reduced
In general, an oxidizing agent gains an electron from the reducing agent, and itself gets reduced. The charge of an oxidizing agent becomes more negative. Similarly, a reducing agent loses an electron to the oxidizing agent, and itself gets oxidized. The charge of a reducing agent becomes more positive.
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
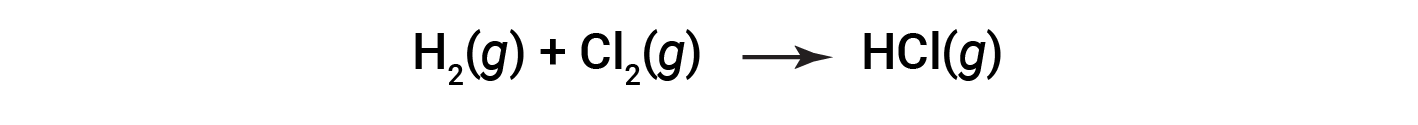
The product of this reaction is a covalent compound, so the transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined.
This text is adapted from Openstax, Chemistry 2e, Section 4.2: Classifying Chemical Reactions.
