Semi-Automated Planimetric Quantification of Dental Plaque Using an Intraoral Fluorescence Camera
概要
This study presents a semi-automated digital image analysis procedure for the planimetric quantification of disclosed dental plaque based on images acquired with an intraoral fluorescence camera. The method allows for the rapid and reliable quantification of dental plaque in the research environment.
Abstract
Dental plaque accumulation is quantified using clinical indices or, otherwise, the planimetric plaque index (PPI), which measures the relative area of a tooth that is covered by plaque deposits. Compared to clinical indices, the PPI has a higher discriminatory power, but traditional planimetry is a time-consuming analysis, as the plaque-covered and clean tooth areas have to be determined manually for each image using image-processing software. Here, we present a method for the semi-automated planimetric quantification of dental plaque, which allows for the rapid processing of up to 1,000 images simultaneously. The method exploits the enhanced contrast between disclosed plaque, sound tooth surfaces, and soft tissues in fluorescence images acquired with an intraoral camera. Careful execution of the clinical procedures and accurate image acquisition are crucial steps for the successful semi-automated identification of the plaque-covered areas. The method is suitable for planimetry on sound facial and oral tooth surfaces, on most composite resin restorations, and on teeth with orthodontic brackets, but not on metallic restorations. Compared to traditional PPI recordings, semi-automated planimetry considerably reduces the amount of time spent on the analysis, as well as the subjective human input, thus increasing the reproducibility of planimetric measurements.
Introduction
The quantification of dental plaque in the research environment is either performed using clinical indices or, otherwise, by recording the planimetric plaque index (PPI)1. Clinical indices, such as the Turesky modified Quigley-Hein plaque index, rely on the visual assessment of plaque coverage by an operator and the subsequent assignment of a score on an ordinal scale2. While the scoring is quick, the use of clinical indices requires laborious inter-examiner and intra-examiner calibration, and the rating always suffers from a certain degree of subjectivity3,4,5. Moreover, as the number of scores is limited, clinical indices may fail to detect relevant differences in plaque coverage6.
For planimetric recordings, the extent of plaque coverage is determined on digital images by dividing the plaque-covered area by the total area of the tooth surface7. The use of a continuous scale increases the accuracy and shows high discriminatory power in statistical analysis8,9,10. Moreover, one may argue that planimetry is less subjective, as the index is calculated and not estimated by the examiner11. Traditionally, plaque-covered and total tooth areas have been determined manually for PPI recordings by drawing regions of interest in each image using image-processing software7,12. Consequently, planimetric analysis was previously very time-consuming, which reduced its applicability for larger clinical studies6.
On traditional white-light images, the contrast between plaque-covered areas, clean tooth areas, and the surrounding tissues is faint, and, thus, automated image processing, which typically relies on the intensity-based detection of objects, is severely hampered13,14. Images that are acquired with a fluorescence camera show a significantly enhanced contrast between disclosed plaque, clean teeth that auto-fluoresce strongly in the green spectrum, and non-fluorescent soft tissues1.
Here, we present a method for semi-automated planimetry that greatly reduces the time spent on image analysis compared to traditional PPI recordings. The method employs standard disclosing procedures, a commercially available fluorescence camera, and an image analysis freeware. The parameters important for image acquisition and image analysis, as well as typical mistakes and limitations of the method, are discussed.
Protocol
The study was approved by the Ethical Committee of Region Midtjylland (1-10-72-259-21) and performed in compliance with the Helsinki Declaration and its amendments.
1. Fabrication of a custom-made spacer (optional)
NOTE: A custom-made 3D-printed spacer may be used during image acquisition to standardize the positioning of the camera head. The spacer is not mandatory for the recording of the fluorescence images.
- Design of the spacer
- Design a spacer that fits the camera head of the intraoral fluorescence camera. To do so, perform a scan of the camera head with a digital scanner. Import the scan into dedicated software.
- Design the spacer to fit the camera head with the desired morphology and positioning distance to the camera head (i.e., 4 mm). Export as an STL file (an example of a design is attached as Supplemental File S1).
- Additive manufacturing of the spacer
- Open the printer-associated additive manufacturing software, and select the basic settings. Click on Printer | Select the available 3D printer | Next | Shape: Clear | Next | Print mode: 50 microns | Next | Build Style: Standard | Next.
- Import the STL file by clicking on File | Import | Select the STL file | Open.
- Define the position of the spacer on the print platform; click on Transform, and drag the spacer to a corner of the platform as close to the surface of the platform as possible.
- To print additional spacers, click on Copy | Linear pattern. Adjust the count and distance to fit additional objects to the print platform, and click on Set.
- To design the support of the objects, click on Smart support | Style: General | Generate | Type: Gate | Create support.
- Send the print job to the 3D printer. Click on Add to queue. The software automatically performs a quality check of the STL file to identify errors when adding to the queue. Then, click on Add to queue | Job name | F4X | Add to queue.
- Mount a clean print platform on the 3D printer, and add an appropriate resin. Click on Start job, and scan the QR code of the resin. Confirm that the print platform is empty and clean, that the resin tray is full, and that the resin has been stirred before addition. Click on Start job.
- When the print job is finished, remove the spacers from the print platform.
- Clean the spacers in an ultrasound bath with isopropanol for 3 min. Repeat the cleaning using fresh isopropanol. Air-dry the spacers.
- Secure the total polymerization of the material by polymerizing the spacers in a post-curing oven for 10 min.
- Remove the support material, and stain the spacers to prevent light from penetrating through the material.
2. Plaque disclosure and image acquisition
- Mount the custom-made spacer on the fluorescence camera (optional). Connect the intraoral camera to a computer, and open the camera software.
- Click on Patient | New patient to create the patient in the system. Fill out the patient information. Click on Patient | Save to save the patient data. Click on Video. The intraoral camera is now ready to use.
- Dim the room lights.
- Apply a red disclosing dye (i.e., 5% erythrosine) with a cotton pellet on the tooth surfaces of interest to disclose the plaque.
- Instruct the patient to rinse with water for 10 s to remove excess dye. Remove any gingival stain using a cotton pellet. Air-dry each tooth for 3 s.
- Place the intraoral camera in a horizontal position in front of the tooth of interest, with the spacer touching the gingiva/adjacent teeth. Acquire the fluorescence image by pressing the camera button.
NOTE: Ensure that the entire tooth surface of interest is in focus and captured in the image without including antagonist or contralateral tooth surfaces. - Repeat steps 2.4-2.6 for all the teeth of interest.
- Mark all the images in the camera software. Click on Save images/videos in the menu.
NOTE: Make sure that the images are saved in "plaque"-mode and not in "caries"-mode. The symbol P/C in the menu indicates the current mode. - To export the images, go to the Viewer. Choose the images to be exported. Click on File | Export (save as…) | All images of the patient to export the images. In the export window, choose the following settings: Mode: Standard | Export path: Choose the desired folder | Image type selection: Check off the left box | Image state: Original data. Expand the export window to display more options. Select the following: File name contains: Cardnumber OR User input OR Patient name | Format: TIF. Click on OK to export the images.
- Alternatively, set up an automated file export prior to imaging. Click on Options | Show configuration | Modules | Viewer | Export/Email | Export options | Mode: Autoexport| Export path: Choose the desired folder | Image state: Original data. Select the following: File name contains: Cardnumber OR User input OR Patient name | Format: TIF. Click on OK to set up the default export settings. When the automated file export is set up, the images will automatically be exported when saved (step 2.8).
3. Digital image analysis
NOTE: The digital image analysis can be performed at any time after the image acquisition. Batches of up to 1,000 fluorescence images can be processed in parallel. If the analysis of large image batches exceeds the computing power, the image size may be reduced prior to analysis.
- Quantification of the total tooth area
- Rename all the images with sequential index numbers (i.e., Planimetry_001, Planimetry_002,…). Import the fluorescence image series into a dedicated image analysis software (i.e. Daime15) in Red-Green-Blue (RGB) mode by clicking on File | Import images | Import as color.
- Perform a threshold-based segmentation of the image series by clicking Segment | Automatic segmentation | Custom threshold. Set the "Low" threshold above the intensity of the oral soft tissues (i.e., 80). Leave the "High" threshold at 255. Thus, only the teeth (both clean and plaque-covered areas) are recognized as objects in the software. Click on Apply | OK | Segment! to initiate the segmentation.
- Open the visualizer by double-clicking on the name of the image series. Enter the object editor (OBJ). Perform a visual quality control of the segmented images, and delete artifacts by rejecting and deleting such objects.
- Merge the remaining objects in all the images (In all images | Merge selected objects). Now, there is one object per image. Quantify the total tooth area in each image (Analysis | Measure objects | Clear all | Pixels). Export the data.
- Quantification of the plaque-covered areas
- Import the fluorescence image series again into the software, this time with split red, green, and blue color channels (File | Import images | Import as grey). Close the blue channel images. Transfer the object layer from the RGB images to the red channel images (Segment | Transfer object layer).
- Delete non-object pixels in the red channel images using the object editor (In all images | Delete non-object pixels (voxels)). Soft tissues are now removed from the images.
- To enhance the contrast between plaque-covered and clean tooth areas, multiply the red channel image series by a factor of two (Edit | Image calculator | Multiplication | Parameters: Factor 2.00 | Apply | OK).
- To remove clean tooth areas from the images, subtract the green channel image series from the enhanced red channel image series (Edit | Image calculator | Second operand images: Planimetry_green | Subtraction | Apply | OK).
- To identify the plaque-covered areas on the teeth, perform a threshold-based segmentation of the resulting image series (Segment | Automatic segmentation | Custom threshold). Set the "Low" threshold above the intensity of the clean tooth areas (i.e., 80). Leave the "High" threshold at 255. Only plaque-covered areas are recognized as objects in the software. Click on Apply | OK | Segment! to initiate the segmentation.
- Perform a visual quality control of the segmented images in the object editor, and delete artifacts by rejecting and deleting such objects. Merge the remaining objects in all the images (In all images | Merge selected objects). Quantify the plaque-covered area in each image (Analysis | Measure objects | Clear all | Pixels). Export the data.
- Open the exported data tables in a dedicated software. Calculate the PPI according to equation (1):
Equation (1)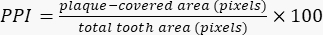
Representative Results
The presented method allows for the rapid, semi-automated planimetric quantification of plaque-covered areas on teeth (Figure 1). Plaque deposits are visualized by erythrosine, while clean tooth areas as well as the acquired pellicle are left unstained16 (Figure 2A). When images are acquired with a fluorescence camera, the contrast between the clean tooth areas, plaque-covered areas, and surrounding soft tissues is enhanced considerably (Figure 2B,C). The fluorescence camera operates with two detection windows, one in the green and one in the red spectrum. Compared to the clean tooth areas, the plaque-covered areas appear slightly brighter in the red channel (Figure 2D,E). In the green channel, the autofluorescence of the tooth is masked considerably in the plaque-covered areas (Figure 2F). This masking effect is exploited during image analysis, when the green channel images are subtracted from the red channel images (Figure 2G). The strong contrast between the clean and plaque-covered areas in the resulting images (Figure 2H) allows for an intensity threshold-based, semi-automated determination of the PPI. Up to 1,000 fluorescence images can be processed simultaneously.
A custom-made 3D-printed spacer may be used to improve the standardized positioning of the camera head at an identical distance from the tooth of interest. The spacer also shields the tooth from ambient light and thereby enhances the contrast between the disclosed plaque, clean tooth areas, and surrounding soft tissues in the acquired images. The spacer is mounted on the camera head with the help of three retention elements (Figure 3).
The described method can be used for planimetric recordings of the supragingival plaque and calculus on both facial and oral tooth surfaces (Figure 4A–D). Depending on the curvature of the dental arch, it can be difficult to position the spacer in close contact with the gums and thereby keep the same distance between the camera head and the tooth. As the plaque area coverage is determined relative to the total tooth area, such differences are unlikely to affect the PPI recordings. Different tooth-colored materials fluoresce in the green spectrum with varying intensities17,18,19. Hence, the PPI can usually be determined with the standard image analysis algorithm on teeth with glass ionomer cements and composite resin restorations (Figure 4E–H). In contrast, amalgam and cast restorations usually emit faintly in both the red and the green channels, and it is, thus, not possible to determine the plaque coverage on such surfaces (Figure 4I,J). The same holds true for metallic orthodontic brackets, but since the bracket surface is typically excluded from PPI recordings, semi-automated planimetry is suitable for orthodontic patients (Figure 4K,L).
The successful semi-automated identification of plaque-covered areas on fluorescence images is highly dependent on the careful execution of all the steps of the clinical procedure. If too much ambient light enters the images, the brightness of the background in the red channel is increased, which renders the differentiation between the teeth and soft tissues difficult (Figure 5A,B). Therefore, the room lights need to be dimmed during the image capture. If the patient does not open the mouth sufficiently during the image acquisition, antagonist teeth may be imaged along with the tooth of interest and hamper the semi-automated processing (Figure 5C). When planimetry is performed on premolars or molars, the correct angulation of the camera is important to avoid imaging parts of the occlusal surface (Figure 5D,E). Once the plaque deposits are disclosed, the operator should immediately proceed to the image acquisition. Otherwise, the erythrosine may be washed out, and the contrast between the plaque-covered and clean tooth areas may become too faint. In some instances, however, the disclosing solution may strongly stain the gingiva, and the stain may not be removed during the following rinse (Figure 5F). To avoid an overestimation of the plaque-covered area, the stain may be reduced by an additional rinse or by gentle wiping of the gingiva with a cotton pellet.

Figure 1: Workflow for the semi-automated quantification of plaque coverage on tooth surfaces. Abbreviation: PPI = planimetric plaque index. Please click here to view a larger version of this figure.
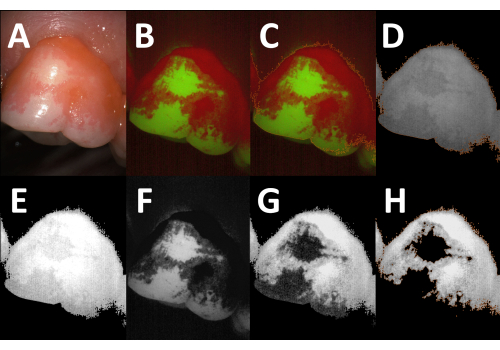
Figure 2: Digital image analysis procedure. (A) White-light image of disclosed plaque (tooth 26, facial aspect). (B) Corresponding image acquired with a fluorescence camera (Red-Green-Blue [RGB] mode). Note the enhanced contrast between the plaque-covered and clean tooth areas. (C) The total tooth area, marked by the orange outline, is identified by intensity threshold-based segmentation. (D) The object layer from the RGB image is transferred to the red channel image (orange outline), and the non-object pixels (background, soft tissues) are deleted. (E) The brightness of the red channel images is enhanced by a factor of two. (F) The green channel image. Note the reduced autofluorescence in the plaque-covered areas. (G) After subtraction of the green channel image (F) from the modified red channel image (E), the contrast between the plaque-covered areas and clean tooth areas is evident. (H) After intensity threshold-based segmentation, the plaque-covered areas are identified as objects (orange outline), and the planimetric plaque index (PPI) can be calculated (PPI = 81.6%). Please click here to view a larger version of this figure.

Figure 3: Custom-made spacer. A custom-made spacer viewed from the (A) front, (B) side, and (C) back. (D) Fluorescence camera with the mounted spacer (orange outline). Please click here to view a larger version of this figure.
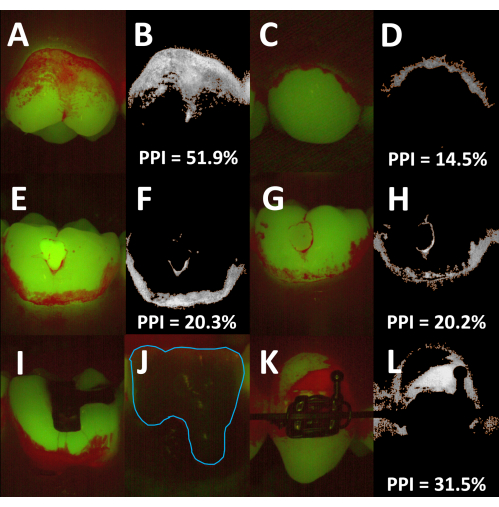
Figure 4: Applications and limitations of semi-automated planimetry. (A) Fluorescence image of a facial tooth surface. (B) Corresponding processed image showing the plaque-covered areas (orange outline; planimetric plaque index [PPI] = 51.9%). (C) Fluorescence image of an oral tooth surface. (D) Corresponding processed image showing the plaque-covered areas (orange outline; PPI = 14.5%). (E–H) Images of teeth with composite resin restorations. The restoration in E fluoresces strongly in the green spectrum, whereas the restoration in G appears slightly fainter than the surrounding clean tooth areas. In both images, the PPI can be determined using the standard image analysis algorithm. (F,H) Processed images showing the plaque-covered areas (orange outlines; PPI = 20.3% and 20.2%, respectively). (I,J) Fluorescence images of a tooth with an amalgam restoration (I) and a tooth with a metal-ceramic crown (J, blue outline, added manually). Both restorations are non-fluorescent, and the plaque deposits cannot be quantified by semi-automated planimetry. (K) Fluorescence image of a tooth with a metallic orthodontic bracket. As the bracket is excluded from the analysis, the PPI can be determined using the standard image analysis algorithm (L, orange outline, PPI = 31.5%). Please click here to view a larger version of this figure.
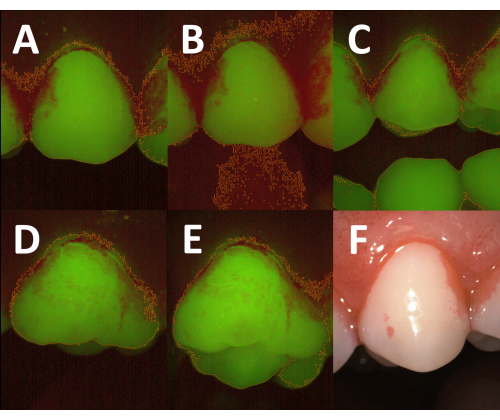
Figure 5: Influence of the clinical procedures on image quality and the results of semi-automated planimetry. (A) Fluorescence image acquired with dimmed room lights. The total tooth area is determined correctly after threshold-based image segmentation (orange outline). (B) Fluorescence image of the same tooth acquired with the room lights turned on. Due to an increased background emission in the red spectrum, the threshold-based segmentation fails to differentiate accurately between the tooth surfaces and the surrounding soft tissues (orange outline). (C) Fluorescence image acquired with insufficient mouth opening. The undisclosed antagonist teeth are visible in the image and, thus, included in the total tooth area (orange outlines). To obtain a correct planimetric plaque index, they have to be removed manually during the image analysis. (D) Fluorescence image acquired with optimal positioning of the camera head. The total tooth area (orange outline) is limited to the facial aspect. (E) Fluorescence image of the tooth in D acquired with suboptimal angulation of the camera head. Part of the occlusal surface is captured, resulting in an increased total tooth area (orange outline). (F) White-light image of disclosed plaque with prominent staining of the gingiva. The high emission in the red spectrum may lead to an overestimation of the plaque-covered area. Please click here to view a larger version of this figure.
Supplemental File S1: Please click here to download this File.
Discussion
The presented method for semi-automated planimetry based on fluorescence images constitutes an improvement in dental plaque quantification on sound tooth surfaces in the research environment compared to traditional planimetry20. Semi-automated planimetry allows for the simultaneous determination of the PPI in up to 1,000 images using a pre-determined post-processing algorithm. Thereby, the method is considerably more time-efficient than conventional planimetry, where the total tooth areas and plaque-covered areas are determined manually by drawing regions of interest in an image processing software7,12. Additionally, the extent of human judgment in the image analysis is reduced to the choice of a brightness threshold for image segmentation. Thereby, all the images are treated alike, and the influence of examiner subjectivity is greatly reduced11.
The critical steps in the protocol are predominantly related to the clinical procedures, which need to be performed in a highly standardized fashion for optimal image quality. The disclosing solution must be applied gently and uniformly, and the images should be acquired right after rinsing and air-drying to avoid a washout of the dye and, thus, a loss of image contrast. Moreover, gingival bleeding needs to be avoided, since hemoglobin may enhance the recorded fluorescence in the red channel19. The image capture should be performed with the room lights dimmed to reduce the interference of ambient light, and the patients need to open their mouths sufficiently, such that the antagonist teeth do not appear in the images. The camera head must be positioned perpendicularly to the tooth axis to avoid capturing part of the occlusal surface and contralateral teeth.
Artifacts that result from sub-optimal image acquisition can – in most cases – be removed during the image analysis, although at the expense of a considerably increased processing time. Some artifacts that are recognized as objects during segmentation can be cleared by simple deletion in the object editor. If artifacts are confluent with the areas recognized as plaque, the resulting objects have to be split in the object editor prior to removal. In extreme cases, the operator may have to recur to the manual determination of the clean tooth and plaque-covered areas by drawing regions of interest in the software. If all the clinical procedures are performed accurately, the only subjective input of the operator during the image analysis consists of determining the cutoff values for the threshold-based segmentations. Generally, the plaque-covered and clean tooth areas are well-defined in the images, but it needs to be mentioned that small differences in the chosen thresholds do influence the calculated PPI values, albeit to a relatively low extent. As all images acquired for a particular study can be segmented with identical thresholds, the subjective choice of cutoff values does not affect the differences between the treatment or patient groups.
Just like manual planimetry, semi-automated planimetry is not suitable for longitudinal recordings of plaque build-up due to the use of a disclosing solution. Erythrosine may interfere with biofilm growth through an antibacterial activity21,22,23, but most importantly, the prominent stain necessitates professional plaque removal before the patient is sent home. However, the described method may be used for the regular quantification of habitual plaque levels in the clinic. Another limitation of semi-automated planimetry arises due to the size differences between individual teeth. Although the distance between the camera and tooth surface and, hence, the size of the field of view can be standardized, the acquired images may include parts of the neighboring teeth. These cannot be removed by a batch operation but only by manual cropping of the images during the analysis. While semi-automated planimetry is appropriate for the quantification of supragingival plaque and calculus24 on sound tooth surfaces, future work will have to determine how the described method is affected by developmental defects25, cavitated and non-cavitated caries lesions, as well as severe stain.
In conclusion, semi-automated planimetry is a method that allows for the rapid and reliable quantification of plaque area coverage using a fluorescence camera. It may be employed in clinical trials that assess de novo plaque formation in different patient groups or the effect of different treatment regimens on plaque removal.
開示
The authors have nothing to disclose.
Acknowledgements
The authors thank Dirk Leonhardt for his excellent assistance in the additive manufacturing of the custom-made spacers. Lene Grønkjær, Javier E. Garcia, Charlotte K. Vindbjerg, and Sussi B. Eriksen are acknowledged for their technical support during the study. The authors would also like to thank Matthias Beck for technical support on the use of the fluorescence camera and Mette R. Jørgensen for fruitful discussions.
Materials
| 3D Sprint Basic | 3D systems | Additive manufacturing software | |
| 5% erythrosine; Top Dent Rondell Röd | Top Dent Lifco Dental AB | 6327 | Disclosing solution |
| D1000 lab scanner | 3 Shape | Lab scanner used to scan the camera head | |
| DBSWIN 5.17.0 | Dürr Dental | Software for VistaCam | |
| Digital image analysis in microbial ecology (Daime), version 2.2.2 | Freeware for image analysis | ||
| LC-3D Print Box | NextDent | Polymerization unit | |
| Meshmixer 3.5 | Autodesk | Freeware for designing custom-made spacer | |
| NextDent 5100 | 3D systems | 3D-printer | |
| NextDent Ortho IBT | 3D systems | Material for spacer | |
| Ultrasound bath T660/H | Elma Schmidbauer GmbH | ||
| VistaCam iX HD Smart intraoral camera | Dürr Dental | Coupled with a fluorescence camera head |
参考文献
- Pretty, I. A., Edgar, W. M., Smith, P. W., Higham, S. M. Quantification of dental plaque in the research environment. Journal of Dentistry. 33 (3), 193-207 (2005).
- Turesky, S., Gilmore, N. D., Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. Journal of Periodontology. 41 (1), 41-43 (1970).
- Marks, R. G., et al. Evaluation of reliability and reproducibility of dental indices. Journal of Clinical Periodontology. 20 (1), 54-58 (1993).
- Matthijs, S., Sabzevar, M. M., Adriaens, P. A. Intra-examiner reproducibility of 4 dental plaque indices: Dental plaque indices. Journal of Clinical Periodontology. 28 (3), 250-254 (2001).
- Shaloub, A., Addy, M. Evaluation of accuracy and variability of scoring-area-based plaque indices. Journal of Clinical Periodontology. 27 (1), 16-21 (2000).
- Söder, P. -. &. #. 2. 1. 4. ;., Jin, L. J., Söder, B. Computerized planimetric method for clinical plaque measurement. European Journal of Oral Sciences. 101 (1), 21-25 (1993).
- Lang, N. P., Ostergaard, E., Loe, H. A fluorescent plaque disclosing agent. Journal of Periodontal Research. 7 (1), 59-67 (1972).
- Staudt, C. B., et al. Computer-based intraoral image analysis of the clinical plaque removing capacity of 3 manual toothbrushes. Journal of Clinical Periodontology. 28 (8), 746-752 (2001).
- Smith, M. R. Parametric vs. nonparametric. Analyzing the periodontal and gingival indicies. Journal of Periodontal Research. 17 (5), 514-517 (1982).
- Quirynen, M., Dekeyser, C., van Steenberghe, D. Discriminating power of five plaque indices. Journal of Periodontology. 62 (2), 100-105 (1991).
- Al-Anezi, S. A., Harradine, N. W. T. Quantifying plaque during orthodontic treatment. The Angle Orthodontist. 82 (4), 748-753 (2012).
- Smith, R. N., Brook, A. H., Elcock, C. The quantification of dental plaque using an image analysis system: reliability and validation. Journal of Clinical Periodontology. 28 (12), 1158-1162 (2001).
- Kang, J., Ji, Z., Gong, C. Segmentation and quantification of dental plaque using modified kernelized fuzzy C-means clustering algorithm. 2010 Chinese Control and Decision Conference. , 788-791 (2010).
- Klaus, K., Glanz, T., Glanz, A. G., Ganss, C., Ruf, S. Comparison of quantitative light-induced fluorescence-digital (QLF-D) images and images of disclosed plaque for planimetric quantification of dental plaque in multibracket appliance patients. Scientific Reports. 10 (1), 4478 (2020).
- Daims, H., Lücker, S., Wagner, M. Daime, a novel image analysis program for microbial ecology and biofilm research. Environmental Microbiology. 8 (2), 200-213 (2006).
- Arnim, S. S. The use of disclosing agents for measuring tooth cleanliness. Journal of Periodontology. 34 (3), 227-245 (1963).
- Meller, C., Klein, C. Fluorescence properties of commercial composite resin restorative materials in dentistry. Dental Materials Journal. 31 (6), 916-923 (2012).
- Kiran, R., Chapman, J., Tennant, M., Forrest, A., Walsh, L. J. Detection of tooth-colored restorative materials for forensic purposes based on their optical properties: An in vitro comparative study. Journal of Forensic Sciences. 64 (1), 254-259 (2019).
- Shakibaie, F., Walsh, L. J. Fluorescence imaging of dental restorations using the VistaCam intra-oral camera. Australian Journal of Forensic Sciences. 51 (1), 3-11 (2019).
- Rey, Y. C. D., Rikvold, P. D., Johnsen, K. K., Schlafer, S. A fast and reliable method for semi-automated planimetric quantification of dental plaque in clinical trials. Journal of Clinical Periodontology. , (2022).
- Baab, D. A., Broadwell, A. H., Williams, B. L. A comparison of antimicrobial activity of four disclosant dyes. Journal of Dental Research. 62 (7), 837-841 (1983).
- Begue, W. J., Bard, R. C., Koehne, G. W. Microbial inhibition by erythrosin. Journal of Dental Research. 45 (5), 1464-1467 (1966).
- Marsh, P. D., et al. Antibacterial activity of some plaque-disclosing agents and dyes (short communication). Caries Research. 23 (5), 348-350 (1989).
- Shakibaie, F., Walsh, L. J. Dental calculus detection using the VistaCam. Clinical and Experimental Dental Research. 2 (3), 226-229 (2016).
- Seow, W. Developmental defects of enamel and dentine: Challenges for basic science research and clinical management. Australian Dental Journal. 59, 143-154 (2014).

