概要
Giving in to temptation of tasty food may result in long-term overweight problems. This protocol describes how to reduce imprudent preference for edible commodities during hypothetical intertemporal choices in women by associating them with errors.
Abstract
Nowadays, the increasing incidence of eating disorders due to poor self-control has given rise to increased obesity and other chronic weight problems, and ultimately, to reduced life expectancy. The capacity to refrain from automatic responses is usually high in situations in which making errors is highly likely. The protocol described here aims at reducing imprudent preference in women during hypothetical intertemporal choices about appetitive food by associating it with errors. First, participants undergo an error task where two different edible stimuli are associated with two different error likelihoods (high and low). Second, they make intertemporal choices about the two edible stimuli, separately. As a result, this method decreases the discount rate for future amounts of the edible reward that cued higher error likelihood, selectively. This effect is under the influence of the self-reported hunger level. The present protocol demonstrates that errors, well known as motivationally salient events, can induce the recruitment of cognitive control, thus being ultimately useful in reducing impatient choices for edible commodities.
Introduction
Nowadays, it is crucial to help people face the rise of eating disorders1-4. These disorders reflect an overestimation of the incentive motivation associated with appetitive food, which induces individuals to seek and consume it as soon as possible (this has been shown especially with sweet high-fat foods5-6). This happens at the expense of future benefits that can result from being on a diet for a while, but for which the ability to exert eating control is necessary7-8. Indeed, people showing these abnormal behaviors have increased attentional bias toward edible cues9-10 and experience enhanced incentive value for primary rewards11. Moreover, even just looking at appetizing foodstuffs can cue the desire for consuming the food immediately, both in individuals with eating disorders and in the normal population12-13. In order to refrain from immediate gratification and not forego the long-term outcome (e.g., losing weight after a few months of diet), one must exercise great self-controlled and resist the innate, evolutionarily determined impulse to give into temptation and consume immediately. Exerting self-control, a concept that is interrelated to the notion of cognitive control in the field of the Neuroscience, means that one is capable of overcoming innate impulses for further consideration and, possibly, implementation of other, more appropriate behaviors14.
How do individuals engage in self-control strategies? Research has highlighted over the years that the capacity to refrain from automatic responses is heightened in error-full contexts15. Errors are well considered highly arousing and aversive events that, when encountered, elicit compensatory responses16. Specifically, they cue failure/loss in both performance and utility, thereby signaling that one needs to adjust levels of control over current and future behavior accordingly17. Moreover, errors can cue aversive learning, as a warning way to escape from error-prone, maladaptive behaviors, thus inducing the implementation of optimal choice responses18-21.
The present protocol shows how the association between tasty food items and errors can signal that engaging in a certain behavior would lead to a cost (i.e., reward loss), thus encouraging the implementation of compensatory self-control strategies, and thereby reducing impulsive food choice20,22. In the present protocol adapted from23, participants are preliminarily asked to self-report their hunger level at the time of the experiment and to rate six different food items. Based on the ratings, two foods with equivalent incentive value for each subject are selected for the subsequent task. Then, participants perform an error task (see reference24) in which the two previously selected food items cue different error rates (high and low) associated with performance: The error task is programmed so that in one trial condition, cued by one food, participants make a small number of errors, and in the other trial condition, cued by the other food, they make a much larger number of errors. Afterwards, participants' intertemporal choice for each of the two primary rewards is measured (adapted from reference25). The ability to seek larger but delayed reinforcements instead of sooner gratification, which is crucial when facing tempting foods while being on a diet, is indeed crisply captured by intertemporal choice paradigms26. The longer the time one needs to wait for a good to be received and consumed, the more the subjective evaluation of this potential reward is weakened (i.e., the so-called temporal discounting phenomenon27-34). Poor decisions (i.e., higher propensity to choose close gratifications, namely increased temporal discounting for future gains) are considered as a core feature of impulsivity35 and a landmark of numerous disorders, including drug addiction and obesity36-45. After undergoing the procedure described in this protocol, participants show reduced impatient choice for the stimulus cueing high error rate, selectively. The effect is more evident when the hunger level reported by the subjects is low23. This occurs because hunger affects the immediate evaluation of food items46-49 by heightening the motivational value of primary rewards and, in turn, the discount rate of future amounts of those rewards7,50-52.
The advantage of this method first lies in its easy applicability. The error training preceding the intertemporal decision tasks is almost completely effortless, making it possible to be employed in diverse clinical settings. Second, the method produces the desired effect with hypothetical edible items, without the need to use real food. Third, participants were mainly unaware of the food-error association, making the subsequent effect on food preferences genuine, which might affect food decisions reliably in real life as well. Finally, participants tested in the study23 were all young females, but there is good reason to speculate that the effect of food-error pairing on intertemporal decisions would be the same on young males too, mainly because subjects in the present study were unaware of the intended effect.
Protocol
Ethics statement: All procedures described in this protocol were developed and tested following ethical approval from the Ethics Committee of the Psychology Department of Bologna University (see also the Declaration of Helsinki23,53).
1. Participants
- Select a sample of healthy young adult females.
- Recruit participants who are not on a diet, not taking psychoactive drugs, free of current or past psychiatric or neurological illness as determined by history, and naïve as to the purpose of the experiment.
- Invite volunteers to sit in a quiet room and collect their demographics, including gender, age, level of education, fasting hours, and height and weight.
- Calculate participants' body mass index (BMI)45,54,55.
2. Hunger and Motivation Ratings
- Collect subjects' self-ratings about their feeling of hunger at that very moment on an 11-point Likert scale56,57.
- Use a similar scale as the one used in the previous step for self-rating the motivation to consume at that very moment six foods, one by one. Choose these foods among those with high appetitive value58-62 in the female population (based on the cultural context).
- Display the six foods in pictures, randomly, by using a slide show presentation program, so that their appearance and size match as much as possible those in the real word.
3. Food Stimuli Selection
- Select, individually for each participant, the two highest rated food items in the previous step.
- Make sure, at the group level, that participants are equally motivated to eat the two foods, namely, that there is no significant difference between the two items in the motivation rating (see steps 2.2 and 2.2.1).
4. Errors as a Way to Enhance Cognitive Control
- Use the Error task24 modified as follows to associate the two preselected stimuli with two different error rates.
- Program and run the task with an appropriate stimulus delivery software, such as E-Prime. The task should last at least 15 min. See Supplemental Code Files for an example script.
- Include two conditions in which one predicts (i.e., it cues) a Low probability of committing an Error (LE), namely, in which participants will make a small number of errors, and one predicts a High probability of committing an Error (HE), namely, in which participants will make a larger number of errors (see steps 4.5.2 and 4.5.3).
- Use one of the two preselected food items as the cue for the LE condition (LEFood); use the other food item as the cue for the HE condition (HEFood). Intersperse, randomly, the same number of trials for both conditions.
- Set the task so that each trial starts with a grayscale picture of one of the two food items, that, after 1,000 msec, becomes colored (Go signal), thus requiring the subject to respond.
- For 33% of the total number of trials, superimpose a red circle on the colored image (Stop signal) after a variable stop-signal delay (SSD) (see section 4.4) relative to the Go signal onset, thus requiring the subject not to respond.
- Once the Stop signal appears, leave both Go and Stop signals on the screen for a maximum response time of 1,000 msec after the Go signal onset (time window in which subjects are allowed to respond).
- After giving a feedback (see step 4.4.), separate the trials with a blank screen of variable interstimulus interval (500-2,000 msec).
- Instruct participants to respond to the Go signal as quickly as possible via button press, and to refrain when a Stop signal occurs. Instruct them about all possible feedback (i.e., ''Correct!'', ''Error!'', ''Too early!'').
- Define the error likelihoods for the LE and the HE conditions by changing their SSDs independently, by the means of a staircase algorithm, based on participant's performance. The LE has shorter SSDs, whereas the HE has longer SSDs24.
- Start the SSD at 200 msec in both LE and HE after the first Go signal onset.
- During the LE, increase the SSD by 5 msec on the subsequent trial if the subject succeeds in avoiding the button press after the Stop signal; decrease the SSD by 50 msec on the subsequent trial if the subject fails.
- During the HE, increase the SSD by 50 msec on the next trial if the subject succeeds, otherwise, decrease the SSD by 50 msec on the next trial if the subject fails63.
- For the analyses, compare Go and Stop trials and LE and HE conditions for accuracy and response time24. Do not consider missed responses. Use Statistica or any other statistical software to analyze the data.
5. Intertemporal Choice as a Measure of Cognitive Control
- Administer participants two Temporal Discounting tasks, separately and in a counterbalanced order, soon after the Error task ends (see below for details). Measure in one Temporal Discounting task the subjective value for the LEFood, and in the other the subjective value for the HEFood. Run the tasks on E-Prime or any other stimulus delivery software. See Supplemental Code Files for an example script.
- In each task, present participants, on each trial, a choice between 40 units of fictive food25,64,65 that can be obtained after a variable delay (i.e., 2 days, 2 weeks, 1 month, 3 months, 6 months, and 1 year, for a total of six delay conditions25,66,67) and a smaller amount of the same food available in the immediate present.
- Include five choices in each delay condition. Deliver blocks of choices pertaining to the six delay conditions in a random order across subjects.
- Instruct participants to make a choice between the two options by imagining to receive the specified amount of reward at the specified time, and to indicate their preference by pressing one of two buttons25,68.
- Make clear to participants that they are making choices toward hypothetical foods; there are no correct or incorrect choices but they are free to choose the option they prefer the most; they have no time limit to decide, but they should make their choice as soon as they feel to prefer one reward more than the other.
- Program the tasks so that, for each delay condition, the amount of the smaller-immediate option is adjusted, trial by trial, based on participants' previous choice, using a titration procedure that converges on the amount of the immediate reward that has equivalent subjective value as the delayed reward25,67 (see steps 5.3.1 to 5.3.5).
- First, always submit participants, for each delay condition, to a choice between 20 units (bites) of food available "now" and 40 units of the same food available at that specific delay67, so that, in the first trial of each delay condition participants always face a choice between these two specific amounts67.
- Then, decrease, after the first choice, the amount of the smaller option on the next trial if participant chose the smaller option in the previous trial, otherwise increase the amount of the smaller option on the next trial if the participant chose the larger option in the previous trial.
- Reduce the size of the adjustment of the smaller option with successive choices: The first adjustment is half of the difference between the smaller and the larger rewards, whereas for subsequent choices it is half of the previous adjustment23.
- Repeat the procedure in the above step until the participant has made five choices at one specific delay condition, after which the participant begins a new series of choices at another delay condition.
- For each trial in a block, consider the smaller immediate amount as the best guess as to the subjective value of the larger later reward. Therefore, take the immediate amount that would have been presented on the sixth trial of a delay block as the estimate of the subjective value of the later reward at that delay and use it for the analysis (i.e., the so-called indifference point; see step 5.6)25.
- Fit, through nonlinear, least-squares analysis, the hyperbolic equation V = 1/(1 + kT) (i.e., V = subjective value; k = constant of the best fitting; T = time delay in days) to participants' indifference points23, independently for each Temporal Discounting task. Do this in order to calculate the discount rate (k) of the subjective value for each reward under consideration as a function of time69-71. Use Statistica or any other statistical software to perform this non-linear estimation.
- Log-transform k values in order to normalize data, and then run parametric analyses23,25.
Note: A larger k value corresponds to a steeper discounting function, thereby indicating that the subjects are more inclined to choose the smaller-immediate option over the larger later one. - Compare the k values for the LEFood and the HEFood by correcting for hunger rating, self-report questionnaire score of sensitivity to external edible cues (see step 6), and BMI45,54,55. Use Statistica or any other statistical software to analyze the data.
6. External Eating Behavior Subscale of the Dutch Eating Behavior Questionnaire (DEBQEEB)
- Use the DEBQEEB72 to measure volunteers' sensitivity to external edible cues, to control for possible confounding during the experiment.
7. Debriefing
- Debrief participants as to the purpose of the experiment at the end of the session. Ask them if they were somehow aware of the error manipulation during the Error task and if they think they chose somehow in a different way during the Temporal Discounting tasks according to the two different foods.
Note: Any impressions or feelings about the entire procedure (e.g., whether they think there was an association between the two main tasks) are welcome.
Representative Results
Representative results from the application of the protocol described above are reported here.
Error task
The validity of the Error task has been determined by the following results. Concerning the percentage of errors committed by participants, they showed a significantly higher number of errors in the HE than in the LE condition, a significantly higher number of errors during Stop trials than during Go trials, and a significantly higher number of errors during Stop trials in the HE condition than in the LE condition (all ps <0.0001). Furthermore, no significant difference in the number of errors during Go trials across HE and LE conditions was detected (p = 0.98). Concerning participants' RTs for correct responses, they showed significantly slower reactions in the HE than in the LE condition, significantly higher RTs during Go trials than during Stop trials, and significantly slower RTs for correct responses during Stop trials in the HE than in the LE condition (all ps <0.0001). Moreover, no significant difference in RTs for correct responses during Go trials across HE and LE conditions was detected (p = 0.98). These results, displayed in Figure 1 and in line with previous findings24, indicate that the Error task is effective in producing different patterns of errors, and support the idea that during the HE condition, participants experience higher conflict than in the LE condition.
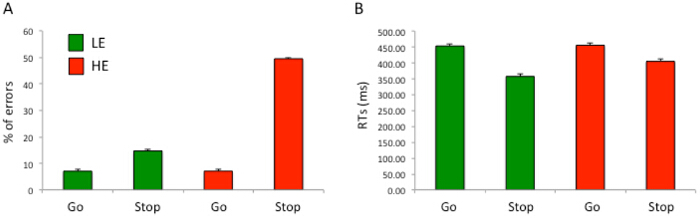
Figure 1: Error Percentage and RTs for Correct Responses in the Error Task. (A) shows the error percentage (%) across LE and HE conditions, separated by Go and Stop trials. (B) shows the RTs (in msec) for correct responses across LE and HE conditions, separated by Go and Stop trials. Error bars indicate the SEM. Please click here to view a larger version of this figure.
Temporal Discounting tasks
Figure 2 shows representative results from the two Temporal Discounting tasks that follow the Error task. An analysis on hyperbolic log-transformed k values for LEFood and HEFood, by including baseline hunger scores as covariate to control data for participants' hunger state, revealed that the discount rate of the HEFood was significantly smaller than the discount rate of the LEFood, as determined by baseline hunger (-0.81 vs. -0.61) (all ps ≤0.01). BMI and DEBQEEB scores did not significantly co-vary with the data (all ps >0.23, data not shown).
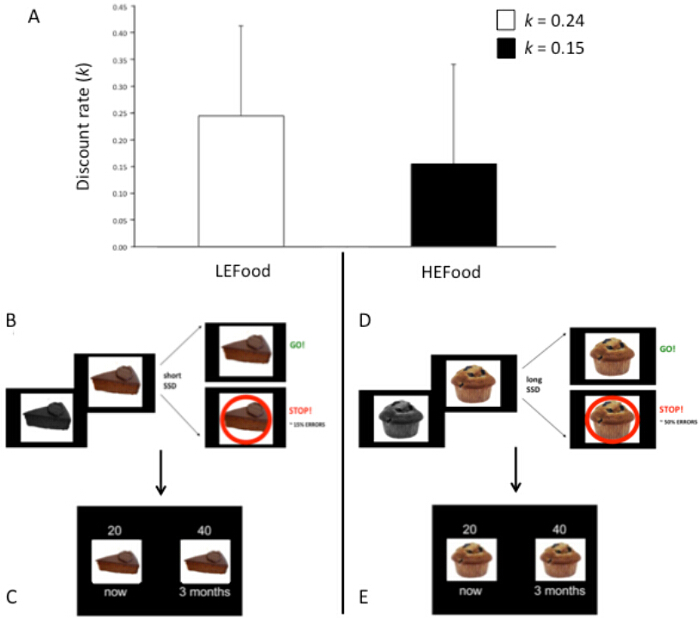
Figure 2: Discount Rates of the LEFood and the HEFood. (A) the k value for each bar reflects the geometric mean (raw k) of the sample for the LEFood and for the HEFood, and error bars indicate the SEM. (B) shows an example of a trial in the LE condition of the Error task followed by a trial example of the Temporal Discounting task for the LEFood (C); (D) shows an example of a trial in the HE condition of the Error task followed by a trial example of the Temporal Discounting task for the HEFood (E). This figure has been modified from Sellitto & di Pellegrino (2014)23. Please click here to view a larger version of this figure.
Moreover, to clarify the modulatory effect of baseline hunger on the discount rate for future outcomes, a regression analysis between hunger ratings and log-transformed k values for the LEFood and the HEFood showed a significant association between the hunger level and the discount rate of the HEFood only (p = 0.002), as displayed in Figure 3.
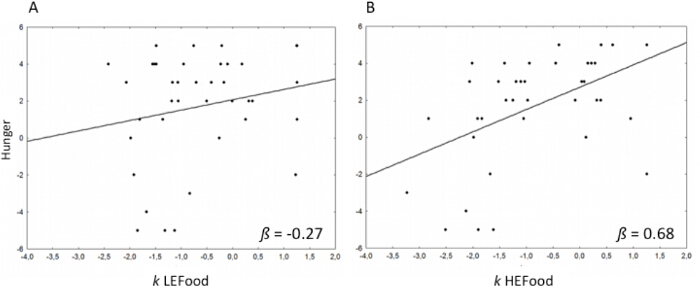
Figure 3: Relation Between Baseline Hunger Level and Discount Rate (k) of the LEFood and the HEFood. The log-transformed k values for the LEFood (A) and for the HEFood (B) entered in a regression analysis with the hunger scores (11-point Likert scale ranging from -5 to 5). This figure has been modified from Sellitto & di Pellegrino (2014)23. Please click here to view a larger version of this figure.
As predicted, the error manipulation in the first phase of the experimental protocol induced volunteers to show, in the second phase, a reduced preference for quantities of food immediately available (i.e., shallower TD) when the item was previously paired with a high-error likelihood as compared with the item that was previously paired with a low-error likelihood. Baseline hunger had a role in the resulting effect: the lower the motivational state linked to food (i.e., hunger) experienced at the time of the experiment, the less subjects showed imprudent preferences when it came to the item previously associated with high-error likelihood. Conversely, the higher their hunger state, the more they did not differentiate between the two edible items (i.e., similar discount rate), indicating that participants assigned a comparable appetitive salience to the two.
Supplemental Code File: Temporal Discounting Task Script. Please click here to download this file.
Supplemental Code File: Error Task Script. Please click here to download this file.
Discussion
This article describes in detail a novel protocol aimed at reducing impulsive food choice in healthy young adult women. Critical steps in this protocol include sampling participants from the healthy female population, collecting self-report hunger level at the time of the experiment, selecting two foods with equivalent incentive value for each subject, submitting participants to an error task where each of two different error likelihoods (high and low, randomly interspersed across trials) are associated with one of the two preselected foods, and measuring participants intertemporal choice for each preselected food separately. The ultimate purpose of this procedure is to heighten the use of control strategies (i.e., increased self-control by the means of the Error task) to encourage more optimal food decision-making (as measured by means of the Temporal Discounting tasks).
The first crucial step in this protocol is to record the level of participants' hunger at the beginning of the session. The protocol, indeed, was successful mainly on participants with low hunger level. Conversely, individuals who experienced high hunger showed comparable discount rates for the two foods. This suggests that the exposure to the error task does not have a strong influence on promoting choice of future outcomes (reduced discount rate) previously paired with high-error likelihood when hunger is high.
The second crucial step in this protocol is the two error-likelihoods (high and low) manipulation. In the field of neuroscience, errors are well known to be salient in terms of incentive motivation, and to act as warning signals anticipating the need for cognitive control73. When making a decision where time matters, the desire for sooner gratification driven by more visceral and automatic processes competes with long-term gain maximization26-28,74,75. Thus, the organism is required to pay attention to the benefit/cost tradeoff linked to potential actions and outcomes for optimal future planning (namely, in order to get the larger outcome). In our view, this manipulation is very powerful in reducing the discount rate for future reward since errors might warn the subject that heightened attention is required in a situation with an increased probability of committing an error. This would in turn heighten cognitive control to avoid further losses in utility17,24,76,77. Second, errors, by means of their intrinsic aversive nature, would signal the increased probability of losing a reward or a gain22,78-80. Thereby, the subject would increase self-control in order to suppress the usually overwhelming and automatic response of trying to attain immediate gratification81.
Neuroscience research has recently provided evidence for a close link between disordered eating behavior and drug addiction82. They would indeed share behavioral features like impulsivity, executive dysfunction83, and a dysregulated reward circuit3,84-86. In the realm of behavioral techniques aimed at reducing impulsivity toward food by the means, for instance, of cue exposure therapy or response-inhibition training87-89, the novel protocol described here seems to be extremely promising if used in the future as clinical training for people with a diagnosis of obesity or other eating disorders90,91. The advantages of this method as compared to others available rely, first, in its easy implementation. The error training preceding the intertemporal decision task is almost completely effortless: it is relatively quick, has easy instructions, and therefore it is possible to employ it in diverse clinical settings, even on a daily basis. Moreover, the method produces the desired effect with hypothetical edible items, with no need for using real food. Furthermore, subjects are mainly unaware of food-error association, as reported at the time of debriefing, thus making the subsequent effect on food preferences likely automatic, implicit, and genuine92. In this regard, one could speculate that the present procedure might affect food decisions reliably in the real life as well.
Nevertheless, the present protocol carries some disadvantages and limitations. First, it has been tested on healthy females only, so that it is unclear whether the same effect can also be found in males. Second, fictive food was used in this study. Thus, there is no evidence that this procedure would affect actual food choices or impulsivity for real food, and that implementation in the clinical population would necessarily be successful. Therefore, further investigation is needed, for instance by implementing this protocol under more controlled conditions of hunger/satiation (e.g., by sating or fasting subjects) than mere self-rating report as used here. Moreover, other modifications can be made in light of the effectiveness of this protocol by using, for instance, a wider range of food items (which should still be well-known to the population under investigation, thus ensuring that all foods selected are very salient to the subjects), or using real food as reward for the two temporal discounting tasks.
The present protocol shows that error-food manipulation has a long-term effect, at least sufficient to influence subsequent intertemporal food decisions in a healthy young adult female population. The present paradigm is in a way similar to a previous study that aimed at the reduction of alcohol abuse: Cues related to alcohol were repeatedly associated with action refraining93 and, as a result, positive effect towards those stimuli was reduced, while control over drinking behavior was increased. There is good reason to believe that the paradigm described here can promote healthier food choice. Indeed, previous research showed that subjects with higher ability in foregoing immediate gratification during the classic "marshmallow test" (which involved food as in the present protocol) had, years later, higher educational scores and social skills, as well as lower BMI, better rational, attentive, and planning abilities, and a higher capacity to deal well with frustration and stress36,94,95. Future investigation could be also directed towards the entire realm of primary rewards (e.g., drug, alcohol, etc.) by adapting the present protocol to each specific condition.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by a Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (PRIN) grant from Ministero Istruzione Università e Ricerca (PRIN 2010, protocol number: 2010XPMFW4_009) awarded to GdP. We are also grateful to Caterina Bertini and Raffaella Marino for proofreading the manuscript and performing in the video.
Materials
| E-Prime | PST | Stimulus Delivery Software | |
| Statistica | Statsoft | Statistical Software |
参考文献
- Haslam, D. W., James, W. P. Obesity. Lancet. 366 (9492), 1197-1209 (2005).
- Knight, J. A. Diseases and disorders associated with excess body weight. Ann Clin Lab Sci. 41 (2), 107-121 (2011).
- Fortuna, J. L. The obesity epidemic and food addiction: clinical similarities to drug dependence. J Psychoactive Drugs. 44 (1), 56-63 (2012).
- Bowden, D. J., Kilburn-Toppin, F., Scoffings, D. J. Radiology of eating disorders: a pictorial review. Radiographics. 33 (4), 1171-1193 (2013).
- Davis, C., et al. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 48 (1), 12-19 (2007).
- Dalton, M., Blundell, J., Finlayson, G. Effect of BMI and binge eating on food reward and energy intake: Further evidence for a binge eating subtype of obesity. Obes Facts. 6 (4), 348-359 (2013).
- Epstein, L. H., Salvy, S. J., Carr, K. A., Dearing, K. K., Bickel, W. K. Food reinforcement, delay discounting and obesity. Physiol Behav. 100 (5), 438-445 (2010).
- Appelhans, B. M., et al. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 19 (11), 2175-2182 (2011).
- Svaldi, J., Tuschen-Caffier, B., Peyk, P., Blechert, J. Information processing of food pictures in binge eating disorder. Appetite. 55 (3), 685-694 (2010).
- Brooks, S., Prince, A., Stahl, D., Campbell, I. C., Treasure, J. A systematic review and meta-analysis of cognitive bias to food stimuli in people with disordered eating behaviour. Clin Psychol Rev. 31 (1), 37-51 (2011).
- Schebendach, J., Broft, A., Foltin, R. W., Walsh, B. T. Can the reinforcing value of food be measured in bulimia nervosa. Appetite. 62, 70-75 (2013).
- Hawk, L. W., Baschnagel, J. S., Ashare, R. L., Epstein, L. H. Craving and startle modification during in vivo exposure to food cues. Appetite. 43 (3), 285-294 (2004).
- di Pellegrino, G., Magarelli, S., Mengarelli, F. Food pleasantness affects visual selective attention. Q J Exp Psychol. 64 (3), 560-571 (2011).
- Miller, E. K., Cohen, J. D. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24, 167-202 (2001).
- Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., Cohen, J. D. Conflict monitoring and cognitive control. Psychol Rev. 108 (3), 624-652 (2001).
- Hajcak, G., Foti, D. Errors are aversive: defensive motivation and the error-related negativity. Psychol Sci. 19 (2), 103-108 (2008).
- Ridderinkhof, K. R., van den Wildenberg, W. P. M., Segalowitz, S. J., Carter, C. S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 56 (2), 129-140 (2004).
- Holroyd, C. B., Coles, M. G. H. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 109 (4), 679-709 (2002).
- Yeung, N., Botvinick, M. M., Cohen, J. D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 111 (4), 931-959 (2004).
- Shackman, A. J., et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 12 (3), 154-167 (2011).
- Frank, M. J., Woroch, B. S., Curran, T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 47 (4), 495-501 (2005).
- Fujita, K., Han, H. A. Moving beyond deliberative control of impulses: the effect of construal levels on evaluative associations in self-control conflicts. Psychol Sci. 20 (7), 799-804 (2009).
- Sellitto, M., di Pellegrino, G. Errors affect hypothetical intertemporal food choice in women. PLoS ONE. 9 (9), 108422 (2014).
- Brown, J. W., Braver, T. S. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 307 (5712), 1118-1121 (2005).
- Sellitto, M., Ciaramelli, E., di Pellegrino, G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci. 30 (49), 16429-16436 (2010).
- Takahashi, T., Ikeda, K., Hasegawa, T. A hyperbolic decay of subjective probability of obtaining delayed rewards. Behav Brain Funct. 3, 52 (2007).
- Frederick, S., Loewenstein, G., O’Donoghue, T. Time discounting and time preference: a critical review. J Econ Lit. 40 (2), 351-401 (2002).
- Sellitto, M., Ciaramelli, E., di Pellegrino, G. The neurobiology of intertemporal choice: insight from imaging and lesion studies. Rev Neurosci. 22 (5), 565-574 (2011).
- Samuelson, P. A. A note on measurement of utility. Review Econ Stud. 4 (2), 155-161 (1937).
- Ainslie, G. W. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 82 (4), 463-496 (1975).
- Myerson, J., Green, L. Discounting of delayed rewards: models of individual choice. J Exp Anal Behav. 64 (3), 263-276 (1995).
- Cardinal, R. N., Pennicott, D. R., Sugathapala, C. L., Robbins, T. W., Everitt, B. J. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 292 (5526), 2499-2501 (2001).
- Kalenscher, T., et al. Single units in the pigeon brain integrate reward amount and time-to-reward in an impulsive choice task. Curr Biol. 15 (7), 594-602 (2005).
- Peters, J., Büchel, C. Neural representations of subjective reward value. Behav Brain Res. 213 (2), 135-141 (2010).
- Takahashi, T. Loss of self-control in intertemporal choice may be attributable to logarithmic time-perception. Med Hypotheses. 65 (4), 691-693 (2005).
- Mischel, W., Shoda, Y., Peake, P. K. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 54 (4), 687-699 (1988).
- Davis, C., Levitan, R. D., Muglia, P., Bewell, C., Kennedy, J. L. Decision-making deficits and overeating: A Risk model for obesity. Obes Res. 12 (6), 929-935 (2004).
- Davis, C., Patte, K., Curtis, C., Reid, C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 54 (1), 208-213 (2010).
- Bickel, W. K., et al. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depen. 90 (1), 85-91 (2007).
- Weller, R. E., Cook, E., Avsar, K., Cox, J. Obese women show greater delay discounting than healthy-weight women. Appetite. 51 (3), 563-569 (2008).
- Manwaring, J. L., Green, L., Myerson, J., Strube, M. J., Wilfley, D. E. Discounting of various types of rewards by women with and without binge eating disorder: Evidence for general rather than specific differences. Psychol Rec. 61 (4), 561-582 (2011).
- Appelhans, B. M., et al. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 59 (2), 576-584 (2012).
- Kishinevsky, F. I., et al. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 58 (2), 582-592 (2012).
- Bickel, W. K., et al. Using crowdsourcing to compare temporal, social temporal, and probability discounting among obese and non-obese individuals. Appetite. 75, 82-89 (2013).
- Schiff, S., et al. Impulsivity toward food reward is related to BMI Evidence from intertemporal choice in obese and normal-weight individuals. Brain Cogn. , 1-8 (2015).
- Kringelbach, M. L. Food for thought: hedonic experience beyond homeostasis in the human brain. 神経科学. 126 (4), 807-819 (2004).
- Seibt, B., Hafner, M., Deutsch, R. Prepared to eat: How immediate affective and motivational responses to food cues are influenced by food deprivation. Eur J Soc Psychol. 37, 359-379 (2007).
- Stafford, L. D., Scheffler, G. Hunger inhibits negative associations to food but not auditory biases in attention. Appetite. 51 (3), 1-15 (2008).
- Piech, R. M., Hampshire, A., Owen, A. M., Parkinson, J. A. Modulation of cognitive flexibility by hunger and desire. Cogn Emot. 23, 528-540 (2009).
- Lappalainen, R., Epstein, L. H. A behavioral economics analysis of food choice in humans. Appetite. 14 (2), 81-93 (1990).
- Epstein, L. H., Paluch, R., Coleman, K. J. Differences in salivation to repeated food cues in obese and nonobese women. Psychosom Med. 58 (2), 160-164 (1996).
- Epstein, L. H., Truesdale, R., Wojcik, A., Paluch, R. A., Raynor, H. A. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav. 78 (2), 221-227 (2003).
- . International Committee of Medical Journal Editors Statements from the Vancouver group. Brit Med J. 302, 1194 (1991).
- Smalley, K. J., Knerr, A. N., Kendrick, Z. V., Colliver, J. A., Owen, O. E. Reassessment of body mass indices. Am J Clin Nutr. 52 (3), 405-408 (1990).
- Borghans, L., Golsteyn, B. H. H. Time discounting and the body mass index: Evidence from the Netherlands. Econ Hum Biol. 4 (1), 39-61 (2006).
- Likert, R. A technique for the measurement of attitudes. Arch Psychol. 140, 1-55 (1932).
- Sibilia, L. The cognition of hunger and eating behaviours. Psihologijske Teme. 19, 341-354 (2010).
- Asmaro, D., Jaspers-Fayer, F., Sramko, V., Taake, I., Carolan, P., Liotti, M. Spatiotemporal dynamics of the hedonic processing of chocolate images in individuals with and without trait chocolate craving. Appetite. 58, 790-799 (2012).
- Asmaro, D., Liotti, M. High-caloric and chocolate stimuli processing in healthy humans: An integration of functional imaging and electrophysiological findings. Nutrients. 6, 319-341 (2014).
- Lawrence, N. S., Hinton, E. C., Parkinson, J. A., Lawrence, A. D. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. 63 (1), 415-422 (2012).
- Siep, N., Roefs, A., Roebroeck, A., Havermans, R., Bonte, M. L., Jansen, A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 198, 149-158 (2009).
- Piech, R. M., et al. Neural correlates of appetite and hunger-related evaluative judgments. PloS one. 4 (8), 6581 (2009).
- Logan, G. D., Cowan, W. B. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 91 (3), 295-327 (1984).
- Bickel, W. K., Pitcock, J. A., Yi, R., Angtuaco, E. J. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 29 (27), 8839-8846 (2009).
- Johnson, M. W., Bickel, W. K. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 77 (2), 129-146 (2002).
- Kirby, K. N., Herrnstein, R. J. Preference reversals due to myopic discounting of delayed reward. Psychol Sci. 6 (2), 83-89 (1995).
- Myerson, J., Green, L., Hanson, J. S., Holt, D. D., Estle, S. J. Discounting delayed and probabilistic rewards: Processes and traits. J Econ Psychol. 24, 619-635 (2003).
- Estle, S. J., Green, L., Myerson, J., Holt, D. D. Discounting of monetary and directly consumable rewards. Psychol Sci. 18 (1), 58-63 (2007).
- Mazur, J. E. An adjusting procedure for studying delayed reinforcement. Quantitative analyses of behavior: The effect of delay and of intervening events on reinforcement value. 5, 55-73 (1987).
- Rachlin, H., Raineri, A., Cross, D. Subjective probability and delay. J Exp Anal Behav. 55 (2), 233-244 (1991).
- Green, L., Myerson, J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 130 (5), 769-792 (2004).
- Van Strien, T., Bergers, G. P. A., Defares, P. B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disorder. 5 (2), 295-315 (1986).
- Botvinick, M. M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 7 (4), 356-366 (2007).
- McClure, S. M., Ericson, K. M., Laibson, D. I., Loewenstein, G., Cohen, J. D. Time discounting for primary rewards. J Neurosci. 27 (21), 5796-5804 (2007).
- Bickel, W. K., Yi, R., Houser, D., McCabe, K. Temporal discounting as a measure of executive function: Insights from the competing neurobehavioral decision system hypothesis of addiction. Neuroeconomics: Advances in health economics and health services research. , 289-310 (2008).
- Cook, E. W., Turpin, G., Lang, P. J., Simons, R. F., Balaban, M. Y. Differentiating orienting, startle, and defense responses: The role of affect and its implications for psychopathology. Attention and orienting: Sensory and motivational processes. 23, 137-164 (1997).
- Notebaert, W., et al. Post-error slowing: an orienting account. Cognition. 111, 275-279 (2009).
- Luu, P., Collins, P., Tucker, D. M. Mood , personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen. 129 (1), 43-60 (2000).
- van der Helden, J., Boksem, M. A., Blom, J. H. The importance of failure: feedback-related negativity predicts motor learning efficiency. Cereb Cortex. 20 (7), 1596-1603 (2010).
- Schultz, W. Predictive reward signal of dopamine neurons. J Neurophysiol. 80 (1), 1-27 (1998).
- Figner, B., et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 13, 538-539 (2010).
- Volkow, N. D., Wang, G. J., Fowler, J. S., Tomasi, D., Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 11, 1-24 (2011).
- Guerrieri, R., Nederkoorn, C., Jansen, A. Disinhibition is easier learned than inhibition. The effects of (dis)inhibition training on food intake. Appetite. 59 (1), 96-99 (2012).
- Avena, N. M., Rada, P., Hoebel, B. G. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. 神経科学. 156 (4), 865-871 (2008).
- Gearhardt, A. N., et al. Neural correlates of food addiction. Arch Gen Psychiat. 68 (8), 808-816 (2011).
- Umberg, E. N., Shader, R. I., Hsu, L. K., Greenblatt, D. J. From disordered eating to addiction: the ”food drug” in bulimia nervosa. J Clin Psychopharm. 32 (3), 376-389 (2012).
- Daniel, T. O., Stanton, C. M., Epstein, L. H. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 24 (11), 2339-2342 (2013).
- Lawrence, N. S., Verbruggen, F., Morrison, S., Adams, R. C., Chambers, C. D. Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite. 85, 91-103 (2015).
- Wessel, J. R., Tonnesen, A. L., Aron, A. R. Stimulus devaluation induced by action stopping is greater for explicit value representations. Front Psychol. 6, 1-10 (2015).
- Anderson, B. A., Laurent, P. A., Yantis, S. Value-driven attentional capture. Proc Natl Acad Sci USA. 108 (25), 10367-10371 (2011).
- Wessel, J. R., Doherty, J. P. O., Berkebile, M. M., Linderman, D., Aron, A. R. Stimulus devaluation induced by stopping action. J Exp Psychol Gen. 143 (6), 1-14 (2014).
- Marteau, T. M., Hollands, G. J., Fletcher, P. C. Changing human behavior to prevent disease: The importance of targeting automatic processes. Science. 337, 1492-1495 (2012).
- Houben, K., Nederkoorn, C., Wiers, R. W., Jansen, A. Resisting temptation: decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depen. 116 (1-3), 132-136 (2011).
- Mischel, W., Shoda, Y., Rodriguez, M. I. Delay of gratification in children. Science. 244, 933-938 (1989).
- Schlam, T. R., Wilson, N. L., Shoda, Y., Mischel, W., Ayduk, O. Preschoolers’ delay of gratification predicts their body mass 30 years later. J Pediatr. 162 (1), 90-93 (2013).

