14.3:
Reacties
20,550 Views
•
•
Homogeneous Equilibria for Gaseous Reactions
For gas-phase reactions, the equilibrium constant may be expressed in terms of either the molar concentrations (Kc) or partial pressures (Kp) of the reactants and products. A relation between these two K values may be simply derived from the ideal gas equation and the definition of molarity. According to the ideal gas equation:
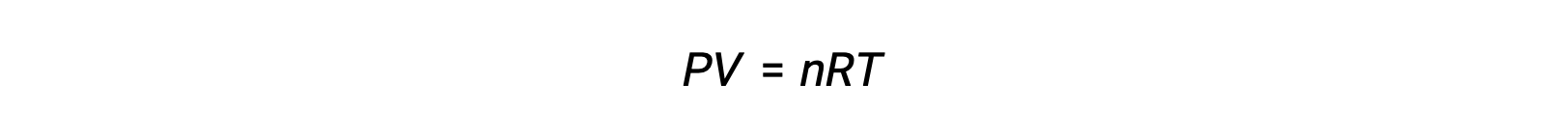
Molar concentration or molarity is given by number of moles divided by the volume:
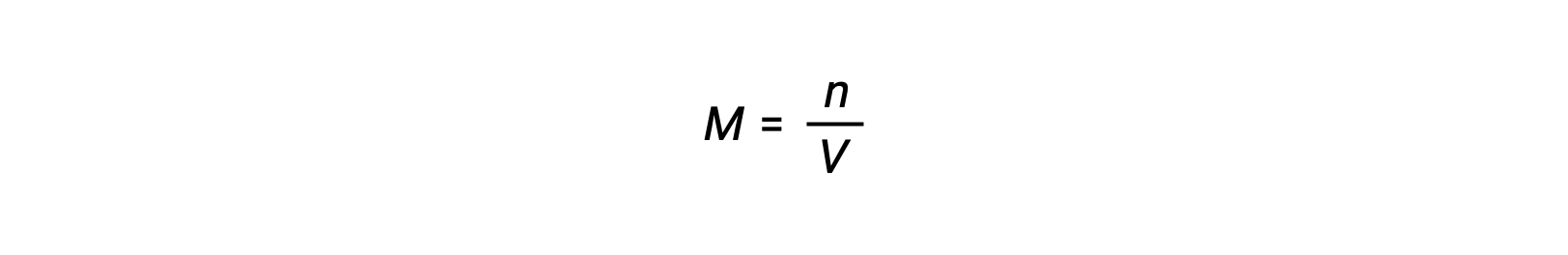
Thus,
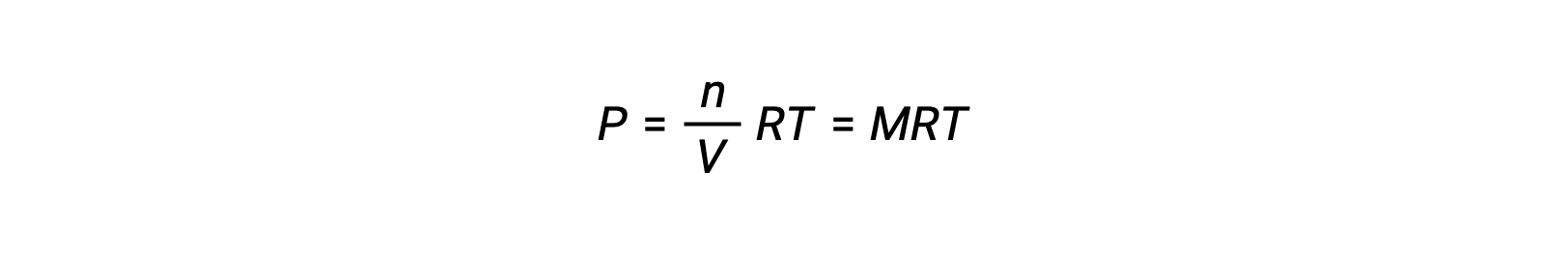
where P is partial pressure, V is volume, n is number of moles, R is the gas constant, T is temperature, and M is molar concentration.
For the gas-phase reaction: m A + n B ⇌ x C + y D
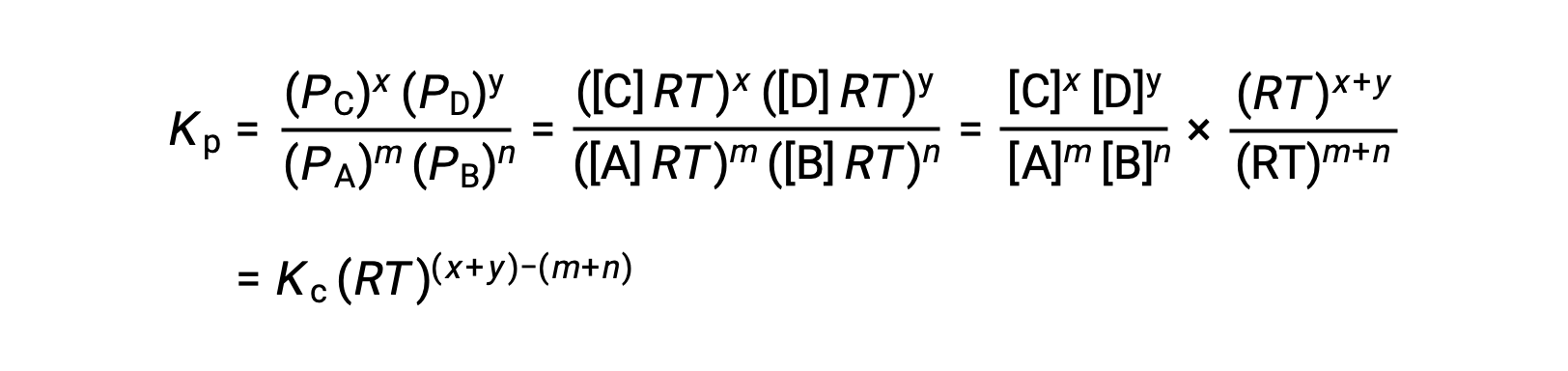
And so, the relationship between Kc and KP is
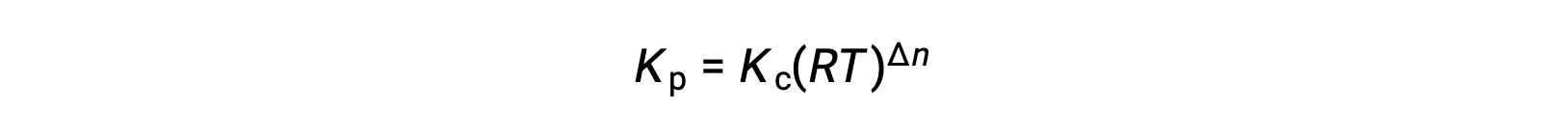
where Δn is the difference in the molar amounts of product and reactant gases, in this case:
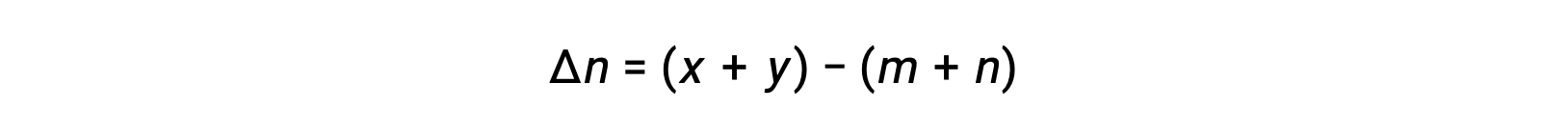
This text has been adapted from Openstax, Chemistry 2e, Section 13.2 Equilibrium Constants.
