Culture of Piglet Intestinal 3D Organoids from Cryopreserved Epithelial Crypts and Establishment of Cell Monolayers
概要
Here, we describe a protocol to culture pig intestinal 3D organoids from cryopreserved epithelial crypts. We also describe a method to establish cell monolayers derived from 3D organoids, allowing access to the apical side of epithelial cells.
Abstract
Intestinal organoids are increasingly being used to study the gut epithelium for digestive disease modeling, or to investigate interactions with drugs, nutrients, metabolites, pathogens, and the microbiota. Methods to culture intestinal organoids are now available for multiple species, including pigs, which is a species of major interest both as a farm animal and as a translational model for humans, for example, to study zoonotic diseases. Here, we give an in-depth description of a procedure used to culture pig intestinal 3D organoids from frozen epithelial crypts. The protocol describes how to cryopreserve epithelial crypts from the pig intestine and the subsequent procedures to culture 3D intestinal organoids. The main advantages of this method are (i) the temporal dissociation of the isolation of crypts from the culture of 3D organoids, (ii) the preparation of large stocks of cryopreserved crypts derived from multiple intestinal segments and from several animals at once, and thus (iii) the reduction in the need to sample fresh tissues from living animals. We also detail a protocol to establish cell monolayers derived from 3D organoids to allow access to the apical side of epithelial cells, which is the site of interactions with nutrients, microbes, or drugs. Overall, the protocols described here is a useful resource for studying the pig intestinal epithelium in veterinary and biomedical research.
Introduction
The intestinal epithelium is formed by a monolayer of cells covering the digestive mucosa at the interface with the luminal environment. This position is associated with diverse functions, such as nutrient absorption and barrier function, that are supported by the presence of stem cells and multiple differentiated epithelial cell types (absorptive, enteroendocrine, Paneth, and goblet cells)1. Immortalized cell lines traditionally used to study epithelial cells have major limitations, since they do not reflect the cellular complexity of the intestinal epithelium and present genomic abnormalities2. The development of three-dimensional (3D) organoids by Sato et al.3 provided a new model to study the intestinal epithelium with an improved physiological relevance. Indeed, intestinal organoids are derived from non-transformed stem cells, are composed of multiple cell types, and recapitulate the functionality of the intestinal epithelium. Intestinal organoids are increasingly being used to understand the development and functions of the intestinal epithelium and its interactions with pathogens, nutrients, toxins, drugs, the microbiota, and its metabolites2.
Initially developed for humans and mice, the methods used to culture intestinal organoids have recently been adapted to other species, including pigs4. Gonzales et al.5 were the first to culture pig organoids from the jejunum ; since then, porcine organoids have been described for other gut segments (duodenum, ileum, and colon)6,7,8, and have been shown to retain a location-specific phenotype9,10,11. Pig intestinal 3D organoids are now commonly used to study the effect of nutrients12,13 or enteric infections6,8,14.
Most of the studies have described the culture of intestinal organoids starting from freshly isolated epithelial crypts. However, this is not always feasible for logistical reasons, notably when working with large animals such as pigs. Indeed, animal facilities for pigs can be located far from the lab where organoids are cultured, which complicates the work organization. Moreover, organoid culture is time-consuming; thus, it is not practical to simultaneously grow multiple organoid lines, for instance, from different gut segments or several animals. To circumvent these issues, a few studies in humans, horses, and pigs have described methods to culture organoids from frozen intestinal tissues (or biopsies) or from isolated epithelial crypts4,15,16,17. These methods allow the cryopreservation of intestinal epithelial stem cells from multiple gut segments of a single animal, which can then be used to grow organoids when needed. Moreover, this allows a strong reduction in the number of live animals used as donors of stem cells, since large stocks of cryopreserved crypts can be created (principles of 3R). Another advantage of this method is the growth of intestinal organoids only from animals of interest after obtaining phenotypic or genotypic results, which is highly cost-effective.
In vivo, intestinal epithelial cells are polarized, with the apical side directed toward the lumen. In vitro, in 3D organoids, the apical side of epithelial cells is also facing the lumen (i.e., inside the organoids)4. This organization prevents access to the apical side, which is an issue when studying the effects of luminal components (e.g., nutrients, microbes, metabolites) on epithelial cells. To circumvent this disadvantage, several methods have been developed, such as the culture of organoid cells as 2D monolayers, microinjection, and polarity reversal ("apical-out organoids")18,19. The culture of organoid cell monolayers is emerging as the most efficient and tractable system. The principle is to dissociate 3D organoids into single cells and seed them on a cell culture vessel previously coated with a thin layer of extracellular matrix (ECM)20. In these culture conditions, the apical side of the epithelial cells is facing upward, and is thus accessible to experimental treatments20. The culture of organoid cell monolayers was recently adapted for the pig intestine21,22; cell monolayers derived from pig 3D organoids have been used for multiple applications, including the study of enteric infections6,23,24,25, the transport of nutrients9, and digestive disease modeling26.
Here, this study first presents a detailed protocol for the culture and maintenance of pig intestinal 3D organoids derived from cryopreserved epithelial crypts (Figure 1). Then, a protocol is described to establish cell monolayers from pig intestinal 3D organoids. The methods described here provide experimental tools that can be used to study the pig intestinal epithelium for nutrient transport, barrier function, and host-microorganism interactions.
Protocol
This protocol was approved by the local ethics committee (N°TOXCOM/0136/PP) in accordance with the European directive on the protection of animals used for scientific purposes (2010/63/EU). This protocol is described for the jejunum as an example, but it can be used for each segment of the small and large intestine (duodenum, jejunum, ileum, colon).
1. Isolation of epithelial crypts from the piglet intestine
NOTE: Prepare a stock of complete Dulbecco's modified eagle medium (DMEMc) with DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S). Prepare 50 mL aliquots and store them at 4 °C for 1 month.
- Preparation of solutions (to be done on the day of the crypt isolation)
- Prepare the dissociation solution containing phosphate buffered saline (PBS), 3 mM dithiothreitol (DTT), 9 mM ethylenediaminetetraacetic acid (EDTA), 10 µM Y27632 ROCK inhibitor, and 1% penicillin-streptomycin (P/S) and store on ice.
- Prepare the freezing solution containing DMEMc, 10% FBS, 10% dimethyl sulfoxide (DMSO), and 10 µM Y27632 ROCK inhibitor and store on ice (the final FBS concentration is 18%).
- Prepare the transport solution containing cold PBS supplemented with 1% P/S and store it on ice.
- Isolation of epithelial crypts
- Slaughter a piglet by electronarcosis followed by exsanguination.
- Immediately after slaughtering, open the abdomen of the piglet with a scalpel and remove the whole intestine.
- Collect approximately 2 cm of an intestinal segment and store it in cold transport solution. Keep the segment on ice until crypt isolation (up to 2 h).
- Place the tissue in a Petri dish. Open the intestinal segment longitudinally and carefully wash the tissue in cold PBS supplemented with 1% P/S to remove the intestinal content.
- Transfer the tissue to a new Petri dish filled with 10 mL of cold PBS supplemented with 1% P/S.
- Hold the tissue with tweezers and remove the villi and the remaining mucus by scraping with a microscope slide.
NOTE: The removal of the villi (the tongue-shaped structure) can be checked by microscopic observation of the supernatant. - Transfer the tissue to a 15 mL conical tube containing 5 mL of ice-cold dissociation solution and incubate for 30 min at room temperature (RT) on a rotating shaker (15 rpm).
- Transfer the tissue to a new Petri dish and add 10 mL of cold PBS supplemented with 1% P/S.
- Isolate the crypts mechanically by firmly scraping the mucosa with a microscope slide.
NOTE: Under a microscope, verify the presence of epithelial crypts in PBS (Figure 2A) . - Aspirate the crypt solution with a serological pipet and filter through a 100 µm cell strainer in a 50 mL conical tube.
- Pipet 10 µL of the solution and verify the presence of crypts under a microscope. Centrifuge at 300 x g for 5 min at 4 °C.
- Under a sterile biosafety cabinet, discard the supernatant and resuspend the pellet of crypts in 10 mL of cold DMEMc supplemented with 10 µM Y27632 ROCK inhibitor.
- Pipet 10 µL of the crypt solution into a 48-well plate. Manually count the number of crypts under a microscope with a 10x magnification, and calculate the concentration of crypts per mL of solution.
NOTE: The isolated crypts can be used directly to culture intestinal organoids. However, it is often more convenient to cryopreserve a large batch of crypts from each piglet and use them later for organoid culture.
- Freezing of epithelial crypts
- Transfer a volume corresponding to 900 crypts in a 15 mL conical tube. Centrifuge at 300 x g for 5 min at 4 °C.
- Discard the supernatant and resuspend the pellet of crypts in 1 mL of the freezing solution. Transfer to a cryotube and place the vial in a cell-freezing container.
- Store the cell freezing container at -80 °C for 24 h and then transfer the vials to liquid nitrogen for long-term storage.
2. Establishment of piglet intestinal 3D organoids from frozen epithelial crypts
NOTE: Piglet intestinal 3D organoids are cultured in a commercial culture medium formulated for the growth of human organoids, supplemented with 1% P/S and 100 µg/mL of an antimicrobial agent for primary cells, and stored at 4 °C for up to 1 week. A tumor-derived extracellular matrix (ECM) is used for the culture of 3D organoids. All references of commercial products are presented in the Table of Materials.
- Preparation of materials
- Place the pipet tips at -20 °C (at least overnight).
- Place the frozen aliquots of the ECM (500 µL) at 4 °C at least 1 h in advance.
- Pre-warm a 48-well plate in a 37 °C, 5% CO2 incubator.
- Place the culture medium at RT.
- Pre-warm a water bath at 37 °C.
- Place a small ice bucket under the hood under aseptic conditions.
- Thawing of frozen epithelial crypts
- Quickly thaw a vial containing 900 frozen crypts in water bath at 37 °C (less than 5 min).
- Transfer the crypt solution into a 15 mL conical tube.
- Centrifuge at 300 x g for 5 min at RT. Remove the supernatant
- Add 150 µL of the ECM with cooled tips to obtain a final concentration of 150 crypts per 25 µL of ECM. Pipet up and down 10 times to obtain a homogenous suspension of crypts in the ECM.
NOTE: Always keep the ECM on ice to avoid polymerization. Always use pre-chilled pipet tips at -20 °C to manipulate the ECM. Pipet slowly to avoid making air bubbles in the ECM. An undiluted ECM is used at this step to prevent the small drops from collapsing. - Seed six wells with a 25 µL drop per well with cooled tips in a pre-warmed 48-well plate.
NOTE: Keep the tip vertical, in the center of the well, and pipet slowly without introducing air to obtain a dome. Here, 48-well plates are used, since the organoid number is usually low when starting from frozen crypts. - Incubate for 30 min in a 37 °C, 5% CO2 incubator for polymerization of the ECM.
- Add 250 µL per well of culture medium at RT. Incubate in a 37 °C, 5% CO2 incubator and change the culture medium every 2-3 days
NOTE: The crypts are usually not visible after the thawing procedure, and most of the cells are dissociated in the ECM (Figure 2B).
3. Passage of piglet intestinal 3D organoids derived from frozen crypts
NOTE: The time to obtain organoids from frozen crypts is usually longer than when starting from fresh crypts. Organoids are usually ready for splitting 10 days after thawing (Figure 2B).
- Preparation of materials
- Place the frozen aliquots of the ECM (500 µL) at 4 °C for at least 1 h.
- Pre-warm the 24-well plates at 37 °C.
- Pre-warm the PBS and the enzyme dissociation reagent supplemented with 10 µM Y27632 ROCK inhibitor in a water bath at 37 °C.
- Place the culture medium at RT.
- Place a small ice bucket under the hood under aseptic conditions.
- Passage of 3D organoids derived from frozen crypts
- Remove the culture media and wash with 250 µL of pre-warmed PBS at 37 °C.
- Add 250 µL of pre-warmed enzyme dissociation reagent supplemented with 10 µM Y27632 ROCK inhibitor at 37 °C in each well.
NOTE: Due to the low number of organoids, the dissociation of organoids is performed directly in each well. - Detach the organoids in the ECM by scraping with a P1000 pipet, and homogenize carefully by pipetting five times.
- Incubate for 5 min in a 37 °C, 5% CO2 incubator. Dissociate the cells by pipetting up and down 10 times using a P1000 pipet.
NOTE: The objective is to obtain isolated cells or small cell clusters (<10 cells). Verify the dissociation under a microscope. If large fragments of organoids are still observed, repeat step 3.2.4. - Add 500 µL of DMEMc in each well containing dissociated cells, and pool up to 12 wells in a 15 mL conical tube containing 3 mL of cold DMEMc.
- Centrifuge at 500 x g for 5 min at 4 °C. Discard the supernatant and resuspend the pellet in 1 mL of cold DMEMc.
- Count the cells with a dilution of 1:2 in Trypan blue with a cell counter.
NOTE: The automated cell counter can count the cells within small clusters, if present. - Centrifuge the necessary volume of the cell solution to have 3,000 live cells per dome (one dome per well of the 24-well plate) at 500 x g for 5 min at 4 °C.
- Resuspend the cells with 17 µL of cold DMEMc per 3,000 live cells on ice. Adjust the volume to the required number of wells.
- Slowly add 33 µL of cold ECM with cooled tips per 3,000 live cells and homogenize on ice without making bubbles. Adjust the volume to the required number of wells.
NOTE: The cells are resuspended in a solution containing 1/3 DMEMc and 2/3 ECM. For each dome, 50 µL of this solution is needed. Diluted ECM is cheaper and easier to pipet. - Seed the wells with 50 µL of the ECM-cell suspension per well with cooled tips in a pre-warmed 24 well-plate.
- Incubate for 30 min in a 37 °C, 5% CO2 incubator for polymerization of the ECM.
- Add 500 µL of the culture medium per well. Incubate in a 37 °C, 5% CO2 incubator and change the culture medium every 2-3 days
NOTE: The organoids can either be used directly for i) experiments, ii) maintenance of the 3D organoid culture, iii) freezing, or iv) seeding of the organoid cell monolayers (Figure 1). Check the growth of the organoids with a microscope every day to choose the optimal timing for splitting the organoids. The organoids must have a clear and empty lumen and well-defined edges. Mature organoids with black debris in the lumen should not be used for splitting (Figure 3).
4. Maintenance of organoid culture in 3D
NOTE: For passaging, organoids should appear clear with an empty lumen. Black debris appears in the lumen approximately 5 days after splitting and indicates the presence of dead cells. Limiting the number of dead cells at the time of passaging is preferable for optimal maintenance of the culture. The schedule should thus be adapted to avoid reaching this stage of maturity.
- Preparation of material
- Place the aliquots of the ECM (500 µL) at 4 °C for thawing for at least 1 h.
- Pre-warm a 24-well plate at 37 °C.
- Pre-warm the enzyme dissociation reagent supplemented with 10 µM Y27632 ROCK inhibitor in a water bath at 37 °C.
- Place the culture medium at RT.
- Place a small ice bucket under the hood under aseptic conditions.
- Passage of intestinal 3D organoids
- Detach the organoids with the ECM by scraping with a P1000 pipet. Homogenize carefully by pipetting in the culture medium, and transfer to a 15 mL conical tube containing 5 mL of cold DMEMc on ice.
NOTE: One 15 mL conical tube is required for a pool with 12 wells of organoids cultured in 50 µL domes in 24-well plates. - Centrifuge the collected organoids at 500 x g for 5 min at 4 °C.
NOTE: The organoids form a white pellet at the bottom of the tube. If the organoids are still in the suspension in the ECM after centrifugation, carefully aspirate the upper supernatant (without touching the ECM layer), homogenize by pipetting 10 times with a P1000 pipet, and repeat the centrifugation step. The ECM layer should not be visible after this procedure. - Carefully aspirate the supernatant and resuspend the cell pellet in 1 mL of pre-warmed enzyme dissociation reagent supplemented with 10 µM Y27632 ROCK inhibitor. Pipet up and down 10 times to initiate dissociation of the organoids.
- Incubate for 5 min in a 37 °C water bath for enzymatic digestion.
- Mechanically disrupt the organoids by pipetting 10 times with a P1000 pipet. Check the cell suspension under the microscope.
NOTE: The objective is to obtain isolated cells or small cell clusters. If large organoid fragments are still observed, repeat the incubation (step 4.2.4) and the mechanical disruption (step 4.2.5). - Add 4 mL of ice-cold DMEMc. Centrifuge at 500 x g for 5 min at 4 °C
- Discard the supernatant and resuspend the organoid cell pellet in 1 mL of DMEMc.
- Proceed as described above (steps 3.2.7 to 3.2.13) to count the cells and seed the organoid cells in 50 µL of ECM domes containing 3000 live cells in pre-warmed 24-well plates.
- Detach the organoids with the ECM by scraping with a P1000 pipet. Homogenize carefully by pipetting in the culture medium, and transfer to a 15 mL conical tube containing 5 mL of cold DMEMc on ice.
5. Freezing of 3D organoids
- Prepare the necessary volume (1 mL for a pool of two domes) of freezing solution containing DMEMc supplemented with 10% FBS, 10% DMSO, and 10 µM Y27632 ROCK inhibitor and store on ice (the final FBS concentration is 18%).
- Remove the culture medium from the wells to be frozen.
- Add 1 mL of the freezing solution to the first well to be frozen, detach the ECM by scraping, and homogenize with the pipet tip.
- Transfer the organoid suspension from the first well to the second well to be frozen.
- Transfer the pool of the two wells to a cryotube and place the vial in a cell-freezing container.
- Store the cell freezing container at -80 °C for 24 h, and then transfer the vials to liquid nitrogen for long-term storage.
6. Culture of cell monolayers derived from 3D organoids
NOTE: Monolayers of pig organoid cells are cultured in a 2D medium composed of the culture medium used for 3D organoids supplemented with 20% FBS.
- Preparation of materials
- Prepare the 2D medium and keep it at RT.
- Pre-warm the enzyme dissociation reagent supplemented with 10 µM Y27632 ROCK inhibitor in a water bath at 37 °C.
- Coating of the culture insert
- Sterilize a pair of tweezers and transfer them to the biosafety cabinet.
- Place the cell culture inserts (0.33 cm2) into a 24-well plate with the tweezers.
- Prepare the coating solution containing collagen IV diluted at 50 µg/mL in cold PBS. Pipet up and down to mix
- Add 150 µL of the diluted collagen IV solution to each cell culture insert, which corresponds to 22.7 µg/cm2.
NOTE: Carefully orient the pipet vertically into the center of the permeable membrane and check that the collagen solution covers all the membrane. - Place the plate in a 37 °C, 5% CO2 incubator and leave overnight (or for a minimum of 3 h).
- Seeding of 3D organoid cells into cell culture inserts
- Prepare a cell suspension from the 3D organoids, as described in steps 4.2.1 to 4.2.7.
- Count the cells with a dilution of 1:2 in Trypan blue with a cell counter, and calculate the necessary volume to seed 2.5 x 105 cells per culture insert, corresponding to 7.6 x 105 cells/cm2.
- Centrifuge the necessary volume of the cell suspension at 500 x g for 5 min at 4 °C.
- During centrifugation, carefully aspirate the coating solution from the culture inserts, and allow to dry at RT under the hood without the lid for 5 min.
- After centrifugation, discard the supernatant and resuspend the cell pellet in the necessary volume of 2D medium supplemented with 10 µM Y27632 ROCK inhibitor. A volume of 200 µL of 2D medium containing the cells is needed for each insert.
- Seed 200 µL of the cell suspension (2.5 x 105 cells) onto the coated permeable membrane (apical side) (Figure 4A).
NOTE: Pipet slowly in the center of the membrane and keep the tip vertical. - Add 500 µL of the 2D medium supplemented with 10 µM Y27632 ROCK inhibitor to the lower compartment (basal side). Incubate in a 37 °C, 5% CO2 incubator.
- One day after seeding, replace the apical and basal media with fresh 2D medium without the Y27632 ROCK inhibitor.
- Change the 2D medium each day. The monolayer becomes confluent 1 day after seeding, and can then be used for experiments.
NOTE: A transepithelial electrical resistance (TEER) value above the blank (insert without cells) confirms that confluency is reached (Figure 4B).
7. Immunostaining of organoid cell monolayers
- Preparation of solutions
NOTE: Adjust the volume of solutions according to the number of wells to be stained; 200 µL of the solution is required at each step for one well. References to all commercial products are provided in the Table of materials.- Prepare a 4% paraformaldehyde (PFA) solution under a chemical hood by adding 5 mL of 32% PFA to 35 mL of PBS. Prepare 10 mL aliquots and store at -20 °C.
CAUTION: Always manipulate the PFA under the chemical hood, while wearing nitrile gloves. - Prepare a solution of 0.2% Triton X100-PBS, by adding 2 µL of Triton X100 to 1 mL of PBS immediately before use. Keep at RT.
- Prepare a 10% bovine serum albumin (BSA)-PBS solution by adding 100 mg of BSA to 1 mL of PBS. Keep at RT.
- Prepare a 1% BSA-PBS solution by adding 10 mg of BSA to 1 mL of PBS. Keep at RT.
- Prepare a solution of the occludin primary antibody diluted at 1:200 by adding 5 µL of primary antibody to 995 µL of 1% PBS BSA. Keep the solution on ice.
- Prepare a solution of the secondary antibody diluted at 1:1,000 by adding 1 µL of secondary antibody to 999 µL of 1% BSA-PBS. Keep the solution on ice, protected from light.
- Prepare a solution of phalloidin TRITC at 10 µg/mL by adding 10 µL of TRITC at 1 mg/mL to 990 µL of PBS. Keep on ice, protected from light.
- Prepare a 4% paraformaldehyde (PFA) solution under a chemical hood by adding 5 mL of 32% PFA to 35 mL of PBS. Prepare 10 mL aliquots and store at -20 °C.
- Immunostaining
NOTE: Unless indicated otherwise, all the incubations are performed at RT, under slow agitation on a rocking platform (30 rpm). The immunostaining is performed directly in the cell culture insert.- Remove the basal and apical medium. Place the plate under the chemical hood.
- Wash the monolayers twice with 200 µL of PBS at RT and incubate for 5 min.
- Fix the cell monolayers with 200 µL of 4% PFA at RT and incubate for 20 min at RT.
- Wash the monolayers twice with 200 µL of PBS at RT and incubate for 5 min.
NOTE: After the fixation and wash steps, the monolayer can be kept at 4 °C in PBS for 1 week. - Remove the PBS, permeabilize with 200 µL of 0.2% Triton X100-PBS, and incubate for 20 min.
- Wash the monolayers twice with 200 µL of PBS at RT and incubate for 5 min.
- Add 200 µL of primary antibody solution in 1% BSA-PBS and incubate overnight at 4 °C, under slow agitation on a rocking platform. Include a negative control well by adding only 1% BSA-PBS without the primary antibody.
- Wash the monolayers three times with 200 µL of PBS at RT and incubate for 5 min.
- Add 200 µL of secondary antibody in 1% BSA-PBS and incubate at RT for 2 h, protected from the light.
- Wash the monolayers three times with 200 µL of PBS at RT and incubate for 5 min.
- Add 200 µL of phalloidin TRITC at 10 µg/mL and incubate for 10 min.
- Wash the monolayers twice with 200 µL of PBS at RT and incubate for 5 min.
- Remove the PBS and cut the membrane with a scalpel.
- Recover the membrane with a pair of tweezers and place it on a microscope slide, with the apical side facing upward.
- Add 15 µL of the mounting medium supplemented with DAPI at 1:1,000 directly on the membrane. Place a coverslip and seal.
- Store at 4 °C, protected from light, until imaging.
Representative Results
Following the protocol described above, epithelial crypts are obtained from the pig intestine and cryopreserved for long-term storage in liquid nitrogen (Figure 1 and Figure 2A). After thawing, the crypt stem cells are seeded in the ECM (Figure 2B). The crypt structure is usually lost after this step, due to the disintegration of the crypt structure in the ECM. The organoids can be observed within 3-4 days, and then rapidly grow and develop budding structures (Figure 2B). We successfully obtained organoids after thawing frozen crypts in >80% of attempts. Approximately 10 days after thawing (according to the growth rate of organoids), a passage of organoids is performed to expand the culture (Figure 3). The organoids grow faster after splitting, and they present diverse morphologies, with some cystic and a majority of budding organoids. For optimal maintenance of the culture, the organoids used for passaging must present a clear and empty lumen and well-defined edges (examples indicated by green arrows) with no black debris in the lumen (indicated by red arrows), as observed in mature organoids (Figure 3). We found that black cell debris starts accumulating around day 6 post-passaging. Thus, splitting or freezing the organoids at day 4-5 post-passaging is recommended.
The maturity stage of the organoids is also an important point in obtaining cell monolayers. Organoids with advanced maturity (indicated by the presence of black cell debris in the lumen) are not optimal to seed monolayers. We usually dissociate 3D organoids 4 days after passage to collect cells for 2D culture. Approximately one to three wells of 3D organoids cultured at 3,000 cells per 50 µL dome of the ECM are needed for seeding one culture insert with 2.5 x 105 cells. Cells attach and form a fully confluent monolayer within 1 day (Figure 4A), which is confirmed by the high TEER of around 700 Ω·cm2 (Figure 4B). However, the cell edges are difficult to visualize by brightfield microscopy at this early time point, probably due to a low differentiation level. The TEER remains high for 3 days (Figure 4B).
Actin staining indicates that the apical side of the epithelial cells is oriented toward the lumen in 3D organoids (Figure 5A). Organoid cells seeded in culture insert form a confluent single layer of epithelial cells, with the apical side oriented toward the upper compartment (Figure 4B,C). Occludin staining reveals the presence of tight junctions at the apical side of the epithelial cells in 3D organoids and in cell monolayers (Figure 5A–C).
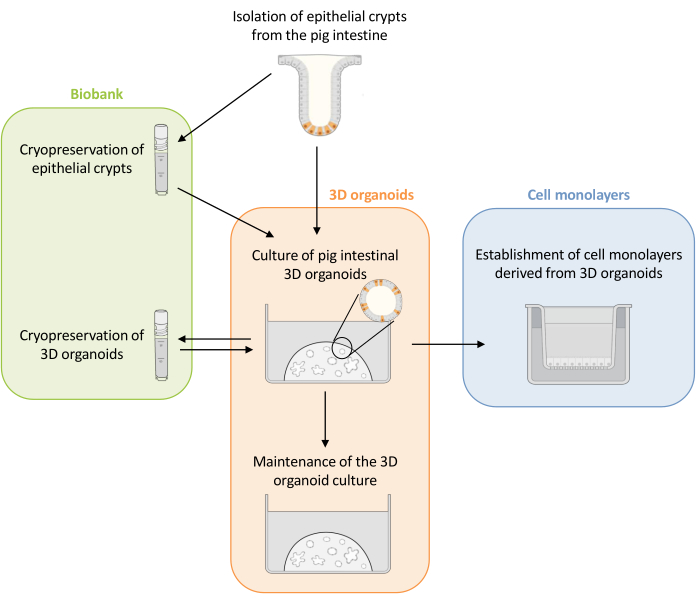
Figure 1: Schematic representation of the methods used to culture 3D organoids and cell monolayers derived from organoids. Epithelial crypts are isolated from the piglet intestine. These crypts can i) be used immediately to culture 3D organoids or ii) be frozen and stored in a biobank in liquid nitrogen. Cryopreserved crypts can be thawed and used to culture 3D organoids. The 3D organoid culture can be maintained with successive splitting, or frozen and stored in the biobank. Cell monolayers can be obtained from the 3D organoid culture to allow access to the apical side of the cells and study the epithelial barrier function. Please click here to view a larger version of this figure.
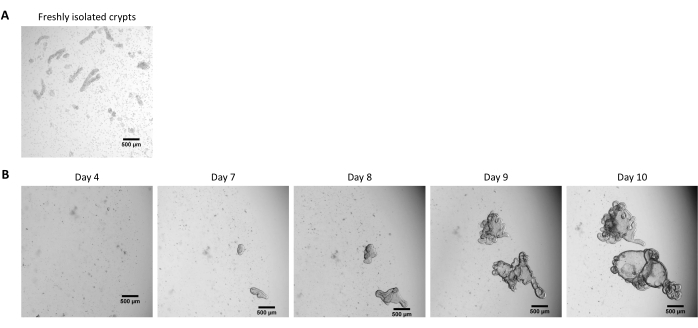
Figure 2: Culture of pig intestinal 3D organoids from cryopreserved epithelial crypts. (A) Representative microscopic image of freshly isolated jejunal crypts. (B) Representative microscopic images of 3D organoids obtained after thawing of the jejunal crypts. The organoids were cultured in a 25 µL dome of ECM in a 48-well plate. The figure shows images of the 3D organoids at 4, 7, 8, 9, and 10 days post-seeding. The scale bar represents 500 µm. Please click here to view a larger version of this figure.
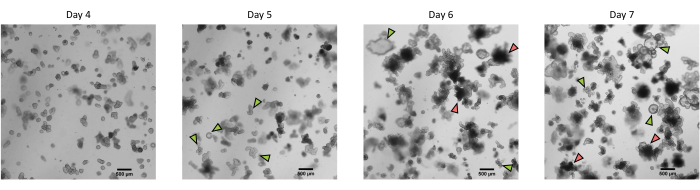
Figure 3: Morphology of pig intestinal 3D organoids derived from cryopreserved epithelial crypts after the first passage. Representative microscopic images showing the development of organoids derived from cryopreserved jejunum crypts after the first passage from day 4 to day 7. Green arrows indicate clear organoids appropriate for the passage or seeding of monolayers. Red arrows indicate mature organoids not suitable for splitting or seeding monolayers; thus, wells should be used before the apparition of this morphology. The scale bar represents 500 µm. Please click here to view a larger version of this figure.
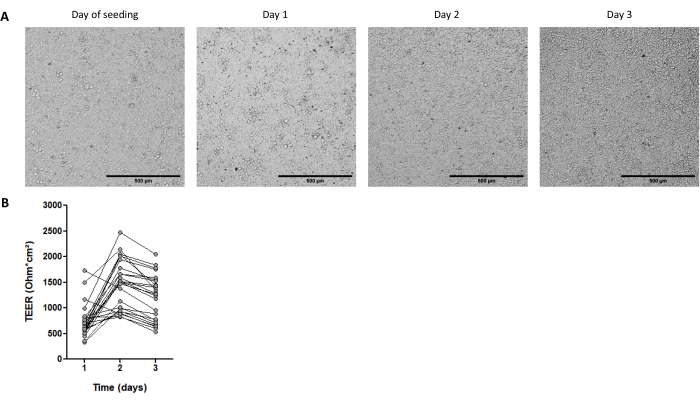
Figure 4: Characteristics of cell monolayers derived from pig intestinal 3D organoids. (A) Representative microscopic images of the monolayer morphology over 3 days. Jejunum organoid cells were seeded at 2.5 x 105 cells into 0.33 cm2 cell culture inserts coated with collagen IV at 50 ng/mL. The scale bar represents 500 µm. (B) Transepithelial electrical resistance (TEER) of organoid cell monolayers over 3 days. Dots connected by a line correspond to the same well at different times. Please click here to view a larger version of this figure.
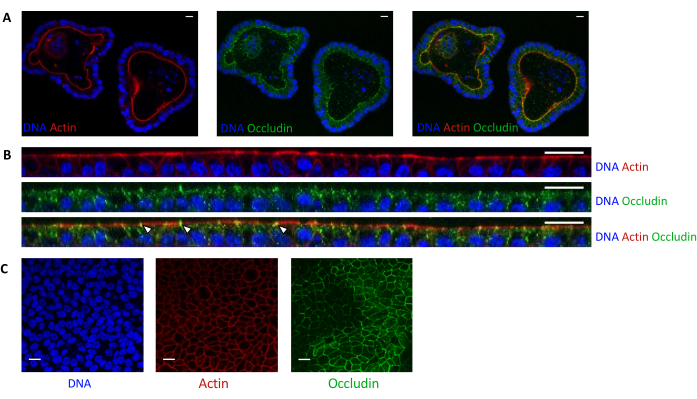
Figure 5: Imaging of pig jejunum organoids cultured in 3D or 2D. Confocal microscopy imaging of (A) 3D organoids 5 days after splitting and (B,C) organoid cell monolayers 3 days after seeding (B: XZ section; C: XY section). DNA (blue) was stained with DAPI. Actin (red) was stained with phalloidin. Occludin (green) was stained with a polyclonal antibody. White arrows indicate occludin localized at the tight junction. The scale bar represents 20 µm. Please click here to view a larger version of this figure.
Discussion
This protocol describes a method used to cryopreserve epithelial crypts from the piglet intestine for the long-term storage and subsequent culture of 3D organoids. This protocol uses a freezing solution containing DMSO, FBS, the Y27632 ROCK inhibitor, DMEM, and antibiotics. Another study in pigs obtained organoids from crypts cryopreserved in a similar freezing solution but without the ROCK inhibitor15. The Y27632 ROCK inhibitor was included to prevent apoptosis and maintain the stem cell pool since, after thawing, epithelial crypt cells are dissociated, which may lead to cell death ('anoikis')27,28. Interestingly, equine enteroids have been obtained from epithelial crypts frozen in a culture medium containing only DMEM and DMSO16; this simple method has not been tested for pig epithelial crypts yet. Other methods have been published to grow human and pig organoids from frozen tissues or biopsies instead of epithelial crypts4,17. The advantage of this method is the ability to directly cryopreserve the intestinal tissues without performing the crypt isolation procedure, which requires time and laboratory equipment. This might be convenient when the tissues have to be collected far from the lab. However, when isolating the crypts immediately after slaughter, large segments of the intestine can be processed to obtain a very high number of crypts, which is not the case when starting from small frozen tissue fragments. After the thawing of epithelial crypts, organoids were observed from 3-4 days post-seeding and split after 10 days. This is a slower growth rate than when starting the culture from fresh epithelial crypts, for which the organoids were obtained from day 1 post-seeding, and can usually be split at around day 511. Khalil et al. also reported a delayed growth of pig enteroids when starting from frozen crypts15, suggesting that stem cells might require time to recover their proliferative capacity. We also obtained a lower number of organoids when starting from frozen crypts compared to fresh crypts, which might be due to the death of stem cells during the freezing process. In some attempts of crypts thawing (<20%), we did not obtain organoids from frozen crypts, probably due to a suboptimal cryopreservation procedure (e.g., delayed freezing after crypt isolation of probably more than 1 h). Thus, we recommend keeping the crypts on ice until counting, and freezing them as quickly as possible.
For 3D organoids, we chose to use a commercial organoid culture medium formulated for humans. Indeed, previous reports have showed that pig intestinal organoids grow efficiently with this medium8,11,14,19,25,26,29,30. It is of interest for this culture medium to be ready-to-use and have a standardized concentration of growth factors within a batch. However, this culture medium is costly, its composition is undisclosed, and it is thus not possible to modulate its composition. In contrast, other studies have cultured pig intestinal organoids in customized media containing pharmacological inhibitors, recombinant growth factor, and/or conditioned media5,6,7,21. Although highly flexible and cheaper, this method is time-consuming for the production of conditioned media and might lack reproducibility due to potential variability in the concentration of growth factors in conditioned media. Thus, the quality of each batch of conditioned media should be validated by measuring organoid growth or marker gene expression31.
A study has showed that pig jejunal organoids cultured in the same commercial organoid culture medium used here grew faster and seemed less differentiated, compared to enteroids cultured with media containing recombinant growth factor and/or conditioned media23. A high proliferative state facilitates the culture of 3D organoids, but might require inducing differentiation to be more representative of intestinal physiological characteristics. In this protocol, for the passage of 3D organoids, the cells are fully dissociated for counting, allowing to control the number of cells seeded in ECM. This increases the reproducibility of the phenotype of organoids, which is highly influenced by their density. Moreover, counting the cells avoids obtaining too low or an overcrowded culture, that requires adapting the culture schedule. Most other studies prepared organoid fragments not fully dissociated to single cells, and used a dilution ratio for passaging. This method is more straightforward, but might induce variability according to the organoid density of the culture.
For the culture in monolayers, organoid cells are seeded in culture inserts precoated with a thin layer of ECM, that allows the attachment of cells but avoids the growth of organoids in 3D. This protocol used collagen type IV as an ECM protein, as described previously in pigs23. Other studies with pig organoid monolayers used the same tumor-derived ECM used here to culture 3D organoids6,8,9,21,25,30. The advantage of using collagen is the ability to standardize the protein concentration with a fully defined composition, which is not the case in the tumor-derived ECM. A critical step for the success of the culture of cell monolayers is to pay attention to the visual appearance of the precursor 3D organoids, which should have well-defined edges and an empty lumen without black debris. Indeed, organoids with a high maturation level and a low proliferative rate are not an appropriate source of cells for 2D culture. Thus, the timing of the dissociation of 3D organoids into single cells is crucial for the success of this step.
The culture of 2D monolayers from single cells allows standardizing the number of cells seeded, which is more difficult when starting from organoid fragments, as performed in some other methods. We seeded 7.6 x 105 cells per cm2, which is high compared to most other studies21,22,23 in pigs that used a lower cell density, ranging from 0.25 x 105 cells per cm2 to 1.78 x 105 cells per cm2. The requirement of a high number of organoid cells constitutes a limitation of this protocol, but it allowed us to quickly obtain a confluent monolayer, fully covering the culture insert after 1 day. In contrast, Vermeire et al.23 obtained confluency after 4-7 days with a lower density of cells seeded (from 0.25 x 105 cells/cm2 to 0.4 x 105 cells/cm2). Some studies have also used pig organoid cell monolayers that did not fully cover the culture surface for infections with viruses8,30. In these conditions, the apical side of epithelial cells is accessible for treatments, but fully confluent monolayers are required if the objective is to study nutrient absorption or epithelial permeability.
For organoid cell monolayers, a commercial organoid culture medium supplemented with 20% FBS was used, based on a recent study on bovine enteroid-derived monolayers32. In our tests, the supplementation with 20% FBS was necessary to obtain fully confluent monolayers, probably due to a high growth factor requirement. On the contrary, other studies using the same commercial medium have established monolayers without additional FBS8,25,30, but without reaching full confluency. Other studies have also used supplementation with 20% FBS in a customized medium for the culture of pig organoid cell monolayers21,22. In our experiments, TEER is high 1 day after seeding (around 700 Ω·cm2), and remains high until day 3 (around 1,500 Ω·cm2; this is consistent with the formation of tight junctions, as indicated by the expression of occludin. Van der Hee et al. obtained similar TEER values over 72 h for jejunum organoid cell monolayers21. They also demonstrated that monolayers can be maintained until day 12-15 with daily media changes. In contrast, other studies have reported much lower TEER values (around 200 Ω·cm2) for pig organoid cell monolayers6,22. These differences between studies might be related to the gut segment studied or to the media used that influence epithelial differentiation.
In conclusion, the above protocol to grow pig intestinal 3D organoids from frozen epithelial crypts facilitates the organization of the culture work. It reduces the need for fresh tissues to be obtained from living animals. We also explain how to establish fully confluent cell monolayers derived from pig organoids in less than 3 days. Thus, our protocols could be useful resources for scientists studying the pig intestinal epithelium for veterinary or biomedical research.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by Institut Carnot France Futur Elevage ("OrganoPig" project) and by INRAE HOLOFLUX ("Holopig" project). The authors are grateful to the Genotoul core facilities (TRI). We acknowledge Christelle Knudsen (GenPhySE, INRAE, Toulouse) for careful proofreading.
Materials
| 0.5 M EDTA, pH 8.0 | Thermo Fisher Scientific | AM9260G | Store at room temperature. |
| 15 mL conical tube | Sarstedt | 62.554.502 | |
| 24-well cell culture plate | Corning | 003526 | |
| 48-well cell culture plate | Corning | 003548 | |
| 50 mL polypropylene conical tube | Falcon | 352070 | |
| Bovine Serum Albumine (BSA) | Euromedex | A6003 | Store at 4 °C. |
| Centrifuge Universal 320 R | Hettich | 1406 | |
| Collagene type IV from human placenta | Sigma | C5533 | Prepare the stock solution at 1 mg/mL in acetic acid according to the manufacturer's recommendation. Aliquot (500 µL) and store at -20 °C. |
| CoolCell LX Cell Freezing Container | Corning | 432003 | Used to cryopreserve crypts and organoids. |
| Countess 3 Automated Cell Counter | Thermo Fisher Scientific | 16842556 | |
| Coverslips, 22 mm x 50 mm | VWR | 630-1845 | |
| Cryotube ClearLine 1 mL | Clear line | 390706 | Used to cryopreserve crypts and organoids. |
| DAPI | Invitrogen | D1306 | Prepare the stock solution at 5 mg/mL in water according to the manufacturer’s recommendation. Aliquot (20 µL) and store at -20 °C |
| Dithiothreitol (DTT) | Merck | 10197777001 | Store at 4 °C. |
| DMEM, high glucose, GlutaMAX Supplement, pyruvate | Thermo Fisher Scientific | 31966047 | Store at 4 °C. |
| DMSO (Dimethyl Sulfoxide) | Corning | 25-950-CQC | Store at room temperature. |
| Epredia Superfrost Plus Adhesion microscopic slide | VWR | 631-9483 | |
| Fetal Bovine Serum (FBS) | Thermo Fisher Scientific | 10270-106 | Store 5 mL aliquots at -20 °C. |
| Fisherbrand Sterile Cell Strainers | Thermo Fisher Scientific | 22363549 | Used for crypt isolation. |
| Fixed Tilt 3D Platform Rotator | VWR | 97025-564 | Used for incubations in the immunostaining protocol. |
| Gibco PBS, pH 7.4 | Thermo Fisher Scientific | 10010015 | Store at 4 °C. |
| Gibco TrypLE Express Enzyme (1x), phenol red | Thermo Fisher Scientific | 12605-010 | Enzyme dissociation reagent. Store at room temperature. |
| Goat anti-rabbit IgG, Alexa fluor 488 | Thermo Fisher Scientific | A-11008 | Secondary antibody. Store at 4 °C. Working dilution 1:1000. |
| IntestiCult Organoid Growth Medium (Human) | Stem Cell Technology | 6010 | Organoid culture medium. Store at -20 °C. Thaw the basal medium and organoid supplement at room temperature and mix (1:1). Store the mix at 4 °C for up to 1 week. |
| Insert with PET membrane transparent Falcon for plate 24 wells | Corning | 353095 | |
| Inverted microscope | Nikon | Eclipse TS2 | |
| Matrigel Basement Membrane Matrix | Corning | 354234 | Tumor-derived extracellular matrix used for the 3D culture of organoids. Matrigel polymerizes at room temperature. Use cooled tips to pipet the Matrigel. Prepare 500 µL aliquots and store at -20 °C. |
| Mounting medium for fluorescence with DAPI | Vectashield | H1250 | Store at 4 °C. |
| Occludin polyclonal antibody | Thermo Fisher Scientific | 71-1500 | Primary antibody. Store at -20 °C. Working dilution 1:200. |
| Paraformaldehyde 32% | Electron microscopy science | 15714 | Prepare 4% paraformaldehyde (PFA) solution under chemical hood by adding 5 mL of 32% PFA to 35 mL of PBS. Aliquot by 10 mL and store at -20 °C. |
| Penicillin-Streptomycin | Sigma | P4333 | Antibacterial. Store 5 mL aliquots at -20 °C. |
| Phalloidin TRITC | Sigma | P1951 | Probe for actin staining. 1 mg/mL stock solution. Store at 4 °C. |
| Primocin | InvivoGen | ant-pm-05 | Antimicrobial agent for primary cells acting on bacteria, mycoplasma and fungi. Store at -20 °C. |
| ROCK Inhibitor (Y27632) | ATCC | ACS-3030 | Used to maintain the stem cells. Prepare the stock solution at 10 mM in sterile water according to the manufacturer's recommendation and store aliquots (50 µL) at -20 °C. |
| Rotating shaker mix XL | Clear line | 062646CL | Used for crypt isolation. |
| Stripette Serological Pipets 10 mL | Corning | 4488 | |
| Tissue Culture Dish | TPP | 93100 | |
| Triton X100 | Sigma | 8787 | Store at room temperature. |
| Trypan Blue stain 0.4% | Thermo Fisher Scientific | T10282 | Store at room temperature. |
| Vacuum system Vacusip | Integra | 159000 | Used to remove the medium of organoid wells. |
参考文献
- Peterson, L. W., Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature Reviews Immunology. 14 (3), 141-153 (2014).
- In, J. G., et al. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nature Reviews Gastroenterology & Hepatology. 13 (11), 633-642 (2016).
- Sato, T., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Beaumont, M., et al. Intestinal organoids in farm animals. Veterinary Research. 52 (1), 33 (2021).
- Gonzalez, L. M., Williamson, I., Piedrahita, J. A., Blikslager, A. T., Magness, S. T. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One. 8 (6), 66465 (2013).
- Holthaus, D., Delgado-Betancourt, E., Aebischer, T., Seeber, F., Klotz, C. Harmonization of protocols for multi-species organoid platforms to study the intestinal biology of toxoplasma gondii and other protozoan infections. Frontiers in Cellular and Infection Microbiology. 10, 610368 (2021).
- Powell, R. H., Behnke, M. S. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biology Open. 6 (5), 698-705 (2017).
- Li, L., et al. Porcine intestinal enteroids: a new model for studying enteric coronavirus porcine epidemic diarrhea virus infection and the host innate response. Journal of Virology. 93 (5), 01682 (2019).
- vander Hee, B., Madsen, O., Vervoort, J., Smidt, H., Wells, J. M. Congruence of transcription programs in adult stem cell-derived jejunum organoids and original tissue during long-term culture. Frontiers in Cell and Developmental Biology. 8, 375 (2020).
- Barnett, A. M., et al. Porcine colonoids and enteroids keep the memory of their origin during regeneration. American Journal of Physiology. Cell Physiology. 320 (5), 794-805 (2021).
- Mussard, E., et al. The phenotype of the gut region is more stably retained than developmental stage in piglet intestinal organoids. Frontiers in Cell and Developmental Biology. 10, 983031 (2022).
- Zhu, M., Qin, Y. -. C., Gao, C. -. Q., Yan, H. -. C., Wang, X. -. Q. l-Glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway. Food & Function. 11 (3), 2714-2724 (2020).
- Wang, Z., et al. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. Journal of Animal Science. 98 (2), (2020).
- Derricott, H., et al. Developing a 3D intestinal epithelium model for livestock species. Cell and Tissue Research. 375 (2), 409-424 (2019).
- Khalil, H. A., et al. A novel culture system for adult porcine intestinal crypts. Cell and Tissue Research. 365 (1), 123-134 (2016).
- Stewart, A. S., Freund, J. M., Gonzalez, L. M. Advanced three-dimensional culture of equine intestinal epithelial stem cells. Equine Veterinary Journal. 50 (2), 241-248 (2018).
- Tsai, Y. -. H., et al. A method for cryogenic preservation of human biopsy specimens and subsequent organoid culture. Cellular and Molecular Gastroenterology and Hepatology. 6 (2), 218-222 (2018).
- Wilson, S. S., Tocchi, A., Holly, M. K., Parks, W. C., Smith, J. G. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunology. 8 (2), 352-361 (2015).
- Li, Y., et al. Next-generation porcine intestinal organoids: an apical-out organoid model for swine enteric virus infection and immune response investigations. Journal of Virology. 94 (21), 01006-01020 (2020).
- Moon, C., VanDussen, K. L., Miyoshi, H., Stappenbeck, T. S. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunology. 7 (4), 818-828 (2014).
- vander Hee, B., et al. Optimized procedures for generating an enhanced, near physiological 2D culture system from porcine intestinal organoids. Stem Cell Research. 28, 165-171 (2018).
- Hoffmann, P., et al. Intestinal organoid-based 2D monolayers mimic physiological and pathophysiological properties of the pig intestine. PLoS One. 16 (8), 0256143 (2021).
- Vermeire, B., Gonzalez, L. M., Jansens, R. J. J., Cox, E., Devriendt, B. Porcine small intestinal organoids as a model to explore ETEC-host interactions in the gut. Veterinary Research. 52 (1), 94 (2021).
- Luo, H., et al. Utility evaluation of porcine enteroids as PDCoV infection model in vitro. Frontiers in Microbiology. 11, 821 (2020).
- Resende, T. P., Medida, R. L., Vannucci, F. A., Saqui-Salces, M., Gebhart, C. Evaluation of swine enteroids as in vitro models for Lawsonia intracellularis infection1,2. Journal of Animal Science. 98 (2), 011 (2020).
- Engevik, A. C., et al. Editing myosin VB gene to create porcine model of microvillus inclusion disease, with microvillus-lined inclusions and alterations in sodium transporters. Gastroenterology. 158 (8), 2236-2249 (2020).
- Watanabe, K., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 25 (6), 681-686 (2007).
- Gracz, A. D., Puthoff, B. J., Magness, S. T. Identification, isolation, and culture of intestinal epithelial stem cells from murine intestine. Somatic Stem Cells: Methods and Protocols. 879, 89-107 (2012).
- Ferrandis Vila, M., et al. Dietary fiber sources and non-starch polysaccharide-degrading enzymes modify mucin expression and the immune profile of the swine ileum. PloS One. 13 (11), 0207196 (2018).
- Li, L., et al. IFN-lambda 3 mediates antiviral protection against porcine epidemic diarrhea virus by inducing a distinct antiviral transcript profile in porcine intestinal epithelia. Frontiers in Immunology. 10, 2394 (2019).
- VanDussen, K. L., Sonnek, N. M., Stappenbeck, T. S. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Research. 37, 101430 (2019).
- Sutton, K. M., Orr, B., Hope, J., Jensen, S. R., Vervelde, L. Establishment of bovine 3D enteroid-derived 2D monolayers. Veterinary Research. 53 (1), 15 (2022).

