Analysis of the Lipid Composition of Mycobacteria by Thin Layer Chromatography
概要
A protocol is presented to extract the total lipid content of the cell wall of a wide range of mycobacteria. Moreover, extraction and analytical protocols of the different types of mycolic acids are shown. A thin-layer chromatographic protocol to monitor these mycobacterial compounds is also provided.
Abstract
Mycobacteria species can differ from one another in the rate of growth, presence of pigmentation, the colony morphology displayed on solid media, as well as other phenotypic characteristics. However, they all have in common the most relevant character of mycobacteria: its unique and highly hydrophobic cell wall. Mycobacteria species contain a membrane-covalent linked complex that includes arabinogalactan, peptidoglycan, and long-chains of mycolic acids with types that differ between mycobacteria species. Additionally, mycobacteria can also produce lipids that are located, non-covalently linked, on their cell surfaces, such as phthiocerol dimycocerosates (PDIM), phenolic glycolipids (PGL), glycopeptidolipids (GPL), acyltrehaloses (AT), or phosphatidil-inositol mannosides (PIM), among others. Some of them are considered virulence factors in pathogenic mycobacteria, or critical antigenic lipids in host-mycobacteria interaction. For these reasons, there is a significant interest in the study of mycobacterial lipids due to their application in several fields, from understanding their role in the pathogenicity of mycobacteria infections, to a possible implication as immunomodulatory agents for the treatment of infectious diseases and other pathologies such as cancer. Here, a simple approach to extract and analyze the total lipid content and the mycolic acid composition of mycobacteria cells grown in a solid medium using mixtures of organic solvents is presented. Once the lipid extracts are obtained, thin-layer chromatography (TLC) is performed to monitor the extracted compounds. The example experiment is performed with four different mycobacteria: the environmental fast-growing Mycolicibacterium brumae and Mycolicibacterium fortuitum, the attenuated slow-growing Mycobacterium bovis bacillus Calmette-Guérin (BCG), and the opportunistic pathogen fast-growing Mycobacterium abscessus, demonstrating that methods shown in the present protocol can be used to a wide range of mycobacteria.
Introduction
Mycobacterium is a genus that comprises pathogenic and non-pathogenic species, characterized by having a highly hydrophobic and impermeable cell wall formed by their peculiar lipids. Specifically, the mycobacterial cell wall contains mycolic acids, which are α-alkyl and β-hydroxy fatty acids, in which the α-branch is constant in all mycolic acids (except for the length) and the β-chain, called the meromycolate chain, is a long aliphatic chain that may contain different functional chemical groups described along with the literature (α-, α'-, methoxy-, κ-, epoxy-, carboxy-, and ω-1-methoxy- mycolates), therefore producing seven types of mycolic acids (I-VII)1. Moreover, other lipids with unquestionable importance are also present in the cell wall of mycobacteria species. Pathogenic species such as Mycobacterium tuberculosis, the causative agent of tuberculosis2 produce specific lipid-based virulence factors such as phthiocerol dimycocerosates (PDIMs), phenolic glycolipid (PGL), di-, tri-, and penta-acyltrehaloses (DAT, TAT, and PAT), or sulfolipids, among others3. Their presence on the mycobacterial surface have been associated with the ability to modify the host immune response and therefore, the evolution and persistence of the mycobacterium inside the host4. For instance, the presence of triacylglycerols (TAG) has been associated with the hypervirulent phenotype of Lineage 2-Beijing sub-lineage of M. tuberculosis, possibly due to its capacity to attenuate the host immune response5,6. Other relevant lipids are lipooligosaccharides (LOSs) present in tuberculous and nontuberculous mycobacteria. In the case of Mycobacterium marinum, the presence of LOSs in its cell wall is related to sliding motility and the ability to form biofilms and interferes with recognition by macrophage pattern recognition receptors, affecting uptake and elimination of the bacteria by host phagocytes7,8. Additionally, the absence or presence of some lipids allows members of the same species to be classified into different morphotypes with virulent or attenuate profiles when interacting with host cells. For instance, the absence of glycopeptidolipids (GPL) in the rough morphotype of Mycobacterium abscessus has been associated with the ability to induce intraphagosomal acidification, and consequently cell apoptosis9, unlike the smooth morphotype that possesses GPLs in their surface. Furthermore, the lipid content of the mycobacterial cell wall is related to the ability to modify the immune response in the host. This is relevant in the context of using some mycobacteria to trigger a protective immune profile against different pathologies10,11,12,13. It has been demonstrated, for example, that Mycolicibacterium vaccae, a saprophytic mycobacterium, which is currently in phase III clinical trials as an immunotherapeutic vaccine for tuberculosis, display two colonial morphotypes. While the smooth phenotype, that contains a polyester in its surface, triggers a Th2 response, the rough phenotype devoid of the polyester can induce a Th1 profile when it interacts with host immune cells14. The repertoire of lipids present in the mycobacterial cell not only depends on mycobacteria species, but also on the conditions of mycobacterial cultures: time of incubation15,16 or composition of the culture medium17,18. In fact, changes in the culture medium composition affect the antitumor and immunostimulatory activity of M. bovis BCG and Mycolicibacterium brumae in vitro17. Moreover, the protective immune profile triggered by M. bovis BCG against M. tuberculosis challenge in mice models also depends on the culture media in which M. bovis BCG grows17. These could then be related to the lipid composition of the mycobacteria in each culture condition. For all these reasons, the study of the lipid content of mycobacteria is relevant. A visual procedure to extract and analyze the lipid composition of the mycobacterial cell wall is presented.
Protocol
1. Extraction of the total non-covalent-linked lipids from mycobacteria (Figure 1)
- Scratch 0.2 g of mycobacteria from a solid media and add to a glass tube with a polytetrafluoroethlene (PTFE) liner screw caps. Add a solution consisting of 5 mL of chloroform and 10 mL of methanol (chloroform:methanol, 1:2).
NOTE: When organic solvents are used, only glass recipient should be used. No plastic containers are allowed. Moreover, PTFE liner screw caps for bottles are needed.
CAUTION: Chloroform is a potentially toxic and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Methanol is a potentially toxic and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Leave the tube in constant stirring overnight to extract non-covalent-linked lipids from the mycobacterial cell surface.
NOTE: If an orbital shaking platform is not available, constant stirring can be replaced by periodic manual stirring as frequently as possible. - Cover a glass funnel with a filter paper, filter the organic solvents, and collect them in a glass tube.
- Use a nitrogen gas flux to evaporate the liquid phase in the tube. Fill the tube with nitrogen gas, cover and store it at 4 °C.
NOTE: Connect a glass Pasteur pipette to the stream of nitrogen gas to specifically evaporate the desired tube. Additionally, maintain the tube inside a dry block heater for tubes at 37 °C. When the solvent evaporates, fill the tube with nitrogen gas before closing it. - Add 15 mL of a solution of chloroform:methanol (2:1) to the cellular debris. Leave the tube in constant stirring overnight to extract non-covalent-linked lipids from the mycobacterial cell surface.
NOTE: If an orbital shaking platform is not available, constant stirring can be replaced by periodic manual stirring as frequently as possible. - Let the mixture rest for 1 h. With a Pasteur pipette, recover the organic solvents. Cover a glass funnel with a filter paper and filter the organic solvents and collect them in the same glass tube previously used in step 1.3. Use a nitrogen gas flux to evaporate the liquid phase in the tube. Fill the tube with nitrogen gas, close it and store it again at 4 °C.
2. Mycolic acid extraction by acid methanolysis (Figure 2A)
- Add 2-5 mL of esterifying solution into a hermetic glass tube with a PTFE liner screw cap. Add 0.2 g of mycobacteria biomass into the glass tube.
NOTE: Esterifying solution is formed by mixing 30 mL of methanol, 15 mL of toluene, and 1 mL of sulfuric acid. Mycobacteria cells can be taken from solid cultures or, even from delipidated cells after performing extraction of total non-covalent-linked lipids from mycobacteria (remaining cells after filtering in step 1.6).
CAUTION: Toluene is a flammable and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Sulfuric acid is a corrosive and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Mix the content by vortexing. Let the mixture stand inside a dry bath at 80 °C overnight.
- Allow the tube to cool until it reaches the room temperature and then add 2 mL of n-hexane to the tube. Mix the contents by vortexing for 30 s and allow the tube to settle until two clear phases appear.
CAUTION: n-hexane is a potential flammable, irritant, environmentally damaging, and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Recover the upper phase corresponding to the n-hexane phase. Transfer it to a new tube.
- Repeat the step 2.3. Recover the upper phase again and transfer it to the same tube used in step 2.4.
- Evaporate the contents of the tube using a nitrogen gas flux. Fill the tube with nitrogen gas, close it, and store it at 4 °C.
3. Mycolic acid extraction by saponification and methylation (Figure 2B)
- Scratch 0.2 g of mycobacteria from a solid media and add to a glass tube with a PTFE screw cap.
- Add 2 mL of methanol-benzene solution (80:20) containing 5% potassium hydroxide. Mix the contents by vortexing. Heat the mixture for 3 h at 100 °C.
CAUTION: Benzene is a flammable, carcinogenic, and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Allow the tube to cool to room temperature. Add 20% sulfuric acid to acidify the samples to achieve pH = 1.
- Add 3 mL of diethyl ether. Gently mix the contents by vortexing.
- Let the two phases form by settling. Recover the diethyl ether phase and transfer to a new tube. Repeat the wash step for a total of three times.
- Wash the diethyl ether extract with 2 mL of distilled water and transfer the upper part corresponding to the diethyl ether to a new tube. Repeat the wash step for a total of three times.
- Add 2 g of anhydrous sodium sulfate over the diethyl ether extract to dry it.
- Filter the suspension. Evaporate the content using a nitrogen gas flux.
- To perform the methylation step, dissolve 3 g of N-nitroso-N-methyl urea in a precooled solution formed by 45 mL of diethyl ether and 9 mL of 40% KOH in distilled water.
CAUTION: N-nitroso-N-methylurea is a toxic, irritant, carcinogenic, and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Transfer the supernatant (diazomethane) to a new flask cooled in ice containing potassium hydroxide pellets (approximately 30 g).
NOTE: If the supernatant is not immediately used, it can be stored at -20 °C for a maximum of 1 h.
CAUTION: Potassium hydroxide pellets are an irritant and corrosive substance. This material must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Diazomethane is highly toxic and potentially explosive. It must be used in a laminar flow hood with safety glass wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Add 2 mL of the ether solution containing diazomethane, obtained in step 3.10, into the dried diethyl ether extract that contains mycolic acids, obtained in step 3.8. Incubate for 15 min at room temperature.
- Evaporate the suspension at 40 °C. Fill the tube with nitrogen gas, close it, and store the methylated lipids at 4 °C.
NOTE: Evaporate the diazomethane from the ether solution under the laminar flow hood, until the ether loses the yellow color.
4. Thin layer chromatography (TLC) analysis
- Saturate the glass TLC chamber. To do this, cover one of the walls of the TLC chamber with a piece of filter paper and allow it to be in contact with the mobile phase composed by the mixture of solvents. Place the remaining volume of the solvent onto the bottom of the TLC chamber.
NOTE: The bottom of the TLC chamber must be covered by at least 1 cm of the mobile phase. In the present experiments, different mobile phases were used to develop the TLCs. They consisted of 85 mL of n-hexane plus 15 mL of diethyl ether; 100 mL of dichloromethane; 90 mL of chloroform, 10 mL of methanol, and 1 mL of water; 30 mL of chloroform, plus 8 mL of methanol, and 1 mL of water; 60 mL of chloroform, plus 35 mL of methanol, and 8 mL of water; 95 mL chloroform plus 5 mL of methanol; and 90 mL of petroleum ether (60-80 °C) plus 10 mL of diethyl ether.
NOTE: In the two-dimensional TLC, use n-hexane:acetone (95:5) in the first direction three times, and use a single development with toluene:acetone (97:3) in the second direction to analyze mycolic acid composition. To analyze PIMs, use chloroform:methanol:water (60:30:6) in the first direction once, and use chloroform:acetic acid:methanol:water (40:25:3:6) in the second direction. To analyze PDIM and AG, use petroleum ether (60-80 °C):ethyl acetate (98:2) in the first direction three times, and use a single development with petroleum ether (60-80 °C):acetone (98:2) in the second direction.
CAUTION: Diethyl ether is a potentially toxic and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Dichloromethane is a potentially toxic and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Petroleum ether is a potential flammable, environmentally damaging and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Acetic acid is a potential flammable and corrosive substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Ethyl acetate is a flammable and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Acetone is a flammable and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves). - Close the TLC chamber to saturate it for at least 20 min. Meanwhile, dissolve the lipids present in the glass tube in 0.2-1 mL of chloroform.
NOTE: The volume used to dissolve the lipids can be modified depending on the desired or expected concentration of the sample. - Apply 10 µL of each suspension using a capillary glass tube directly on the TLC plate and let the sample dry for 5 min at room temperature.
NOTE: Samples must be applied at the bottom part of the plate leaving 1 cm on each side. Samples must be separated one from another for at least 0.5 cm. Once the sample is applied on the plate, tubes can be evaporated again with nitrogen gas and stored at 4 °C for further use. - Insert the plate into the saturated TLC chamber containing the mobile phase. Allow the mobile phase to run through the TLC.
NOTE: Any movement applied to the TLC chamber affects the running solvent on the plate and affects lipid mobility. In the case of performing two-dimensional TLC, two TLC chambers are required to contain both elution systems. - Remove the plate from the TLC chamber when the solvent reaches 1 cm distance from the upper end of the plate. Leave the plate under laminar flux until the silica is totally dried.
NOTE: In the case of analyzing the mycolic acid composition, using n-hexane and diethyl-ether (85:15), repeat steps 4.4 and 4.5 two times more, until running the mobile phase three times over the TLC plate. - Reveal the plate with the required stain; heat the plate if required.
NOTE: In the present experiment, 15-20 mL of the following solutions were used to spray the TLC plates: 10% Molybdatophosphoric acid hydrate in ethanol until the plate is bright yellow, followed by heating the plate at 120 °C; 5% in ethanol of 20% α-naphthol in sulfuric acid followed by heating the plate at 120 °C; Molybdenum Blue reagent (1.3% molybdenum oxide in 4.2 M sulfuric acid) until phosphate bands appeared or 1% anthrone in sulfuric acid.
CAUTION: Molybdatophosphoric acid hydrate is a flammable and corrosive substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Ethanol is a potential flammable and hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: 1-Naphthol is a flammable, corrosive, and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
CAUTION: Molybdenum Blue Spray Reagent is a corrosive, toxic, and extremely hazardous substance. It must be used in a laminar flow hood wearing appropriate personal protective equipment (laboratory coat, protective eyewear, and nitrile gloves).
Representative Results
With the aim of showing a wide range of lipids present in different mycobacteria species, M. bovis BCG was selected as it is rough and slow-growing mycobacteria. The rough and fast-growing M. fortuitum and M. brumae were added in the procedure and, finally, the smooth morphotype of M. abscessus was also included. These four species permit us to visualize a broad spectrum of mycobacteria-derived lipids such as acyltrehaloses (AT), GPLs, PDIM, PGL, PIM, TDM, and TMM. Moreover, all four species have different mycolic acid patterns.
After performing the mycolic acid extraction protocols, lipid extracts were analyzed through 1D-TLC analysis using two different, equally valid, elution systems (Figure 3A,B). The first mobile phase (Figure 3A) was composed by n-hexane and diethyl-ether (85:15), and the plate was run three times. The second mobile phase consisted of 100% of dichloromethane and the plate was eluted once (Figure 3B). In both the elution systems, mycolic acids are located approximately in the middle of the TLC plate from the origin of sample application. As Figure 3 shows, M. brumae only possesses type I mycolic acids, a mycolic acid present in all mycobacteria species. M. bovis BCG has type I and IV, M. fortuitum type I and V, and M. abscessus, type I and II mycolic acids profiles. Performing two types of methylation procedures permits us to confirm the presence of type V mycolic acid since type V mycolic acid is cleaved during the acid methanolysis procedure. As Figure 3 shows, only after the saponification procedure was the spot corresponding to type V mycolic acid observed. After methanolysis, TLC showed the derived compounds from type V cleavage that migrated near the application point19. For neophyte researchers, 2D-TLC can allow for a complementary method to identify each mycolic acid type (Figure 3C,D). Mycolic acid extracts must be first run in an elution system formed by petroleum ether (60-80 °C) and acetone (95:5) three times. Then, the plate must be run in the second direction with a mobile phase formed by toluene and acetone (97:3). 2D-TLC combined with mass spectrometry (MS) has been used to identify and chemically characterize the functional groups of mycolic acids and has been used extensively to characterize mycolic acids20,21,22. Therefore, the mycolic acid pattern is one of the biochemical features of value in systematic mycobacterial evaluation in combination with other analyses due to shared mycolic acid patterns among different species.
After performing the above-mentioned procedure to extract the non-covalent linked lipids, different elution systems were selected in function of the polarity and size of the lipid profile found in mycobacteria cells. The ideal combination of solvents in the elution systems should enable to visualize the desired lipids in the middle zone of the TLC plate to facilitate their further purification, if desired. In Figure 4, TLC plates are ordered from the elution system that allows the most apolar lipids to be monitored (Figure 4A) to the elution system that allows the most polar lipids to be visualized (Figure 4E).
Acyl glycerols (AG) and PDIMs are two of the most apolar lipids present in the mycobacterial cell wall and are easily visualized through 1D-TLC analyses using a mobile phase formed by petroleum ether:diethyl ether (90:10). Figure 4A shows that AGs were present in M. bovis BCG, M. fortuitum and M. brumae but not in the smooth morphotype of M. abscessus. Although 1D-TLC suggested the presence of PDIM in M. bovis BCG and M. fortuitum, it was only corroborated in M. bovis BCG when 2D-TLC analysis was performed (Figure 4B). Altogether, these results demonstrate the importance of corroborating the presence of a mycobacterial compound by at least two different elution systems. Another interesting lipid to analyze in mycobacteria composition is PGL. In the chosen mycobacteria, PGL is only present in M. bovis BCG, and it is noticeable when TLC is eluted with the elution system consisting of chloroform and methanol (95:5) (Figure 4C). Following the idea of visualizing more polar components, the elution system consisting of the mixture of 90:10:1 (chloroform:methanol:water) was used to monitor the presence of GPLs (Figure 4D), which are only present in M. abscessus smooth morphotype. In the same TLC: PGL, trehalose dimycolate (TDM), acyl trehaloses (AT), and trehalose monomycolate (TMM), can be also observed. PGL, GPLs, TDM, AT were also observed at the top of the plate when the elution system consisted of 30:8:1 (chloroform:methanol:water) (Figure 4E). TMM is located in the middle of the plate. TDM and TMM were clearly expressed in all mycobacteria studied. Despite phosphatidyl-inositol mannosides (PIMs) are observed at the bottom of the plate, the best elution system to analyze PIMs is 60:35:8 (chloroform:methanol:water) as shown in Figure 5A,B. While all sugar-containing lipids are revealed with anthrone (Figure 5A), PIMs contain phosphate groups that are specifically revealed with Molybdenum Blue reagent (Figure 5B). Similar to mycolic acids, AG, and PDIMs, PIMs can also be easily visualized through 2D-TLC analyses (Figure 5C). Moreover, in the case of analyzing mycobacteria that are able to synthesize LOSs, PIMs and LOSs would be differentiated using the same 2D elution system, as detailed in Ren et al.8.
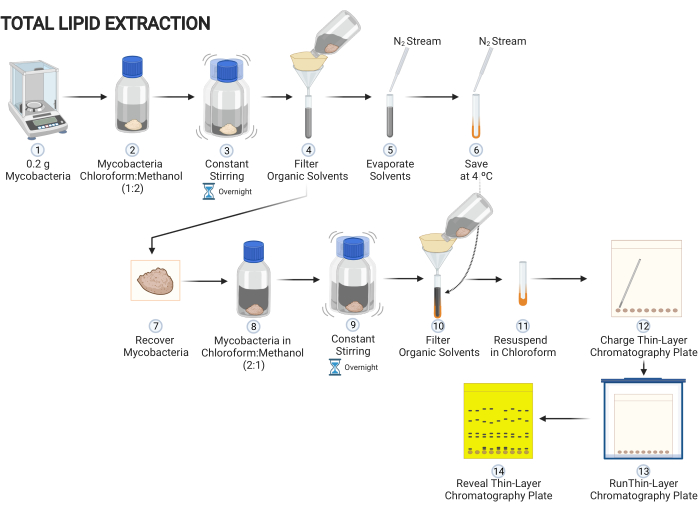
Figure 1: Scheme of the procedure of extracting lipid content of mycobacteria grown on solid media. Main steps to decipher lipids present on mycobacteria cells. Please click here to view a larger version of this figure.
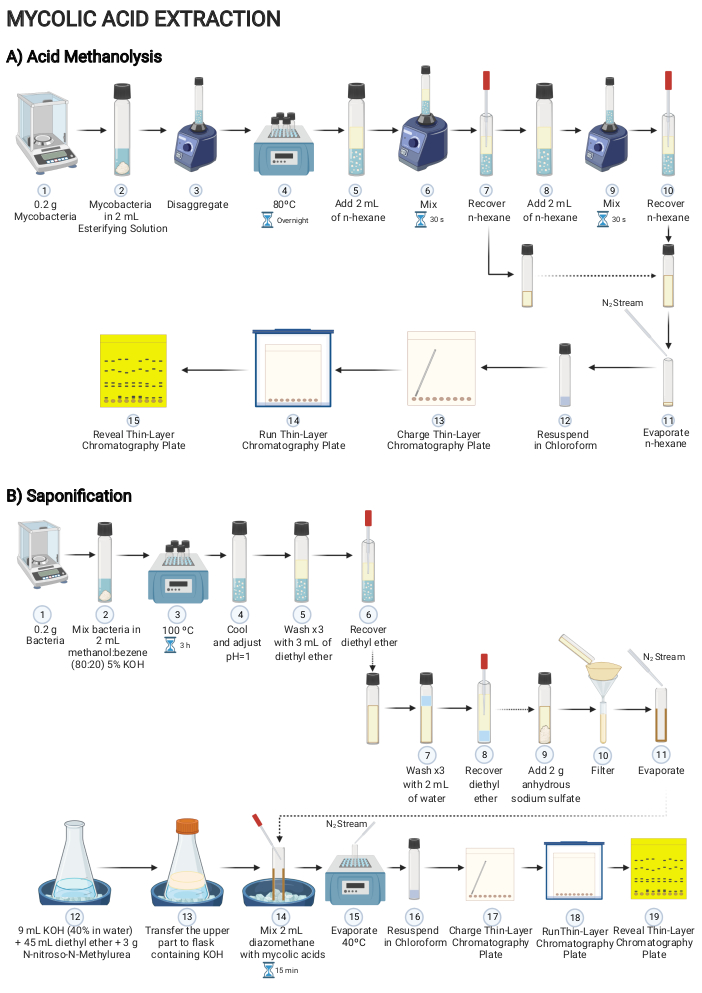
Figure 2: Scheme of the procedure for extracting mycolic acid content of mycobacteria grown on solid media. Main steps to decipher mycolic acids present on mycobacteria cells using either (A) acid methanolysis or (B) saponification. Please click here to view a larger version of this figure.
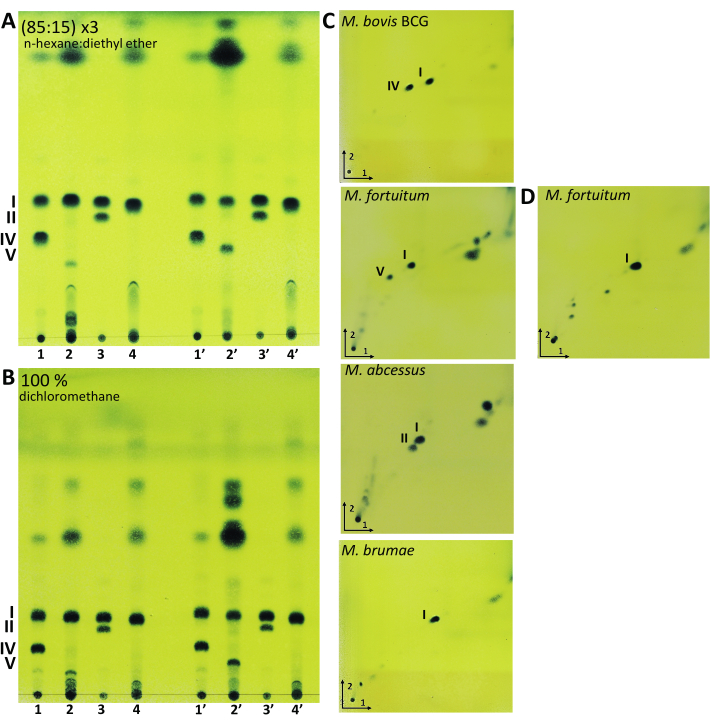
Figure 3: Representative results of lipid extraction from mycobacteria. Thin-layer chromatography (TLC) analysis of mycolic acids developed in (A) 85 mL of n-hexane, plus 15 mL of diethyl ether (three runs), and (B) 100 mL of dichloromethane. (C) Two-dimensional TLC analysis of mycolic acids extracted by acid methanolysis developed in 95:5 (n-hexane:acetone) (three runs) in the first direction and 97:3 (toluene:acetone) in the second direction. (D) Two-dimensional TLC analysis of mycolic acids from M. fortuitum extracted by saponification developed in 95:5 (n-hexane:acetone) (three runs) in the first direction and 97:3 (toluene:acetone) in the second direction. TLCs were revealed with 10% molybdatophosphoric acid hydrate in ethanol followed by heating the plate at 120 °C. M. bovis BCG Connaught (Line 1 and 1'); M. fortuitum (Line 2 and 2'); M. abscessus smooth morphotype (Line 3 and 3') and M. brumae (Line 4 and 4'). 1-4 mycolic acids obtained by acid methanolysis and 1'-4' mycolic acids obtained by saponification. I, α-mycolates; II, α'-mycolates; IV, ketomycolates; V, epoxymycolates. Please click here to view a larger version of this figure.
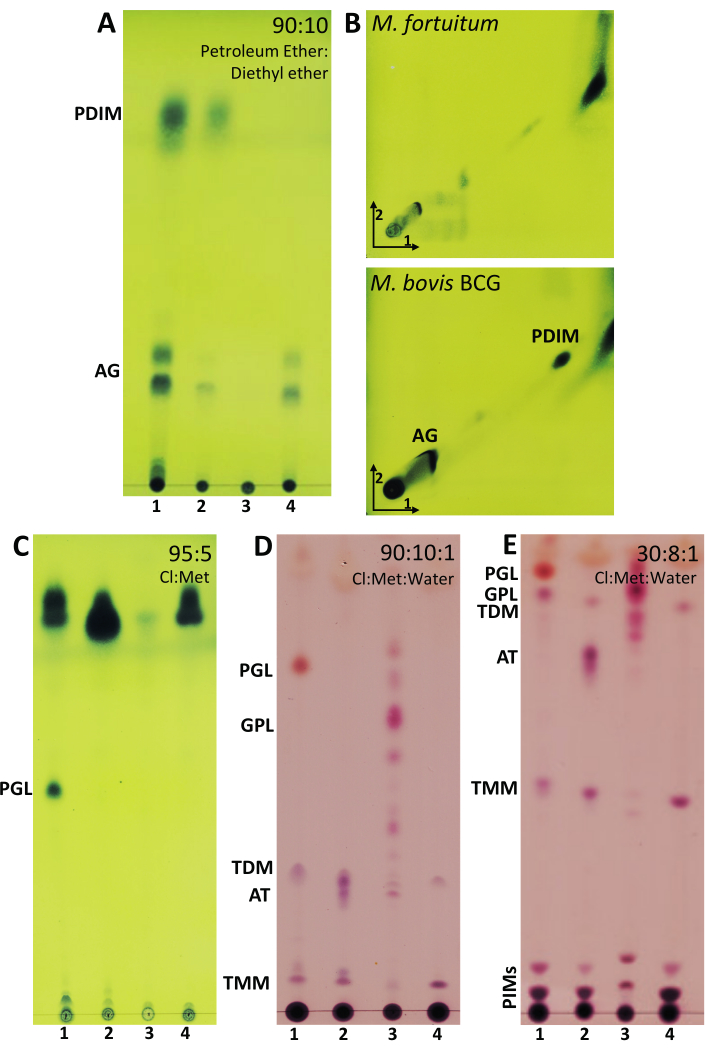
Figure 4: Representative results of lipid extraction from mycobacteria. (A) TLC analysis of acylglycerols (AG) and phthiocerol dimycocerosates (PDIMs) developed in 90:10 (petroleum ether (60-80 °C):diethyl ether). (B) Two-dimensional TLC analysis of PDIMs and AG developed in 98:2 (petroleum ether (60-80 °C):ethyl acetate) (three runs) in the first direction and 98:2 (petroleum ether (60-80 °C):acetone) in the second direction. (C) TLC analysis of phenolic glycolipid (PGL) developed in 95:5 (chloroform:methanol). (D) TLC analyses developed in 90:10:1 (chloroform:methanol:water) of PGL, glycopeptidolipids (GPL), trehalose dimycolate (TDM), acyl trehaloses (AT), and trehalose monomycolate (TMM). (E) TLC analysis of PGL, GPL, AT, TMM, and phosphatidyl-inositol mannosides (PIMs) developed in 30:8:1 (chloroform:methanol:water). A–B–C were revealed with 10% molybdatophosphoric acid hydrate in ethanol followed by heating the plate at 120 °C. D–E were revealed with 5% in ethanol of 20% α-naphthol in sulfuric acid and heated at 120 °C. Line 1: M. bovis BCG Connaught; Line 2: M. fortuitum; Line 3: M. abscessus smooth morphotype; Line 4: M. brumae. Please click here to view a larger version of this figure.
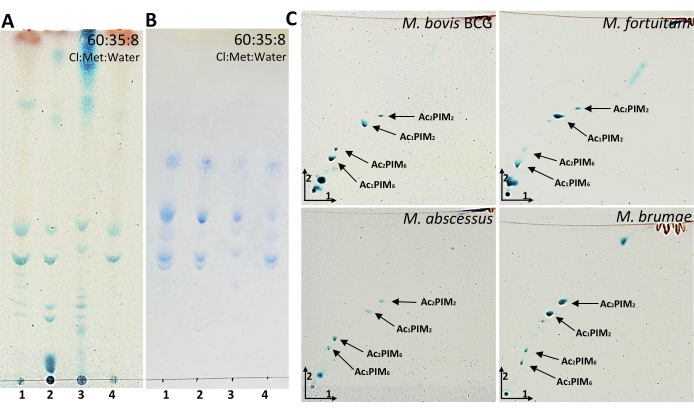
Figure 5: Representative results of PIMs from mycobacteria. (A-B) TLC analysis of PIMs developed in 60:35:8 (chloroform:methanol:water). (C) Two-dimensional TLC analysis of PIMs developed in 60:30:6 (chloroform:methanol:water) in the first direction and 40:25:3:6 (chloroform:acetic acid:methanol:water) in the second direction. A–C were revealed with 1% anthrone in sulfuric acid followed by heating the plate at 120 °C. B was revealed with Molybdenum Blue reagent until phosphate bands appeared. Line 1: M. bovis BCG Connaught; Line 2: M. fortuitum; Line 3: M. abscessus smooth morphotype; Line 4: M. brumae. Please click here to view a larger version of this figure.
Discussion
A simple protocol considered as the gold standard method for the extraction of noncovalently linked lipid compounds from the mycobacterial cell wall is presented. Further visualization by one- and two-dimensional TLCs from the extracted lipids of four different mycobacteria is shown.
Two consecutive combined mixtures of chloroform and methanol to recover the lipidic content of mycobacterial cells is the most widely used solvent mixture23,24,25,26,27,28,29. This mixture permits recovery of a wide range of apolar and polar lipids from the cells. Nevertheless, some other methods have been described in the literature to extract total or specific mycobacterial lipids, which have been recently reviewed by Hameed et al.29. For instance, the Folch method is one of the most widely used protocols developed to recover the total mycobacterial lipids from tissues30 and has also been adapted to pure mycobacterial cultures. It consists of suspending mycobacterial cells in chloroform:methanol (1:2), followed by centrifugation and the addition of chloroform to obtain a ratio of 1:1. Finally, KCl is used to remove nonlipid components from the extract31. In parallel, other protocols have been developed to extract specific lipids. Slayden et al. used a mixture of chloroform:methanol plus acetone to specifically recover glycolipids such as TDM or TMM32. Altogether, published methods are based on exposing mycobacterial cells to different concentrations of solvents, mainly chloroform and methanol. Likewise, some salts are occasionally added to discard other cell components present on the sample.
In addition to noncovalently linked lipids, mycolic acid extraction by two different procedures is also shown. While acidic methanolysis permits the easy extraction of mycolic acids with less hazardous reagents, the saponification procedure preserves the structure of all mycolic acid types, including type V mycolic acid, which is cleaved during the methanolysis procedures. Once the lipids are extracted, 1D- or 2D-TLC are standard methods to monitor them, and the assay utilized varies depending on the physicochemical characteristics of the lipids. The polarity and size of each molecule will determine the selection of the elution system needed, allowing for determination of the lipids that form part of the mycobacterium. One-dimensional TLC can be chosen when the retention factors (Rf) between mycobacterial lipids are different, while 2D-TLC facilitates visualization when different lipids share molecular weight and polarity characteristics. To facilitate the identification, purified lipids should be run in parallel to the extracted sample to compare similar Rf. The identification of a lipid can be achieved when it runs with the same Rf as that of the known purified control at least in two TLC systems (two different mobile phases). Purified lipids can be obtained from commercial suppliers or from mycobacterial research laboratories. Finally, the biochemical nature of the molecule indicates which stain can be used to reveal TLC plates. There are universal staining methods, such as phosphomolybdic acid which enables the visualization of any organic component to be visualized as it binds to carbon bonds. While others such as a-naphthol or anthrone provide specific colors to sugar residues, molybdenum blue specifically binds to phosphate residues.
The most important consideration to analyze the lipid content of mycobacteria is to avoid the use of plastic material throughout the procedures since the contact of organic solvents with plastic can contaminate the samples and can be observed in the TLC plates. It is also relevant to consider that the culture medium composition used for mycobacteria cultivation, as well as temperature or days of incubation, can modify the lipid pattern of each mycobacterium, as previously described16. Mycobacteria grown on either liquid and solid media can be used to extract the non-covalent linked lipids or mycolic acids. When obtaining cells from liquid culture, they should be adequately filtered and dried to avoid the presence of liquid media in the sample. Moreover, when using mycobacteria from liquid media, bacteria must be properly and equally grown between experiments in order to obtain reproducible results over time. Moreover, mycobacterial cells can also be grown on pellicles17,33,34,35,36, from which the most outermost lipids can be recovered using organic solvents and monitored by TLC, as we showed in the present article.
The main limitation of mycobacterial lipid extraction procedures remains the utilization of toxic solvents under safe conditions. The TLC procedure is less sensitive than other techniques, such as gas chromatography or high-performance liquid chromatography. Furthermore, TLC does not permit the quantification of samples, and further techniques need to be applied to identify the structure of the extracted compounds. For instance, nuclear magnetic resonance needs to be performed to distinguish lipid isomers. It is noteworthy that for describing the structure of a mycobacterial lipid for the first time, mass spectrometry or infrared spectroscopy are required. Thus, quantitative and qualitative analysis of lipid classes normally requires combinations of different extraction, derivatization, chromatographic, and detection methods, such as high- or ultra-performance liquid chromatography tandem mass spectrometry and nuclear magnetic resonance spectroscopy37,38,39,40. Recent studies have demonstrated that using a single-step thin-layer chromatography-flame ionization detection technique permits the quantification and preliminary screening of mycolic acids in Actinobacteria41. Nevertheless, TLC is an extremely useful, timesaving, and cheap technique to screen and evaluate the lipidic composition of mycobacteria. Overall, the procedures presented here are highly versatile providing basic tools to analyze the most relevant feature of mycobacteria cells: its complex cell wall.
開示
The authors have nothing to disclose.
Acknowledgements
This research was funded by the Spanish Ministry of Science, Innovation and Universities (RTI2018-098777-B-I00), the FEDER Funds, and the Generalitat of Catalunya (2017SGR-229). Sandra Guallar-Garrido is the recipient of a PhD contract (FI) from the Generalitat de Catalunya.
Materials
| Acetic Acid | Merck | 100063 | CAUTION. Anhydrous for analysis EMSURE® ACS,ISO,Reag. Ph Eur |
| Acetone | Carlo Erba | 400971N | CAUTION. ACETONE RPE-ACS-ISO FOR ANALYS ml 1000 |
| Anthrone | Merck | 8014610010 | Anthrone for synthesis. |
| Benzene | Carlo Erba | 426113 | CAUTION. Benzene RPE – For analysis – ACS 2.5 l |
| Capillary glass tube | Merck | BR708709 | BRAND® disposable BLAUBRAND® micropipettes, intraMark |
| Chloroform | Carlo Erba | 412653 | CAUTION. Chloroform RS – For HPLC – Isocratic grade – Stabilized with ethanol 2.5 L |
| Dry block heater | J.P. Selecta | 7471200 | |
| Dicloromethane | Carlo Erba | 412622 | CAUTION. Dichloromethane RS – For HPLC – Isocratic grade – Stabilized with amylene 2.5 L |
| Diethyl ether | Carlo Erba | 412672 | CAUTION. Diethyl ether RS – For HPLC – Isocratic grade – Not stabilized 2.5 L |
| Ethyl Acetate | Panreac | 1313181211 | CAUTION. Ethyl acetate (Reag. USP, Ph. Eur.) for analysis, ACS, ISO |
| Ethyl Alcohol Absolute | Carlo Erba | 4146072 | CAUTION. Ethanol absolute anhydrous RPE – For analysis – ACS – Reag. Ph.Eur. – Reag. USP 1 L |
| Glass funnel | VidraFOC | DURA.2133148 1217/1 | |
| Glass tube | VidraFOC | VFOC.45066A-16125 | Glass tube with PTFE recovered cap |
| Methanol | Carlo Erba | 412722 | CAUTION. Methanol RS – For HPLC – GOLD – Ultragradient grade 2.5 L |
| Molybdatophosphoric acid hydrate | Merck | 51429-74-4 | CAUTION. |
| Molybdenum Blue Spray Reagent, 1.3% | Sigma | M1942-100ML | CAUTION. |
| n-hexane | Carlo Erba | 446903 | CAUTION. n-Hexane 99% RS – ATRASOL – For traces analysis 2.5 L |
| n-nitroso-n-methylurea | Sigma | N4766 | CAUTION |
| Orbital shaking platform | DDBiolab | 995018 | NB-205L benchtop shaking incubator |
| Petroleum ether (60-80ºC) | Carlo Erba | 427003 | CAUTION. Petroleum ether 60 – 80°C RPE – For analysis 2.5 L |
| Sprayer | VidraFOC | 712/1 | |
| Sodium sulphate anhydrous | Merck | 238597 | |
| Sulfuric acid 95-97% | Merck | 1007311000 | CAUTION. Sulfuric acid 95-97% |
| TLC chamber | Merck | Z204226-1EA | Rectangular TLC developing tanks, complete L × H × W 22 cm × 22 cm × 10 cm |
| TLC plate | Merck | 1057210001 | TLC SilicaGel 60- 20×20 cm x 25 u |
| TLC Plate Heater | CAMAG | 223306 | CAMAG TLC Plat Heater III |
| Toluene | Carlo Erba | 488551 | CAUTION. Toluene RPE – For analysis – ISO – ACS – Reag.Ph.Eur. – Reag.USP 1 L |
| Vortex | Fisher Scientific | 10132562 | IKA Agitador IKA vórtex 3 |
| 1-naphthol | Sigma-Aldrich | 102269427 | CAUTION. |
参考文献
- Watanabe, M., et al. Location of functional groups in mycobacterial meromycolate chains; the recognition of new structural principles in mycolic acids. 微生物学. 148 (6), 1881-1902 (2002).
- Global Health Organization World Health Organization. (2018) Global tuberculosis report. WHO. , (2019).
- Jackson, M. The Mycobacterial cell envelope-lipids. Cold Spring Harbor Perspectives in Medicine. 4 (10), 1-36 (2014).
- Jankute, M., et al. The role of hydrophobicity in tuberculosis evolution and pathogenicity. Scientific Reports. 7 (1), 1315 (2017).
- Reed, M. B., Gagneux, S., DeRiemer, K., Small, P. M., Barry, C. E. The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. Journal of Bacteriology. 189 (7), 2583-2589 (2007).
- Ly, A., Liu, J. Mycobacterial virulence factors: Surface-exposed lipids and secreted proteins. International Journal of Molecular Sciences. 21 (11), 3985 (2020).
- Szulc-Kielbik, I., et al. Severe inhibition of lipooligosaccharide synthesis induces TLR2-dependent elimination of Mycobacterium marinum from THP1-derived macrophages. Microbial Cell Factories. 16 (1), 217 (2017).
- Ren, H., et al. Identification of the lipooligosaccharide biosynthetic gene cluster from Mycobacterium marinum. Molecular Microbiology. 63 (5), 1345-1359 (2007).
- Roux, A. L., et al. The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biology. 6 (11), 160185 (2016).
- Guallar-Garrido, S., Julián, E. Bacillus Calmette-Guérin (BCG) therapy for bladder cancer: An update. ImmunoTargets and Therapy. 9, 1-11 (2020).
- Bach-Griera, M., et al. Mycolicibacterium brumae is a safe and non-toxic immunomodulatory agent for cancer treatment. Vaccines. 8 (2), 2-17 (2020).
- Noguera-Ortega, E., et al. Nonpathogenic Mycobacterium brumae inhibits bladder cancer growth in vitro, ex vivo, and in vivo. European Urology Focus. 2 (1), 67-76 (2015).
- Noguera-Ortega, E., et al. Mycobacteria emulsified in olive oil-in-water trigger a robust immune response in bladder cancer treatment. Scientific Reports. 6, 27232 (2016).
- Rodríguez-Güell, E., et al. The production of a new extracellular putative long-chain saturated polyester by smooth variants of Mycobacterium vaccae interferes with Th1-cytokine production. Antonie van Leeuwenhoek. 90, 93-108 (2006).
- Garcia-Vilanova, A., Chan, J., Torrelles, J. B. Underestimated manipulative roles of Mycobacterium tuberculosis cell envelope glycolipids during infection. Frontiers in Immunology. 10, (2019).
- Yang, L., et al. Changes in the major cell envelope components of Mycobacterium tuberculosis during in vitro growth. Glycobiology. 23 (8), 926-934 (2013).
- Guallar-Garrido, S., Campo-Pérez, V., Sánchez-Chardi, A., Luquin, M., Julián, E. Each mycobacterium requires a specific culture medium composition for triggering an optimized immunomodulatory and antitumoral effect. Microorganisms. 8 (5), 734 (2020).
- Venkataswamy, M. M., et al. et al. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine. 30 (6), 1038-1049 (2012).
- Secanella-Fandos, S., Luquin, M., Pérez-Trujillo, M., Julián, E. Revisited mycolic acid pattern of Mycobacterium confluentis using thin-layer chromatography. Journal of Chromatography B. 879, 2821-2826 (2011).
- Minnikin, D. E., et al. Analysis of mycobacteria mycolic acids. Topics in Lipid Research: From Structural Elucidation to Biological Function. , 139-143 (1986).
- Minnikin, D. E., Hutchinson, I. G., Caldicott, A. B., Goodfellow, M. Thin-layer chromatography of methanolysates of mycolic acid-containing bacteria. Journal of Chromatography A. 188 (1), 221-233 (1980).
- Minnikin, D. E., Goodfellow, M. Lipid composition in the classification and identification of acid-fast bacteria. Society for Applied Bacteriology Symposium Series. 8, 189-256 (1980).
- Muñoz, M., et al. Occurrence of an antigenic triacyl trehalose in clinical isolates and reference strains of Mycobacterium tuberculosis. FEMS Microbiology Letters. 157 (2), 251-259 (1997).
- Daffé, M., Lacave, C., Lanéelle, M. A., Gillois, M., Lanéelle, G. Polyphthienoyl trehalose, glycolipids specific for virulent strains of the tubercle bacillus. European Journal of Biochemistry. 172 (3), 579-584 (1988).
- Singh, P., et al. Revisiting a protocol for extraction of mycobacterial lipids. International Journal of Mycobacteriology. 3 (3), 168-172 (2014).
- Camacho, L. R., et al. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. Journal of Biological Chemistry. 276 (23), 19845-19854 (2001).
- Dhariwal, K. R., Chander, A., Venkitasubramanian, T. A. Alterations in lipid constituents during growth of Mycobacterium smegmatis CDC 46 and Mycobacterium phlei ATCC 354. Microbios. 16 (65-66), 169-182 (1976).
- Chandramouli, V., Venkitasubramanian, T. A. Effect of age on the lipids of mycobacteria. Indian Journal of Chest Diseases & Allied Sciences. 16, 199-207 (1982).
- Hameed, S., Sharma, S., Fatima, Z. Techniques to understand mycobacterial lipids and use of lipid-based nanoformulations for tuberculosis management. NanoBioMedicine. , (2020).
- Folch, J., Lees, M., Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry. 226 (1), 497-509 (1957).
- Pal, R., Hameed, S., Kumar, P., Singh, S., Fatima, Z. Comparative lipidome profile of sensitive and resistant Mycobacterium tuberculosis strain. International Journal of Current Microbiology and Applied Sciences. 1 (1), 189-197 (2015).
- Slayden, R. A., Barry, C. E. Analysis of the lipids of Mycobacterium tuberculosis. Mycobacterium tuberculosis Protocols. 54, 229-245 (2001).
- Ojha, A. K., et al. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Molecular Microbiology. 69 (1), 164-174 (2008).
- Ojha, A. K., Trivelli, X., Guerardel, Y., Kremer, L., Hatfull, G. F. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. The Journal of Biological Chemistry. 285 (23), 17380-17389 (2010).
- Layre, E., et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chemistry and Biology. 16 (1), 82-92 (2009).
- Llorens-Fons, M., et al. Trehalose polyphleates, external cell wall lipids in Mycobacterium abscessus, are associated with the formation of clumps with cording morphology, which have been associated with virulence. Frontiers in Microbiology. 8, (2017).
- Butler, W. R., Guthertz, L. S. Mycolic acid analysis by high-performance liquid chromatography for identification of mycobacterium species. Clinical Microbiology Reviews. 14 (4), 704-726 (2001).
- Teramoto, K., et al. Characterization of mycolic acids in total fatty acid methyl ester fractions from Mycobacterium species by high resolution MALDI-TOFMS. Mass Spectrometry. 4 (1), 0035 (2015).
- Sartain, M. J., Dick, D. L., Rithner, C. D., Crick, D. C., Belisle, J. T. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel “Mtb LipidDB”. Journal of Lipid Research. 52 (5), 861-872 (2011).
- Li, M., Zhou, Z., Nie, H., Bai, Y., Liu, H. Recent advances of chromatography and mass spectrometry in lipidomics. Analytical and Bioanalytical Chemistry. 399 (1), 243-249 (2011).
- Nahar, A., Baker, A. L., Nichols, D. S., Bowman, J. P., Britz, M. L. Application of Thin-Layer Chromatography-Flame Ionization Detection (TLC-FID) to total lipid quantitation in mycolic-acid synthesizing Rhodococcus and Williamsia species. International Journal of Molecular Sciences. 21 (5), 1670 (2020).

