Phase Change Dimethyldioctadecylammonium-Shelled Microdroplets as a Promising Drug Delivery System: Results on 3D Spheroids of Mammalian Tumor Cells
概要
Decafluoropentane microdroplets developed with a shell of dimethyldioctadecylammonium bromide exhibited an exceptional colloidal stability and an actractive biointerface. DDAB-MDs proved to be efficient drug reservoirs characterized by a high affinity to plasma membranes together with enhanced uptake and antitumor activity of Doxorubicin against human triple-negative breast cancer (MDA-MB-231) 3D model.
Abstract
Significant improvement of phase-change perfluorocarbon microdroplets (MDs) in the vast theranostic scenario passes through the optimization of the MDs composition with respect to synthesis efficiency, stability, and drug delivery capability. To this aim, decafluoropentane (DFP) MDs stabilized by a shell of dimethyldioctadecylammonium bromide (DDAB) cationic surfactant were designed. A high concentration of DDAB-MDs was readily obtained within a few seconds by pulsed high-power insonation, resulting in low polydisperse 1 µm size droplets. Highly positive ζ-potential, together with a long, saturated hydrocarbon chains of the DDAB shell, are key factors to stabilize the droplet and the drug cargo therein. The high affinity of the DDAB shell with cell plasma membrane allows for localized chemotherapeutics delivery by increasing the drug concentration at the tumor cell interface and boosting the uptake. This would turn DDAB-MDs into a relevant drug delivery tool exhibiting high antitumor activity at very low drug doses.
In this work, the efficacy of such an approach is shown to dramatically improve the effect of doxorubicin against 3D spheroids of mammalian tumor cells, MDA-MB-231. The use of three-dimensional (3D) cell cultures developed in the form of multicellular tumor spheroids (i.e., densely packed cells in a spherical shape) has numerous advantages compared to 2D cell cultures: in addition to have the potential to bridge the gap between conventional in vitro studies and animal testing, it will improve the ability to perform more predictive in vitro screening assays for preclinical drug development or evaluate the potential of off-label drugs and new co-targeting strategies.
Introduction
Drug-delivery vectors capable of ensuring high antitumor efficacy and reducing side effects are primary goals while remaining a severe chemical-pharmaceutical challenge1,2. To date, their progress is limited at first by the contrast of an insufficient in situ drug release and a critical level of nonspecific toxicity3,4,5. In recent years, several drug delivery systems have been implemented to improve the administration of anticancer agents, including liposomes, polymeric micelles, polymersomes6,7,8,9,10. These systems exhibit potential in increasing circulation time and selectivity of drugs, while reducing distribution and accumulation in healthy organs and tissues. Anyway, the encapsulated formulations of antineoplastic chemotherapy drugs, such as anthracyclines, led to a significantly reduced drug internalization efficiency. Recently, stimuli-responsive micron and submicron carriers such as microbubbles11, microdroplets, hybrid gold nanoparticles12, nano-hydrogels13, PLGA scaffolds, and mesoporous platforms14, have been gaining pharmacological interest for their high versatility in targeting and exerting tumor inhibitory effects using doxorubicin (Dox) and docetaxel. Pioneering experiments to turn these carriers into efficient anticancer soldiers for multimodal tasking (i.e., chemotherapeutic, photothermal, and gene synergistic approaches) and molecular imaging15 have paved the way for personalized theranostic nanomedicine.
In this scenario, phase-change perfluorocarbon microdroplets (MDs) have been evaluated through the key opportunity they offer to conjugate high drug cargo loading, chemical versatility of the MDs shell addressing biological barriers, colloidal stability and synthesis efficiency11,12. As an additional asset, the echogenicity of the MDs promoted by acoustic or optical vaporization of the perfluorocarbon (PFC) core allows to gain in situ imaging and promising therapeutic efficacy. Moreover, MDs core vaporization obtained by the energy release of ionizing particle beams can be exploited for beam tracking and radiation dosimetry.
The present study is aimed to develop decafluoropentane (DFP) microdroplets stabilized by a multiple usable shell of dimethyldioctadecylammonium bromide (DDAB) cationic surfactant. DDAB shelled-MDs meet both physico-chemical and biological expectations. DFP based microdroplets have been demonstrated to be valuable phase-change contrast agents to achieve biocompatible and stable perfluorocarbon MDs16. DDAB crystalline gel saturates long-chains at physiological temperature, deeply penetrating the hydrophobic core, stabilizing the droplet and the drug cargo therein. Moreover, the high positive ζ-potential at the water interface enhances the colloidal stability of the MDs. Biological attractiveness of DDAB shell surface lies in the ability to cause the death of bacteria and fungi, at concentrations that barely affect mammalian cells, and to bind plasma membranes, negatively charged antigenic proteins, nucleotides, DNA, or nanoparticles. The above-mentioned features can be exploited to generate a remarkable immunoadjuvant, gene therapy and antitumor action within mammalian cells17.
Dox-loaded DDAB-MDs (Dox@DDAB-MDs) described herein promote the drug release against highly aggressive, invasive, and poorly differentiated triple-negative breast cancer cells. A simple and rapid protocol is described below based on high power probe insonation to obtain stable and high-density DDAB-MDs with a narrow size distribution with a high loading efficiency of Dox in a one-step formulation. Such characteristics are competitive even for other preparation methods like microfluidic devices and high shear homogenizers16.
The other major limiting issue in designing efficient drug delivery vectors is that the activity of a drug is a function of various parameters (e.g., absorption, distribution, concentrations) obtainable in an actual biological target, which cannot be considered by monolayer cell models18. For this reason, the history of the development of novel antitumor formulations is studded with in vitro studies that unfortunately have resulted to be ineffective already at the level of preclinical models in animals19.
Particularly, the need to move from cell cultures to a more complex and reliable system than in vivo and ex vivo studies is linked to the inherent limitations of pharmacological studies on 2D cultures. In this context, the in vitro 3D systems are included, such as spheroids, organoids, organ-on-chip, which simulate the morphology, activity, and physiological response of more complex structures than the 2D monolayers20. In a preclinical view, 3D cell models mimicking the cellular microenvironment offer the possibility to better understand complex biology in a physiologically more pertinent frame in which traditional monolayer cultures are not effective21,22.
After proving that DDAB-MDs can interact with the cell membrane of human breast cancer cells, favoring drug internalization and cell death at very low (nanomolar) Dox concentration, the efficacy of such methodology against 3D spheroids of mammalian tumor cells, MDA-MB-231, has been tested.
Protocol
NOTE: All the reagents and instruments are listed in the Table of Materials.
1. Fabricating and characterizing microdroplets
- Preparing Dox-loaded DDAB-MDs
- Dissolve the DDAB powder in ethanol to obtain a final concentration of 10 mM and a final volume of 1 mL. Prepare 1 mL of Dox stock solution dissolving 2 mg Dox powder in ethanol.
CAUTION: Dox is known to have acute oral toxicity, category 4 and carcinogenicity, category 1B. Use only under a fume hood with gloves and a health mask. - Add 250 µL of DFP to 300 µL of DDAB solution (oil phase) in a 15 mL plastic graduated centrifuge tube.
NOTE: The purity of DFP is 60% (GC), density 1.6 g/mL (20 °C), boiling point is 55 °C. Dox purity is 98, 0-102, 0% (HPLC). DDAB purity is ≥98% (TLC). Purity of ethanol is 97%. - Gently add 2.15 mL of deionized water on the oil phase resulting in a two-phase water/oil mixture. Gently inject 10 µL of Dox solution directly into the oil phase immediately before the insonation to avoid partitioning of a significant amount of Dox into the water phase.
- Emulsify the biphasic mixture by probe insonation (with a 1/8 in titanium tapered microtip) using a high-intensity ultrasonic liquid processor equipment in a pulse mode (0.7 s On and 0.3 s Off) at 20 kHz, 100 W for 10 s. Immediately dilute freshly prepared MDs with ultrafiltered, deionized water (e.g., MilliQ) by a factor of 1.8.
NOTE: The dilution factor is indicative, empirically chosen. MDs solution can be diluted moreas long as enough pellet is obtained from subsequent centrifugations. - Take 1 mL of the obtained suspension and centrifuge 3x, resuspending in ultrafiltered, deionized water (e.g., MilliQ) to remove the excess of Dox and ethanol. Carry out the first centrifugation at 25 °C, 360 x g for 3 min, and the second and third one at 280 x g at the same temperature and time.
- After the last wash, remove the supernatant water and draw up 5 µL of the pellets and disperse them into 2 mL of the medium for cell treatment, obtaining an equivalent Dox concentration of 10 nM.Protocol steps for Dox@DDAB-MDs synthesis are shown in Figure 1.
NOTE: Equivalent Dox concentration is defined as the amount of free Dox contained into the same volume of a suspension of Dox@DDAB-MDs.
- Dissolve the DDAB powder in ethanol to obtain a final concentration of 10 mM and a final volume of 1 mL. Prepare 1 mL of Dox stock solution dissolving 2 mg Dox powder in ethanol.
- Characterization of Dox-loaded DDAB-MDs
- Measure the size distribution using a Dynamic Light Scattering (DLS) photometer and analyze the obtained correlograms with the CONTIN algorithm to extrapolate the associated decay times. Then use the decay times to determine the distribution of the particles' diffusion coefficients (D) and, convert these in a distribution of hydrodynamic diameters (2RH) using the Stokes-Einstein relationship RH = kBT/6πηD, where kBT is the thermal energy of the system and η the solvent viscosity.
- Check the size and size distribution also using bright field microscopy with an image analysis software (e.g., Image J), measuring the size of at least 100 droplets per frame (for 3 or 4 frames), obtaining an average value and a standard deviation.
NOTE: For DLS measurements dilute the MDs solution in ultrafiltered, deionized water (e.g., MilliQ), PBS and in cell culture medium to avoid backscattering to a fixed concentration of 1.5 x 108 MDs /mL. For ultrafiltered, deionized water, and PBS in cell culture medium, we obtained mean size values of 1.1 ± 0.1 µm, 1.1 ± 0.25 µm, and 1.2 ± 0.2 µm, respectively. MDs recovered from the pellets, after centrifugation, do not show any size changes within the errors. - Use a cell counting chamber slide for microscopy to assess MDs concentration. The chamber is composed of two different counting areas with a thickness of 0.10 mm. Place 10 µL of MDs suspension over the chamber. Count MDs inside the 0.25 x 0.25 nm2 square of the chamber using an optical microscope with a 40x long distance objective and analyze with an image analysis freeware.
- Calculate the MDs concentration, expressed as number of MDs/mL, according to the equation:
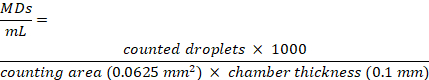
- Check the internalized Dox by Confocal Laser Scanning Microscope (CLSM) images exploiting the Dox autofluorescence at 590 nm. Measure the Dox content using a fluorimetric assay, dispersing the particles in ultrafiltered, deionized water or PBS, centrifuging them at 25 °C, 360 x g for 3 min and then determining the amount of free drug by evaluating the fluorescence of the supernatant at 590 nm with a calibration curve (linearity range for Dox concentration: 2-20 µmol/mL, R2 = 0.99).
NOTE: Subtract the obtained value from the total concentration of Dox to obtain the encapsulated amount of Dox. Perform the experiment in triplicate. The Dox encapsulation efficiency results in 28% ± 2%, calculated by dividing the drug amount of Dox in the MDs with the total content of Dox deployed. - Perform experiments of Dox release over time, by centrifuging and repeating the procedure as per step 1.2.3 to obtain the percentage of Dox released in the supernatant over to the number of encapsulated ones. After the first determination, repeat the procedure by decreasing the centrifugation speed to 280 x g to avoid MDs breaking. Plot a release curve over time. Check that the concentration of Dox released within 24 h in the supernatant reaches a maximum of 20%.
- Measure the ζ-potential at 37 °C using a dedicated apparatus (e.g., NanoZetaSizer) and verify that the obtained values are around 90-100 mV.
2. Fabrication of spheroids in micro-molded nonadhesive substrates
- Casting micro-molds
- Place small 3D molds and 1 g of pure agarose powder in containers suitable for sterilization. Autoclave them for 30 min on a dry cycle (121 °C).
- In a biosafety cabinet, add 50 mL of 0.9% saline (NaCl) solution to the glass bottle containing sterilized agarose and place it into a microwave oven to boil and dissolve the powder. Let the molten agarose cool down to 60 °C and add 500 µL to each micro-mold avoiding creation of bubbles when pipetting.
WARNING: Molten agarose can cause severe skin burns. Appropriate personal protective equipment is needed. - Remove the gelled agarose by carefully flexing the mold and removing the newly formed substrate. Put the substrates in a 12-well plate and add 2 mL of fresh culture medium per well. Incubate for at least 15 min to equilibrate each substrate.
NOTE: Prepare the culture medium in advance and pass it through a 0.22 µm filter to remove possible particles or contaminants.
- Seeding the cells
- Culture MDA-MB 231 cells with complete medium (DMEM supplemented with 1% Pen/Strep, 10% FBS, and 1% L-Glu) in a cell culture incubator at 37 °C, 5% CO2. When the cells reach about 80% confluence in a T75 flask, discard the medium, rinse with 4 mL of DPBS and add 3 mL of trypsin/EDTA. Place the flask in the incubator and wait for cells to detach. Inspect the detachment under an inverted microscope (at 20x) every 5 min.
- Collect the detached cells with 3 mL of DPBS with 10% of FBS in a 15 mL tube and centrifuge at 235 x g for 10 min. Discard the supernatant containing both DPBS and trypsin/EDTA and resuspend the cells pellet in 3 mL of fresh medium. Pipette 10 µL of cell suspension with 10 µL of trypanblue and count the cells using a Neubauer chamber.
NOTE: When harvesting the cells, neutralize the trypsin/EDTA with DPBS containing 10% of FBS to prevent the trypsin from damaging the cells during the centrifugation step. - To obtain a nominal spheroid diameter of 50 µm (~15 cells/spheroid), dilute the cell suspension to a final concentration of 3,840 cells/190 µL, resulting in 256 spheroids in the small mold. Alternatively, for a 200 µm spheroid diameter (~1,000 cells/spheroid) dilute to a final concentration of 81,000 cells/190 µL, resulting in 81 spheroids in the large mold, as per the manufacturer's instructions.
- Remove the culture medium from the 12-well plate and tilt the substrates to carefully remove the medium from the cell seeding chamber. Add 190 µL of cell suspension to each substrate (in a dropwise manner) and wait for cells to settle for 10 min in the tissue culture incubator.
- Add 2 mL of the surrounding medium per well and place the multiwell back to the incubator. Inspect for spheroid formation every 24 h. Replace with a fresh medium when needed.
- Harvesting and processing spheroids
- Place a 35 mm Petri dish containing 2 mL of fresh medium in the incubator to equilibrate for 10-15 min.
- Remove the cell culture medium surrounding the substrate. With a sterile tweezer, remove the substrate from the well and invert it in the Petri dish. Gently tap the bottom of the substrate to make the spheroids fall by gravity.
- Place the Petri dish containing the spheroids back in the incubator for further processing. Protocol steps for spheroids fabrication are shown in Figure 2.
3. Spheroid treatment
- Remove the supernatant from the mold and replace it with 200 µL of Dox@DDAB-MDs dispersion, prepared as described in step 1.1.6.
- After 5 min, add 2 mL/well of surrounding medium to equilibrate. After the desired treatment time, proceed as per step 2.1.
4. Characterization of spheroid size and morphology
- Check the spheroid size after the desired incubation time with a 40x objective combined with the microscope image processing software.
- Measure the size of at least 10 spheroids to collect enough data for suitable statistics and analyze their volumes as reported in step 7.
5. Proliferation/viability assay: fluorescence microscopy with live cell staining
NOTE: Follow the instructions for spheroid fabrication until step 2.2.5.
- Remove the supernatant from the mold and replace it with 200 µL of 4 µM calcein-AM in PBS and incubate for 3 h at room temperature in the dark. During the last 20 min of incubation, add 10 µg/mL of propidium iodide to stain the dead cells.
- Harvest the spheroids by inverting the substrate into the Petri dish as per step 2.3.2.
- Image the spheroids in staining solution using CLSM with 40x/60x objectives and an Ar+ laser, setting the gain and the pinhole in appropriate ways to obtain focused images.
6. Image analysis and acquisition
- Transmission and confocal 2D images
- Open the confocal software (Supplementary File A) and select the objective (60x, 40x, etc). On the right panel click on Trans to select the transmission channel.
- Click on Live to visualize the image and search for the optimized focus. Click on Single to stop the acquisition.
- For the confocal images, click on Red or Green laser channels depending on the fluorescent dye used. On the pinhole section, select S-aperture on the drop-down menu and set the gain section at 6.00 B.
- Click on Live and set the pinhole aperture and gain for optimizing the contrast. Click on Single to stop the acquisition and save the image. Overlap the transmission and confocal image by selecting the correct channel on the top toolbar.
- Confocal 3D images
- Click on Z on the right panel (Supplementary File B). Click on the red button to reset the settings.
- Select the Ref section, click on Live and search the median plan inside the object moving the focus. Select the Top section and move the focus up until the object is out of focus and disappears.
- Repeat step 6.2.2 for the bottom section moving down the focus.
- Set the step size to 0.75 µm. Click on Z-stack and then on Single to start the scanning. Save the images in .ics format.
- Optical images and analysis
- Open the optical microscope software, capture the image with a 40x long focus or 20x objective by pressing Play on the top toolbar. Press Stop and save the image.
- On the top toolbar click on View, Analysis Control, Annotations and Measurements opening a panel with different options (Supplementary file C). In the Annotation and Measurements panel select Semiaxis and click on Ellipse.
- On the Image Search, search for the axis that better fits the object shape and press Enter on the keyboard to transfer the obtained value on the right panel table.
- Repeat step 6.3.3 for at least 10 objects to obtain sufficient data. Click on Export to Excel Data Sheet to export the data and save them.
- 3D Z-Stack image visualization and volume numerical calculation
- Open the 3D image captured with the confocal microscopy in the optical microscope software (Supplementary file D).
- To see the 3D reconstruction, click on Show Volume View in the image toolbar; the object can be rotated in any direction by clicking the left mouse button. To select a portion of the volume, keep pressing ctrl + left mouse keys (Supplementary File E).
- Press the x button on the keyboard to take a snapshot of the 3D image and to save it.
- To calculate the object volume, click on Measure, 3D object measurements opening a panel (Supplementary File, Image F). Click on the panel toolbar Define 3D threshold and set the optimized threshold using also the smooth and clean filters (Supplementary File, Image G). Click on OK obtaining in the 3D object measurements panel different parameters, including the volume.
- Click on Export to Excel to export data and save them.
7. Spheroid data analysis
- For the spheroids volume calculations, approximate the 3D structure with a prolate ellipsoid, estimating the major and minor axis through the 2D projection as shown in the insert of Figure 3.
- Validate the prolate ellipsoid approximation through a comparison between the volume calculated numerically as shown in step 6.4 and the prolate ellipsoid volume formula
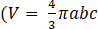 with b>a=c where a, b, and c are the ellipsoid axis).
with b>a=c where a, b, and c are the ellipsoid axis). - Calculate the mean volume of at least 10 spheroids and the respective standard deviations. If the volume distribution of spheroid is normal, apply the Dixon test to identify and reject the outlier value. Thereafter, calculate the volume ratio between the treated and samples together with the error propagation as reported in Figure 6A.
Representative Results
Dox@DDAB-MDs were developed according to protocol (Section 1) as schematically described in Figure 1. The obtained MDs are made of a monolayer of DDAB encapsulating the DFP core (Figure 1A). The cationic charge of DDAB and the sonication procedure avoid the formation of DDAB multilamellar layers stacked at the DFP and water interface23.
The CLSM micrograph (Figure 1B) shows a production of concentrated spherical droplets with a concentration of 1.5 x 1010 MDs /mL, an average diameter of about 1 µm. The red fluorescence from Dox indicates the stable encapsulation and confinement of the drug cargo in the MDs. DLS measurements confirm that the size distribution of the MDs is narrow with a mean value of 1.1 ± 0.1 µm with polydispersity index (PDI) of 0.1 (Figure 1B). ζ-potential of MDs is 90 ± 5 mV.
Droplets with a 1 µm size are considered the best trade-off among stability optimization, their extravasation and flow through capillaries without clogging them. MDs are prepared in ultrafiltered, deionized water in which they are stable and easy to store. The stability of the droplets in 5 mL of PBS and cell culture medium has been assessed within 3 h, where they show a size of 1.10 ± 0.25 µm and of 1.1 ± 0.2 µm, and a concentration of 1.0 x 1010 MDs /mL and 1.3 x 1010 MDs /mL, respectively. The protocol in Figure 1 provides a reproducible way for MDs preparation in terms of size, PDI, concentration, and ζ-potential with average values varying within 4%, 10%, 2%, and 2% respectively, from three independent preparations.
A fluorescence microscopy experiment on the spontaneous release of the drug from DDAB-MDs in the absence of interaction with cells in PBS solution and 37 °C highlights that even after 24 h more than 80% of the drug initially encapsulated remains trapped in the core of the droplets, as shown in Figure 1C. This confirms that not only are the MDs stable in a physiological environment, but also that they behave as good reservoirs without losing significant Dox amount before reaching the target cells. The interaction of Dox@DDAB-MDs with MDA-MB-231 tumor cells was first documented using two-dimensional (2D) cell culture and then on 3D spheroids fabricated as per the protocol shown in Figure 2.
Evidence of a strong affinity of the DDAB shell of the MDs to the MDA-MB-231 plasma membrane is shown in Figure 3A. Interestingly, the droplets once in contact with the cell surface release with a rapid burst the drug contents within the cytosol (Figure 3A). According to confocal fluorescence analysis, as shown in the video with a time duration of about 2 min (Supporting Video S1), the estimated time-lapse of Dox release is about 1 min.
After this time, the droplets, emptied from the Dox, remain adhered to the membrane. The interaction between DDAB-MDs and cell membranes is both electrostatic and hydrophobic. In fact, DDAB particles are known to be used as a transfection agent, which interacts with membrane phospholipids. Analogous to these agents, the DDAB shell of the MDs is strongly coupled with the cell membrane, with dynamic interchange between the DDAB shell and cell membrane lipids. Therefore, the interacting droplet is no longer stable and after a few minutes, MDs change their size and shape features. After 24 h, the droplets disappear from the cell surface and the cells appear morphologically changed (Figure 3B), indicating a suffering state.
A trypan blue test confirmed that, after 24 h incubation of Dox@DDAB-MDs at the equivalent concentration of Dox of 10 nM (i.e., confined within the DDAB-MDS of 109 MDs /mL), a loss of viability equal to 73% occurs. As a control experiment, free Dox showed no cytotoxic effect, since the working concentration of Dox used in the present work is much lower than the IC50 reported in the literature for the cell line tested (equal to 1.2 µM)24. Cytocompatibility of unloaded DDAB-MDs was also successfully evaluated (data not shown).
Given the promising results on two-dimensional cell cultures, the treatment response was investigated on a more realistic tumor model in vitro. In Figure 4 is shown a representative 3D reconstruction of a spheroid that, according to the protocol section, can be approximated to a rotation prolate ellipsoid (Figure 4). Also, in the 3D experiments, a strong affinity between the MDs shell and the MDA-MB-231 plasma membrane was noticed as shown in Figure 5A.
Preliminary tests were carried out on 100 µm diameter spheroids and, after 24 h of treatment with Dox@DDAB-MDs at the relative Dox concentration of 10 nM, the detachment of cell aggregates from the spheroids was observed (Figure 5B). Furthermore, as shown in the 3D CLSM image (Figure 5C), the viability assay with calcein-AM staining showed a non-colocalization of the green (calcein-AM) and red (Dox) color, indicating that not only the detached cells but also some spheroid cells are dead. This effect is more evident in the zoomed image taken with a 100x objective on the spheroid surface (insert in Figure 5C).
To verify the drug release efficacy of the Dox@DDAB-MDs at the equivalent Dox concentration of 10 nM, 100 µm sized spheroids were treated at time 0 h and then harvested after 1 h, 3 h,6 h, 16 h, 24 h, and 48 h. Results in Figure 6A show the decrease of spheroids volume ratio (Vt/Vc), where Vt and Vc are the mean volumes of treated and untreated spheroids, respectively, as a function of the treatment time. A minimum of the (Vt/Vc) ratio after about 6 h is found, indicating a spheroid volume ratio decrease of about one half (Figure 6B);correspondingly treatments performed with free Dox DDAB-MDs did not show any effect on the volume (gray points). Noteworthy, the volume of the Dox treated spheroids, Vt, recovers the control volume, Vc, after 24 h of treatment. This finding shows that Dox can be metabolized in 6 h, and an increase of the spheroid volume is observed afterwards due to cell proliferation.
In general, the spheroid volumes were studied (assuming uniform density in terms of radius of gyration, thus obtaining an equivalent sphere volume), which were sensitive to remarkable dimensional changes. However, the trends of the volume ratios obtained with this method was not significantly different from the ellipsoid approach. It is reasonable to conclude that the variations observed regard the volume of the spheroid rather than their shape. This statistical analysis applied to spheroids produced with this method, shows its reproducibility in terms of shape and size.
These findings also confirm that the formation of dense multicellular spheroids plays a role in determining the sensitivity of MDA-MB-231 cells to the Dox, compared to their resistance in 2D arrangement in agreement to reported MDA-MB-231 spheroids drug resistance studies25. Based on these findings, chronic treatment tests were carried out using Dox concentrations lower than the one used in normal clinical practice.
The second administration of encapsulated Dox in DDAB-MDs after 6 h, when the maximum effect is achieved (see Figure 6A), keeps the (Vt/Vc) ratio at about 0.5 (red point in Figure 6A) for ~24 h. Since the innermost part of a spheroid is generally composed of dead or quiescent cells, the reduction in volume therefore involves a loss of the outermost layers, bringing at the surface the spheroid core, which is now accessible to treatment. The importance of this data should not be underestimated as the observed volume reduction could also be interpreted as a decreased ability of the tumor to metastasize.
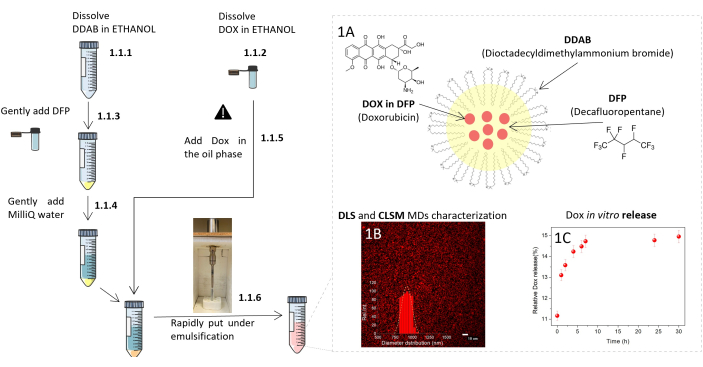
Figure 1: Scheme of the MDs easy protocol procedure and characteristics. Left: detailed representation of the MDs formulation protocol. Numerical sequence is indicated by the digits corresponding to the steps of the protocol. Danger sign means operate with caution. Right: (A) Sketch of the Dox@DDAB-MDs. (B) MDs size distribution centered at 1 µm, by DLS and CLSM determinations. (C) MDs in vitro Dox release kinetics at 37 °C by fluorescence analysis. Protocol scheme was modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/. Please click here to view a larger version of this figure.
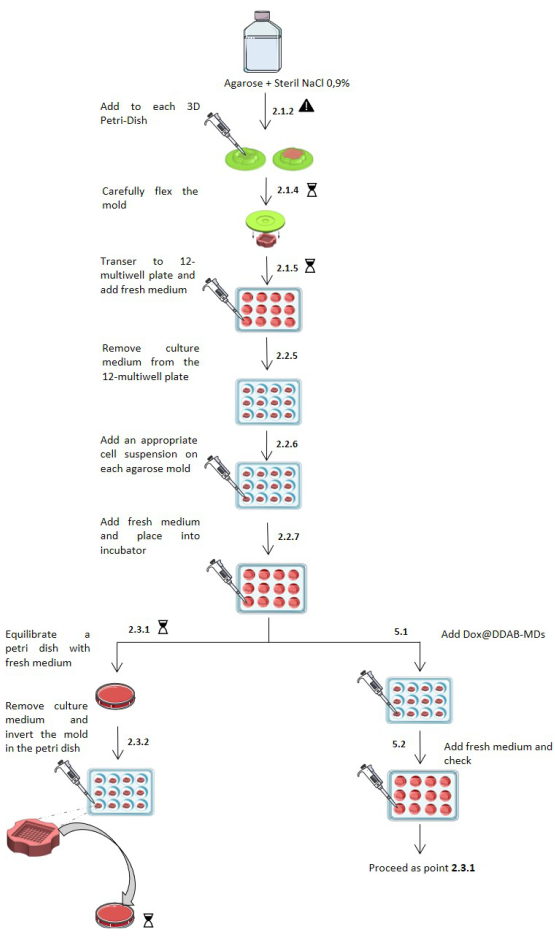
Figure 2: Scheme of micro-molded spheroid fabrication and treatment protocol procedure. Step by step representation of the spheroids' fabrication and treatment protocol. Numerical sequence is indicated by the digits corresponding to the steps of the protocol. The danger sign means operate with caution. The hourglass indicates an incubation time. The protocol sketch was modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/. Images of the 3D PetriDish courtesy of Microtissues, Inc (www.microtissues.com) Please click here to view a larger version of this figure.
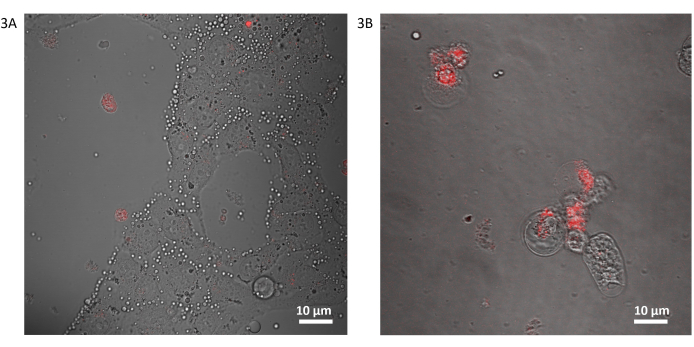
Figure 3: Dox@DDAB-MDs interacting with 2D MDA-MB 231 cell line. Merged transmission and confocal fluorescence micrographs (60x) of the MDA-MB-231 cell line after incubation with Dox@DDAB-MDs: (A) MDA-MB 231 cells after 1 min of treatment, MDs are represented by white spots, all surrounding the cellular membranes. (B) MDA-MB 231 cells after 24 h of treatment; morphological changes and red Dox fluorescence show that cells are sentenced to death. Please click here to view a larger version of this figure.
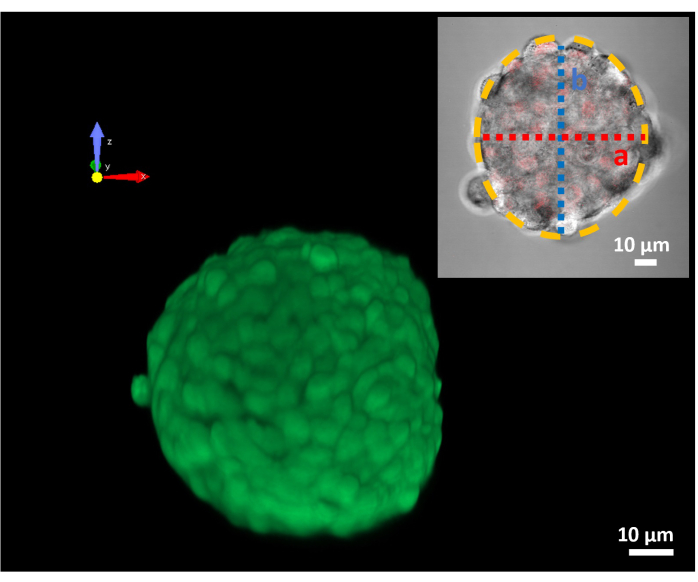
Figure 4: 3D confocal image of an MDA-MB 231 spheroid. Z-stack image reconstruction of a 100 µm spheroid. The green fluorescence is due to the calcein-AM dye; the insert shows the spheroid equatorial section with the ellipsoid approximation (yellow dotted line) applied for the volume calculation. (A) is the minor axis in red and (B) is the major axis in blue. Please click here to view a larger version of this figure.
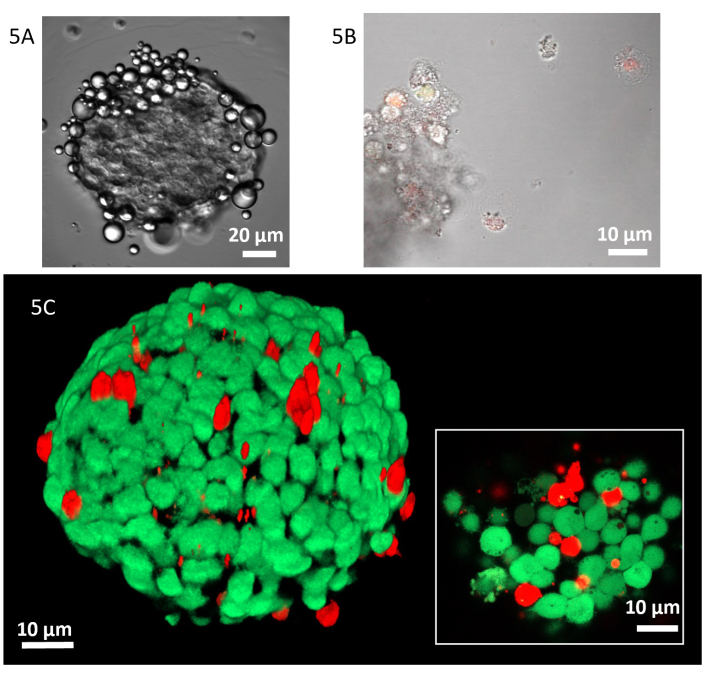
Figure 5: Dox@DDAB-MDs interacting with MDA-MB 231 spheroids. (A) Transmission micrograph (40x) of MDA-MB-231 spheroid after 5 min of treatment in the mold. (B) Merged transmission and confocal fluorescence micrographs (20x) of the MDA-MB-231 cells detached from the spheroids after 24 h incubation with Dox@DDAB-MDs. (C) Z-stack 3D reconstruction of a spheroid after 6 h of treatment, dead and living cells are stained in red (propidium iodide) and green (calcein-AM), respectively. In the insert, confocal zoom image (100x) of a spheroid slide. Please click here to view a larger version of this figure.
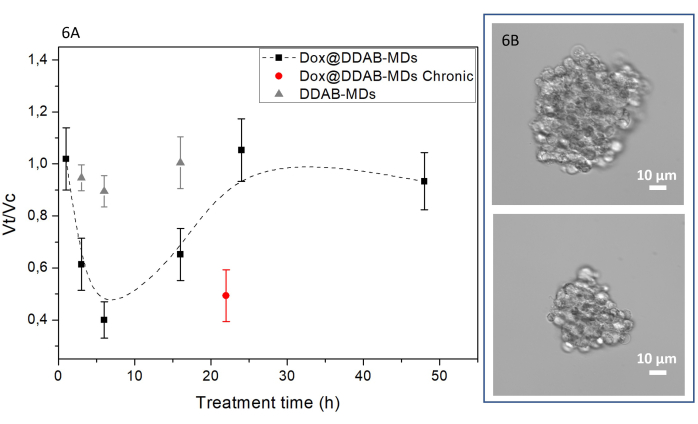
Figure 6: Spheroids volume reduction upon Dox@DDAB-MDs treatment. (A) Black squares represent the trend of volume ratio of treated (Vt) to control (Vc) spheroids as a function of the treatment time (h); the dashed line is an eyes guide. The red circle represents the chronic treatment at time 0 h and after 6 h and analyzed after 22 h. The grey triangles stand for the treatment with Dox free DDAB-MDs. Each point represents the volume ratio between the mean volume of treated and control samples, calculated on at least 10 spheroids. (B) Two representative 40x micrographs of the spheroids before (on top) and after 6 h Dox@DDAB-MDs treatment (below). Please click here to view a larger version of this figure.
Supporting Video S1: Time lapse video of in vitro Dox@DDAB-MDs drug uptake by MDA-MB-231 cells. 2 mL of cell culture medium and relative Dox concentration of 10 nM was incubated in a Petri dish. At the bottom right a timer is shown. After 28 s, droplets were added. At 1.03 min the interaction between droplets and cells takes place and the timer is zeroed. The Dox uptake (red fluorescence increase of cells) is observed after one minute in a rapid burst. Please click here to download this Video.
Supplementary File: Screenshots of the microscope software used to acquire micrographs. (A) Home page of the confocal microscope software. (B) 3D z-stack settings details of the confocal microscope software. (C) Details of the optical microscope software package for the ellipsoid axis measurements. (D, E) Use of the optical microscope software to open and view a 3D z-stack. (F, G) Details of the optical microscope software for the spheroid volume numerical calculation. Please click here to download this File.
Discussion
To improve the efficacy of anthracyclines as antitumor drugs, this work presents the formation of DDAB shelled PFC droplets encapsulating the chemotherapeutic drug doxorubicin (Dox) and the effect of such formulation interacting with the high aggressive triple-negative breast cancer cells, MDA-MB-231.
Building up of DOX@DDAB-MDs
Dox loaded MDs have been formulated by the insonation method with an extremely fast, well reproducible, user-friendly, and efficient protocol. This method allows the obtainment of MDs with a very narrow size distribution centered at about 1 µm and the encapsulation of a good amount of drug. The following steps of the protocol need particular care: a) Step 1.1.3: To maximize the drug loading and storage in MDs. The Dox alcoholic solution is gently injected into the oil phase immediately before the insonation to avoid the partitioning of a significant amount of Dox in the water phase. b) Step 1.1.4: It is advisable to dilute the dispersion immediately after insonation as the high droplets concentration could speed up the coalescence, despite their electrostatic stabilization due to the high measured ζ-potential. c) Step 1.1.6: As mentioned before, the high ζ-potential allows also to depress the spontaneous coalescence. Additionally, it gives enough time to centrifugate and wash the sample without affecting the size distribution or the initial concentration of MDs. However, it is advisable not to leave the dispersion for more than 30 min in the form of a pellet. Moreover, the cumulative drug release from Dox@DDAB-MDs in phosphate buffer (PBS) was studied as a function of time and determined by measuring the fluorescence signal at 590 nm. More than 80% of the loaded Dox remains confined inside the MDs over 24 h (Figure 1C).
Growth and characterization of three-dimensional tumor cell culture as a preclinical model of triple-negative breast cancer
The proposed protocol also describes a new method for the study and screening of drugs in vitro using tumor spheroids. It has long been believed that the growth of tumor cells as spheroids is a better model, closer to in vivo conditions, than as an adherent cell monolayer25. However, their use is still not widespread and possible limitations may include the difficult and time-consuming nature of some of the current techniques and the need for specialized equipment. In fact, should the use of spheroids become standard practice for high throughput screening, the training method should have ease of use and reproducibility as main features. Therefore, to promote such kind of in vitro 3D cell culturing, this study reports a fast, inexpensive, and easily reproducible method of manufacturing spheroids.
However, it is important to consider some fundamental critical points when working with 3D models: a) The dyes incubation time required for the spheroids is longer than the one for 2D culture since penetration is slower. To avoid spheroid loss or disintegration, do not wash dye solution in the mold and caution is requested when pipetting. b) The innermost part of a tumor spheroid, which is visually darker in brightfield, is mostly composed of dead or quiescent cells. In fact, the staining with propidium iodide / Calcein-AM shows a more intense fluorescence in the outermost part of the spheroid, at the expense of an exclusively red color inside. c) It is important to know and keep fixed the size and volume of the spheroids to obtain a good image quality since as the spheroid radius increases, the fluorescent signal decreases. In the present work, all the 3D images were a Z-stack reconstruction performed with 0.75 µm increments using the microscope software.
In conclusion, it has been demonstrated that the affinity of the MDs shell to the cell membrane of MDA-MB-231 cells promotes an intracellular burst drug release along with a significant cytotoxic effect at a Dox dose which is two orders of magnitude below the IC50 of the free drug (it seems to be even lower than that of other encapsulated formulations17). Additionally, the effectiveness of this novel formulation was successfully evaluated on a 3D in vitro model for breast cancer, suggesting a protocol of treatment that provides a short-term volume reduction of the spheroidal tumor.
In future work, the MDs shell could be implemented by co-adding lipids such as lecithins for non-viral gene transfection or gene and drug delivery combined strategy26,27,28. On this line, it could be interesting to substitute DDAB with other cationic lipids (e.g., DC-Cholesterol;1,2-dioleoyl-3-trimethylammonium-propane;1,2-dioleoyl-sn-glycero-3-phosphoethanolamine), which, similarly to DDAB, in the liposomal assembly have already demonstrated28 to establish electrostatic interaction with DNA or RNA strands and cell transfection capabilities.
Moreover, investigating ultrasound and microdroplet for selective drug delivery technology might prove important since targeted drug delivery reduces drug dose adverse effects. In fact, it has been shown that in situ ultrasound-mediated microbubble delivery can reduce the cytotoxicity of Dox in non-cancer cells29. Future research should balance the effectiveness of drug delivery by Acoustic Droplet Vaporization (ADV) with the potential detachment and fractionation of tumor mass induced by ultrasound irradiation.
開示
The authors have nothing to disclose.
Acknowledgements
This work has received funding from the European Union Horizon 2020 research and innovation program under grant agreement AMPHORA (766456).
Materials
| µ-Petri dish | Ibidi | 81156 | 35mm high, IbiTreat |
| 1,1,1,2,3,4,4,5,5,5-Decafluoropentane | Sigma-Aldrich | 138495-42-8 | b.p. 55°C |
| 12-well culture plate | Corning | ||
| 15 ml centrifuge tube | Falcon | 89039-664 | |
| 3D-Petri dishes 12:256 | Microtissues (Sigma-Aldrich) | Z764000-6EA | Small |
| 3D-Petri dishes 12:81 | Microtissues (Sigma-Aldrich) | Z764019-6EA | Large |
| 5%CO2 culture incubator, 37°C | Thermo Scienific | HERAcell 150i | |
| 50 ml centrifuge tube | Falcon | 352070 | |
| Biological safety cabinet, II level | |||
| Calcein | Sigma-Aldrich | ||
| Calcein-AM | Sigma-Aldrich | 148504-34-1 | 4mM stock solution in DMSO |
| cam sCMOS Andor Zyla 4.2 | Andor Instruments | ||
| Centrifuge Hettich Universal 320R | Hettich Lab. Technology | ||
| DAPI | SIgma-Aldrich | ||
| Dimethyldioctadecylammonium bromide powder | Sigma-Aldrich | 3700-67-2 | |
| DMEM (Dulbecco's Modified Eagle Medium) | Corning | 15-013-CV | |
| Doxorubicin hydrochloride | Sigma-Aldrich | 25316-40-9 | |
| DPBS (Dulbecco's Modified PBS) | Corning | 21-030-CV | pH 7,4 |
| Ethanol 70% | Sigma-Aldrich | ||
| EZ-C1 digital ecliplse | Nikon Instruments | Silver version 3.91 | |
| Fetal Bovine Serum (FBS) | Corning | 35-079-CV | |
| Goniometer BI-200SM | Brookhaven Instruments Corporations | ||
| Laser Ar+ | Spectra Physics | ||
| Laser He-Ne | Melles-Griot | ||
| L-Glutammine | Corning | 25-005-CI | |
| Mcroscope Nikon Eclipse Ti | Nikon Instruments | ||
| MDA-MB 231 cell line | ATCC | ||
| Microsoft Excel | Microsoft | ||
| Microplates reader Spark | Tecan group | ||
| NanoZetaSizer ZS | Malvern Instruments LTD | ||
| Neubauer improved chamber | 718605 | ||
| NIS Elements software | Nikon Instruments | AR 4.30 | |
| Pen/Strepto | Corning | 30-002-CI | |
| Photocorrelator BI-9000 AT | Brookhaven Instruments Corporations | 62927-1 | |
| Photometer HC120 | Brookhaven Instruments Corporations | N° 1275 | |
| Pipettors and tips, various size | Gilson | ||
| Propidium Iodide | SIgma-Aldrich | ||
| Serological pipets, various size | Corning | ||
| Solid-state laser | Suwtech Laser | N° 22368 | |
| T25 Flasks | Sarstedt | 83.3910.002 | |
| T75 Flasks | Sarstedt | 83.3911.002 | |
| Trypsin/EDTA 0.05% | EuroClone | ECB3052D | |
| Vibra-Cell VCX-400 | Sonics & Materials, inc | ||
| Water bath | 37°C |
参考文献
- Aryal, S., Park, H., Leary, J. F., Key, J. Top-down fabrication-based nano/microparticles for molecular imaging and drug delivery. International Journal of Nanomedicine. 14, 6631-6644 (2019).
- Peng, Y., et al. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian Journal of Pharmaceutical Sciences. 15, 220-236 (2020).
- Chan, K. S., Koh, C. G., Li, H. Y. Mitosis-targeted anti-cancer therapies: Where they stand. Cell Death and Disease. 3, 411 (2012).
- Raj, S., Franco, V. I., Lipshultz, S. E. Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Current Treatment Options in Cardiovascular Medicine. 16, 315 (2014).
- Iyer, A. K., Singh, A., Ganta, S., Amiji, M. M. Role of integrated cancer nanomedicine in overcoming drug resistance. Advanced Drug Delivery Reviews. 65, 1784-1802 (2013).
- Blanco, E., Shen, H., Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology. 33, 941-951 (2015).
- Davis, M. E., Chen, Z., Shin, D. M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nature Reviews Drug Discovery. 7, 771-782 (2008).
- Lammers, T., Hennink, W. E., Storm, G. Tumour-targeted nanomedicines: Principles and practice. British Journal of Cancer. 99, 392-397 (2008).
- Couvreur, P., et al. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. Journal of Pharmacy and Pharmacology. 31, 331-332 (1979).
- Yordanov, G. G., Dushkin, C. D. Preparation of poly(butylcyanoacrylate) drug carriers by nanoprecipitation using a pre-synthesized polymer and different colloidal stabilizers. Colloid and Polymer Science. 288, 1019-1026 (2010).
- Kooiman, K., Vos, H. J., Versluis, M., De Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Advanced Drug Delivery Reviews. 72, 28-48 (2014).
- Fasolato, C., et al. Antifolate SERS-active nanovectors: Quantitative drug nanostructuring and selective cell targeting for effective theranostics. Nanoscale. 11, 15224-15233 (2019).
- Cerroni, B., et al. Temperature-tunable nanoparticles for selective biointerface. Biomacromolecules. 16, 1753-1760 (2015).
- Chronopoulou, L., et al. PLGA based particles as “drug reservoir” for antitumor drug delivery: characterization and cytotoxicity studies. Colloids Surfaces B: Biointerfaces. 180, 495-502 (2019).
- Calderó, G., Paradossi, G. Ultrasound/radiation-responsive emulsions. Current Opinion in Colloid and Interface Science. 49, 118-132 (2020).
- Capece, S., et al. Complex interfaces in ‘phase-change’ contrast agents. Physical Chemistry Chemical Physics. 18, 8378-8388 (2016).
- Mielczarek, L., et al. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. International Journal of Pharmaceutics. 558, 311-318 (2019).
- Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E., Paul Solomon, F. D. 3D cell culture systems: Advantages and applications. Journal of Cellular Physiology. 230, 16-26 (2015).
- Heinonen, T. Better science with human cell-based organ and tissue models. Alternatives to Laboratory Animals. 43, 29-38 (2015).
- Thoma, C. R., Zimmermann, M., Agarkova, I., Kelm, J. M., Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Advanced Drug Delivery Reviews. 69-70, 29-41 (2014).
- Astashkina, A., Grainger, D. W. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Advanced Drug Delivery Reviews. 69-70, 1-18 (2014).
- Weigelt, B., Ghajar, C. M., Bissell, M. J. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Advanced Drug Delivery Reviews. 69-70, 42-51 (2014).
- Feitosa, E., Karlsson, G., Edwards, K. Unilamellar vesicles obtained by simply mixing dioctadecyldimethylammonium chloride and bromide with water. Chemistry and Physics of Lipids. 140, 66-74 (2006).
- Cancerrxgene. Doxorubicin IC50. Genomics of drug sensitivity in cancer Available from: https://www.cancerrxgene.org/compound/Doxorubicin/133/overview/ic50 (2020)
- Boo, L., et al. Phenotypic and microRNA transcriptomic profiling of the MDA-MB-231 spheroid-enriched CSCs with comparison of MCF- 7 microRNA profiling dataset. PeerJ. 2017, 1-27 (2017).
- Domenici, F., Castellano, C., Dell’unto, F., Congiu, A. Temperature-dependent structural changes on DDAB surfactant assemblies evidenced by energy dispersive X-ray diffraction and dynamic light scattering. Colloids and Surfaces B: Biointerfaces. 95, 170-177 (2012).
- Choosakoonkriang, S., et al. Infrared spectroscopic characterization of the interaction of cationic lipids with plasmid DNA. Journal of Biological Chemistry. 276, 8037-8043 (2001).
- Yin, H., et al. Non-viral vectors for gene-based therapy. Nature Reviews Genetics. 15 (8), 541-555 (2014).
- Lentacker, I., Geers, B., Demeester, J., De Smedt, S. C., Sanders, N. N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Molecular Therapy. 18, 101-108 (2010).

.
