Detecting Amyloid-β Accumulation via Immunofluorescent Staining in a Mouse Model of Alzheimer’s Disease
概要
In the neuropathology of Alzheimer’s disease, one of the most crucial characteristics is the deposition of amyloid-β. In this protocol, we describe the method of immunofluorescent staining in 5×FAD transgenic mouse to detect amyloid-β accumulation in plaques. The process of perfusion, cryosectioning, staining and quantification will be described in detail.
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that contributes to 60-70% dementia around the world. One of the hallmarks of AD undoubtedly lies on accumulation of amyloid-β (Aβ) in the brain. Aβ is produced from the proteolytic cleavage of the beta-amyloid precursor protein (APP) by β-secretase and γ-secretase. In pathological circumstances, the increased β-cleavage of APP leads to overproduction of Aβ, which aggregates into Aβ plaques. Since Aβ plaques are a characteristic of AD pathology, detecting the amount of Aβ is very important in AD research. In this protocol, we introduce the immunofluorescent staining method to visualize Aβ deposition. The mouse model used in our experiments is 5×FAD, which carries five mutations found in human familial AD. The neuropathological and behavioral deficits of 5xFAD mice are well-documented, which makes it a good animal model to study Aβ pathology. We will introduce the procedure including transcardial perfusion, cryosectioning, immunofluorescent staining and quantification to detect Aβ accumulation in 5×FAD mice. With this protocol, researchers can investigate Aβ pathology in an AD mouse model.
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease that causes 60%-70% dementia around the world and costs much social resources1. It is well-known that accumulation of amyloid-β (Aβ) is a pathological hallmark in Alzheimer's disease. Amyloid precursor protein (APP) is an integral membrane protein that exists in many tissues. Aβ peptide, consisting of 36-42 amino acids2, is produced by the subsequent cleavage of β- and γ-secretase in APP3,4. Changes in APP cleavage and mutations in APP gene lead to overproduction of Aβ. Aβ molecules can aggregate to form oligomers or fibrils, which are believed to be neurotoxic5,6. In previous studies, the accumulation of Aβ was demonstrated to be correlated with neuronal death in AD7,8,9.
The 5×FAD (C57BL/6J) transgenic mice contain 3 mutations in APP, and 2 mutations in PSEN1. The accumulation of intracellular Aβ starts as early as 1.5 month of age. Extracellular accumulation of Aβ was found around 2 months, in the cortex and hippocampus. The accumulation increased rapidly with age10. The well-documented Aβ pathology makes it a good animal model for our protocol.
The goal of the described staining method is to visualize and quantify Aβ deposition in the brain of AD mice model. The procedure including transcardial paraformaldehyde perfusion, cryosectioning, immunofluorescent staining and quantification to detect Aβ accumulation in 5×FAD mice will be introduced. This protocol is a reliable and easy method to investigate Aβ pathology in AD mouse model.
Protocol
All experimental procedures were performed with the approval of the Institutional Animal Care and Use Committee of Xuzhou Medical University and in accordance with the guidelines of the Chinese governmental regulations for the care and use of laboratory animals.
1. Perfusion of Mice
NOTE: More details of the perfusion procedure can refer to the video from Wiliam Shain's lab 11.
- Anesthetize 5×FAD (C57BL/6J) transgenic mice with 1% pentobarbital sodium (50 mg/kg body weight) by intraperitoneal injection12,13. Loss of response to hind paw-pinching indicates proper anesthetization. Do not start perfusion before the mouse is properly anesthetized.
- Use four pins to fix the limbs on the polyfoam plank. Set the abdomen of the anesthetized mouse upwards, with its limbs adequately stretched. Use a pair of iris scissors to make an incision at the xiphoid process. Then the diaphragm will be exposed.
- Use surgical scissors to make an incision on the diaphragm, and carefully continue the diaphragm incision to the upper bound of the rib cage. Cut the ribs and thoracic muscles, dissect the attached tissues to expose the heart.
- Find the right atrium of the mouse. Cut open the right atrium using iris scissors.
- Inject 20 mL of 37 °C PBS (0.01 M, pH 7.2-7.4) from the left ventricle of the heart to flush out blood. Then slowly inject 20 mL of 4% PFA of room temperature from the left ventricle to fix the tissues. The injection rate of PBS and 4% PFA is around 5 mL/min. Fixation tremors should be observed within seconds.
CAUTION: 4% PFA can irritate eyes and airways, and may provoke allergic reactions. This step should be done in ventilated places, and the operator should wear safety goggles and face masks. - Use surgical scissors to cut off the head, and carefully remove the cranium use dissecting forceps and extract the brain. Then submerge the brain into 4 mL of 4% PFA in a 5 mL plastic tube for 12-24 h at 4 °C.
2. Embedding and Cryosectioning
- After fixation in 4% PFA, transfer the brain into 4 mL of 15% sucrose in a 5 mL plastic tube at 4 °C. After 12-24 h, the brain should sink to the bottom.
- Transfer the brain into 4 mL of 30% sucrose in a 5 mL plastic tube at 4 °C for 12-24 h. Then the brain is ready for embedding. The purpose of 15% and 30% sucrose stepwise soaking is to dehydrate the brain, which avoids the formation of ice crystals inside the cells during embedding and cryosectioning. The dehydrated brain can be stored at 4°C for one week.
- Leave roughly 1 mL of sucrose with the brain in the tube. Then add the same volume optimal cutting temperature (OCT) compound into the tube and mix properly. This helps OCT compound to wrap the brain more sufficiently.
- Mount a thick layer of OCT compound on the knob surface and freeze at -21 °C before cryosectioning. Cut the brain sagittally from the middle; use either half for sectioning in a cryostat.
- After the OCT compound solidifies, mount the knob on the specimen head and trim it into a platform surface. Lay one half of the brain on the trimmed surface with the middle side downwards.
- Put a tinfoil ring around the brain to avoid leakage of OCT compound, and then fill the ring with OCT compound until the brain is submerged. When OCT compound embedding solidifies, it is ready for sectioning.
- Set the thickness of the section to 20 µm. Set the chamber temperature to -21 °C, and the specimen head temperature to -19 °C. Section the brain from the rostral end to the caudal end.
- Brush some PBS on the glass slides. Pre-coat the glass slides in poly-lysine to prevent the sections from peeling off. Attach the section onto the slide. If the section is folded, use a soft brush to unfold the sections with PBS.
- Dry the sections overnight at room temperature and store at -20 °C. Sections can be kept at -20 °C for up to one month before Aβ staining.
3. Aβ Staining Procedure
- Warm up the sections at room temperature, dry the slide surface, and use a hydrophobic pen to draw a circle around the section for antibody incubation.
- Soak the slide in PBS in a 30 mL plastic staining box to wash off OCT compound. Use at least 20 mL of PBS.
- Add 100 µL of 1x antigen retrieval solution for frozen sections onto the slide. Incubate for 5 min at room temperature.
- Wash the retrieval solution with at least 20 mL of PBS of room temperature for 3 times (5 minutes for each).
- Add diluted primary antibody (6E10) on the sections and incubate at 4 °C for 16-24 h in a wet, dark box (primary antibody dilution solution: 1% BSA, 0.3% Triton X-100, 0.01% sodium azide in PBS, 1:500 dilution). BSA functions as blocking agent.
- Wash the sections with PBS for 3 times, 5 min each time.
NOTE: Perform steps 3.7, 3.8 and 3.9 in a dark place. - Incubate sections in secondary antibody solution (Goat anti-Mouse IgG (H+L) Alexa Flour 594, diluted with PBS 1:200) for 1 h at room temperature in a wet, dark box.
- Wash the secondary antibody off the sections with at least 20 mL of PBS for 3 times, 5 min each time.
NOTE: Do not pour PBS onto sections during washing to prevent sections peeling off. Keep the sections wet. - Absorb the liquid around sections and add a drip of mounting medium on each section. Seal the sections with cover glass, avoiding bubbles.
- Observe the stained sections with a fluorescence microscope. Keep the imaging parameters consistent to avoid derivation. Keep the slides at 4°C in a dark place. Analyze the sections within one week, since the staining will fade with time.
4. Imaging and Quantification of Aβ accumulation by ImageJ
- Capture images by a fluorescence microscope connected with a digital camera and imaging software. We used Image Pro Plus software and the imaging parameter was set as follows: Exp Pvw: 450 ms, Exp Acq: 450 ms; Pvw: 1 × 1, Acq: 1 × 1; Pvw Resolution: Width 1 × Height 1, Acq Resolution: Width 1 × Height 1; Capture Depth: 8-bit mono; Gain: Pvw: 13, Acq: 13, Gamma: Pvw: 1, Acq: 1, Offse: Pvw: -700, Acq: -700.
- In ImageJ Fiji 2.0.0 (https://imagej.net/Fiji), select Plugins | Stitching | MosaicJ in the menu. Then select Files | Open Image Sequence to open the images to be stitched (Figure 1A).
- All the selected images will be displayed on the bottom. After stitching the images manually, select File | Create Mosaic (Figure 1B, C, D).
- Select Image | Adjust | Brightness/Contrast …, and adjust the brightness and contrast of the image (Figure 3A). Then the image is able to be saved (Figure 1E).
- To quantify the accumulation of Aβ, open the image through File | Open… in the menu (Figure 2A).
- Select Image | Type | 8-bit to adjust the image to 8-bit. Then choose the area intended to be counted using Polygon selection (Figure 2B).
- Select Image | Adjust | Threshold to select the proper threshold of signals. Threshold can be adjusted via dragging the scroll bars or change the numbers directly in the textbox (maximum: 255, minimum: 0). When all the Aβ signals in the section has become red, the threshold is appropriate for measurement (Figure 2C). Make sure that Dark Background box is checked.
- Selecting Analyze | Set Measurement… in the menu to choose the parameters that will be shown in the results. Confirm that Integrated density and Limit to Threshold is selected. Integrated density (total intensity) is the target of the measurement (Figure 2D).
- Select Analyze | Measure to get the results. The results will be displayed and ready for statistical analysis (Figure 2E).
Representative Results
We used the above-described immunofluorescent staining procedures to investigate the deposition of Aβ accumulation in 5×FAD mice of different age. Figure 3 represents typical results and suboptimal results using our protocol. Brain slices of 5-month and 8-month heterozygous 5×FAD transgenic mice and 4 to 6-month wild-type control were stained with 6E10 antibody and detected under a fluorescence microscope. Figure 3A shows Aβ accumulation in plaques in the sagittal section of the mouse brain. They are mainly deposited in cortex and hippocampus, with the highest density in subiculum of hippocampus, which is consistent with previous study10. As the image shows, Aβ accumulation was clearly detected by the antibody in 5×FAD mice. The integrated density (integrated density = Area × Mean. Integrated density: the sum of fluorescence intensity of this area; Mean: the average fluorescence intensity in a certain area; Area: the area of positive fluorescence signal in the image) of Aβ accumulation in 8m 5×FAD mice was significantly higher than that in 5m 5×FAD mice (unpaired students' t-test, p = 0.0071), which shows that the accumulation of Aβ will gradually increase with the age. In addition, no Aβ signal was observed in the wild-type control, which shows the specificity of 6E10 antibody. Some suboptimal results (an 8-month heterozygous 5×FAD transgenic mouse and an 8-month wild-type control) are also shown in Figure 3C. The deficits include slice breakage and curled edge. How to prevent this situation will be discussed. In general, immunofluorescent staining using 6E10 anti-Aβ antibody is a specific method that can easily quantify Aβ accumulation in 5×FAD mice.

Figure 1. Stitching brain slice images by ImageJ. (A) In the menu of ImageJ/Fiji, Select Plugins | Stitching | MosaicJ to begin stitching. (B) Select File | Open Image Sequence and select the images to be stitched. Stitch the images manually according to the anatomical structures of the section. (C) After stitching, select File | Create Mosaic to create the whole image of the section. (D) An example of a stitched image. (E) Select Image | Adjust | Brightness/Contrast… to adjust brightness and contrast of the whole image. The middle panel shows the adjustment menu. Select File | Save as… to save the image into Tiff or other format. Please click here to view a larger version of this figure.

Figure 2. Quantification method of Aβ fluorescence. (A) Set the image type as 8-bit through selecting Image | タイプ | 8-bit. (B) Use the Polygon selection in the menu of ImageJ and depict the contour of the whole brain. (C) Select Image | Adjust | Threshold… to exclude the background noise. Make sure that Dark background box is checked. Drag the red lines or scroll bars until the Aβ fluorescence is properly highlighted. (D) Use Analyze | Set Measurements to choose what will be presented in the result table and set counting method. The menu of Set Measurements is shown. Confirm that Integrated density and Limit to threshold are selected. (E) Select Analyze | Measure to get the results. Integrated density (shown as IntDen) represents the total intensity of Aβ fluorescence (Integrated Density = Area × Mean). Please click here to view a larger version of this figure.
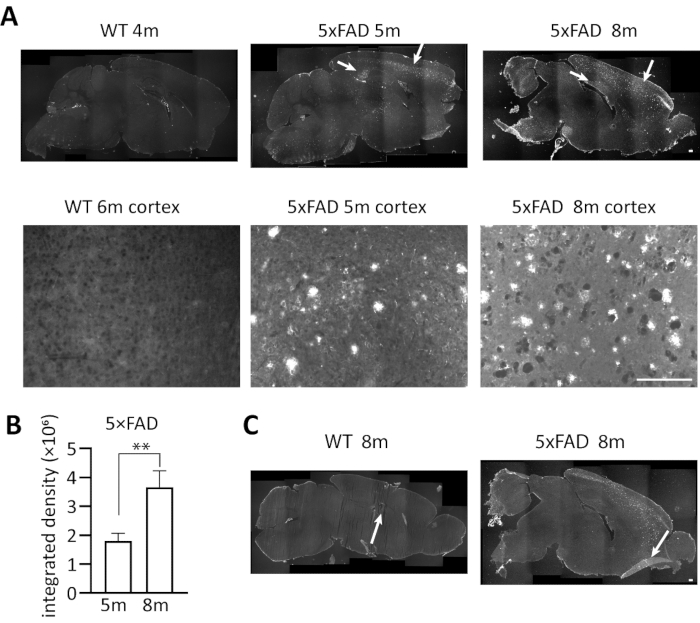
Figure 3. Representative results of immunofluorescent staining of Aβ deposition in 5×FAD mice. (A) The immunostaining of Aβ deposition in the sagittal section of 5-month and 8-month 5×FAD transgenic mice and 4 to 6-month wild-type mice brains using anti-Aβ antibody 6E10. Images were captured by a fluorescence microscope under a 4x or 20x objective. Upper panel, stitched whole brain images in lower magnification. Lower panel, cortex images in higher magnification. As the white arrows indicate, the florescence signals of Aβ deposition were observed clearly in 5×FAD groups, but no Aβ signal was observed in wild-type group. (B) The bar chart shows quantitative analysis of total intensity (Integrated Density = Area × Mean) of Aβ fluorescence in the brains of 5-month and 8-month 5×FAD transgenic mice. Unpaired students' t-test: **, p < 0.01 (n = 3 for each group). Error bars represent SD. (C) Cases of suboptimal slices in immunofluorescent staining, representing an 8-month 5×FAD transgenic mouse and an 8-month wild-type control. Left, section breakage; Right, curled edge of the section. Scale bar, 200 µm. Please click here to view a larger version of this figure.
Discussion
Immunofluorescent staining using 6E10 antibody can specifically detect Aβ accumulation in the brain, which is easy to be quantified by Image J. What is worth noticing is that some crucial steps in this protocol may affect the results.
To prevent slices from peeling off or breakage shown in Figure 3C, a few key points should be noticed. Perfusion should proceed rapidly after making the incision on the diaphragm because of the irreversible pathophysiological effects caused by hypoxia. These effects may profoundly influence the results11. The brain tissue is required to be properly fixed and dehydrated before cryosectioning. Temperature should be properly set for the cryostat (chamber -21 °C, specimen head -19 °C), and the situation of the cutting blade and anti-roll plate should be checked. No dust or frost should be on the cutting blade or anti-roll plate, and they should be positioned correctly. The sections should also be flattened properly before attaching. In addition, slides should be pre-coated to prevent slices from peeling off during staining. Popular methods include poly-lysine or gelatin-coating. Poly-lysine coated microscope adhesion slides can be bought from companies.
Some steps in the staining process will also affect the results. Before incubation, antigen retrieval solution should be applied to the sections to enable full exposure of the antigen. In order to prevent evaporation of the primary antibody during incubation, the sections should be incubated in a wet box. In addition, the sections should be kept in dark after the secondary antibody incubation to avoid fluorescence quenching. Several staining methods have been developed for Aβ detection, such as Thioflavin-S staining, Congo Red staining and Gallyas silver staining, but immunofluorescent staining using 6E10 antibody has unique advantages. Thioflavin-S and Congo Red can bind with all the β-sheets containing proteins, therefore, these chemical dyes are less specific for Aβ detection14,15. Compared with chemical staining, immunofluorescent staining is more specific. The 6E10 anti-Aβ monoclonal antibody is specifically reactive to amino acid residues 4-10 of Aβ, according to a previous high-resolution mapping16. Previous studies also indicated that immunostaining using 6E10 antibody can detect greater plaque deposition than Gallyas silver staining and Thioflavin-S staining, which indicates that Aβ detection using antibody might be more sensitive than chemical staining methods17. However, the critical limitation of 6E10 staining is that this antibody can still recognize full-length APP and other cleaved peptides containing 6E10 epitope, but the fluorescence signal outside Aβ plaques is very weak in 5xFAD mice. Previous studies showed that 6E10 antibody bound with Aβ peptides in both conformation- and sequence-dependent ways16. Despite the limitations, this protocol is still a practical and specific method that can help researchers investigate the Aβ pathology of AD.
開示
The authors have nothing to disclose.
Acknowledgements
This research was supported by a general project of Natural Science Foundation of China (grant number: 81974157) from the Natural Science Foundation of China, a Jiangsu Special Appointed Professorship (to C.L.) from Jiangsu Education Department, a Jiangsu Province Innovative and Entrepreneurial Team Program (to H.Z., A. L., W.W., C.L. and Y.S.), a starting grant of excellent talent (D2019025) and Innovation and Entrepreneurship Training Program (201910313038Z to Z.S., Y.X., M.Z. and J.D.) from Xuzhou Medical University. This research was also supported by National Demonstration Center for Experimental Basic Medical Science Education (Xuzhou Medical University).
Materials
| Anesthetia | |||
| Injection syringe | KLMEDICAL | 1 mL | |
| Pentobarbitual sodium | Sigma-Aldrich | P3761 | 1% in saline for i.p. injection |
| Thoracotomy: | |||
| IRIS-Fine Straight iris scissors (~11.5 cm) | RWD | S12010-11 | |
| Sharp curved surgical scissors(11.5 cm) | RWD | S14016-11 | |
| Straight dissecting forceps (~10.5 cm) | RWD | F12010-10 | |
| Perfusion | |||
| Centrifugal tube (5 mL) | Biosharp | ||
| Injection syringe | KLMEDICAL | 20 mL | |
| Paraformaldehyde | Vicmed | VIH100 | |
| PBS 0.01M (PH7.2-7.4) powder | Vicmed | VC2001P | Add deionized water to make solution |
| Fixation, Dehydration and Cryosectioning | |||
| Adhesion microscope slides | CITOTEST | 188105 | 76.2 mm × 25.4 mm |
| Microtome Cryostat | LEICA | CM1950 | |
| Optimal cutting temperature compound (OCT) | Sakura Finetek | 4583 | |
| Sucrose | VETEC | V900116 | |
| Immunofluorescence staining | |||
| Fluorescence microscope | OLYMPUS | IX-81 | |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | A-11005 | 200X |
| Image-Pro Plus 7.0C | Media Cybernetics | Scientific graphing and data analysis software | |
| Immunohistochemical Wet Box (black) | Sinylab | Customized | 300 mm × 100 mm × 38 mm, groove depth 27 mm, can contain 10 standard slides |
| Pipet | Thermo Scientific | ||
| Pipette tips | Well-offer | ||
| Plastic staining box | Sinylab | Customized | 30 mL, can contain 5 standard slides |
| Primary Antibody Dilution Buffer | made in our lab | 1% BSA 1g, 0.3% Triton X-100 300ul, 0.01% sodium azide 10mg in 100ml PBS | |
| Purified anti beta amyloid,1-16 antibody (6E10) | Biolegend | SIG-39320 | 500X |
| Quick Antigen Retrieval Solution for Frozen Sections | KeyGEN BioTECH | KGIHC005 | 5X |
| Quantification | |||
| Graphpad Prism 8.0.1 | Graphpad | Medical mapping software | |
| Image J Fiji 2.0.0 | National Institute of Health | Scientific graphing and data analysis software | |
参考文献
- Burns, A., Iliffe, S. Alzheimer’s disease. BMJ. 338, 158 (2009).
- Leong, Y. Q., Ng, K. Y., Chye, S. M., Ling, A. P. K., Koh, R. Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metabolic Brain Disease. 35 (1), 11-30 (2020).
- Tiwari, S., Atluri, V., Kaushik, A., Yndart, A., Nair, M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. International Journal of Nanomedicine. 14, 5541-5554 (2019).
- Murphy, M. P., LeVine, H. Alzheimer’s disease and the amyloid-beta peptide. Journal of Alzheimer’s Disease. 19 (1), 311-323 (2010).
- Hardy, J. A., Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 256 (5054), 184-185 (1992).
- Reitz, C. Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. International Journal of Alzheimer’s Disease. 2012, 369808 (2012).
- Bozyczko-Coyne, D., et al. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. Journal of Neurochemistry. 77 (3), 849-863 (2001).
- Canevari, L., Abramov, A. Y., Duchen, M. R. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochemical Research. 29 (3), 637-650 (2004).
- Zhu, X., et al. Oxidative stress signalling in Alzheimer’s disease. Brain Research. 1000 (1-2), 32-39 (2004).
- Oakley, H., et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. Journal of Neuroscience. 26 (40), 10129-10140 (2006).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiments. (65), e3564 (2012).
- An, S., et al. Medial septum glutamatergic neurons control wakefulness through a septo-hypothalamic circuit. Current Biology. , (2021).
- Cao, J. L., et al. Activation of peripheral ephrinBs/EphBs signaling induces hyperalgesia through a MAPKs-mediated mechanism in mice. Pain. 139 (3), 617-631 (2008).
- Ly, P. T., Cai, F., Song, W. Detection of neuritic plaques in Alzheimer’s disease mouse model. Journal of Visualized Experiments. (53), e2831 (2011).
- Klunk, W. E., Jacob, R. F., Mason, R. P. Quantifying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Analytical Biochemistry. 266 (1), 66-76 (1999).
- Hatami, A., Albay, R., Monjazeb, S., Milton, S., Glabe, C. Monoclonal antibodies against Abeta42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. Journal of Biological Chemistry. 289 (46), 32131-32143 (2014).
- Shi, X. Z., Wei, X., Sha, L. Z., Xu, Q. Comparison of beta-Amyloid Plaque Labeling Methods: Antibody Staining, Gallyas Silver Staining, and Thioflavin-S Staining. Chinese Medical Sciences Journal. 33 (3), 167-173 (2018).

.
