Cerebrovascular Reactivity Measurement with Functional Near Infrared Spectroscopy
概要
Presented here is a protocol for imaging and measurement of cerebrovascular reactivity in humans with functional Near Infrared Spectroscopy (fNIRS). fNIRS is a novel imaging modality that captures the concentration changes of hemoglobin species in the brain’s outermost cortex under specific stimuli.
Abstract
Cerebrovascular reactivity (CVR) is the capacity of blood vessels in the brain to alter cerebral blood flow (either with dilation or constriction) in response to chemical or physical stimuli. The amount of reactivity in the cerebral microvasculature depends on the integrity of the capacitance vasculature and is the primary function of endothelial cells. CVR is, therefore, an indicator of the microvasculature’s physiology and overall health. Imaging methods that can measure CVR are available but can be costly, and require magnetic resonance imaging centers and technical expertise. In this study, we used fNIRS technology to monitor changes of oxyhemoglobin (HbO) and deoxyhemoglobin (HbR) in the cerebral microvasculature to assess the CVR of 15 healthy controls (HC) in response to a vasoactive stimulus (inhaled 5% carbon dioxide or CO2). Our results suggest that this is a promising imaging technology that offers a non-invasive, accurate, portable, and cost-effective method of mapping cortical CVR and associated microvasculature function, resulting from a traumatic brain injury or other conditions associated with cerebral microvasculopathy.
Introduction
Vascular health in the cerebral cortex can be measured via the vessels’ ability to constrict or dilate under varying physiological conditions. Measuring vascular reactivity can be useful in the diagnosis and management of neurological conditions associated with cerebral microvascular dysfunction, like dementia, traumatic brain injury (TBI) and even aging1,2,3,4. Additionally, CVR can be used as a predictive and/or pharmacodynamic biomarker for neurological disorders such as Alzheimer's5 or TBI6,7,8,9,10. Well-established imaging methods exist to study CVR in human and animal subjects. A typical method includes functional magnetic resonance imaging (fMRI) in conjunction with an exogenous or endogenous stimulus, such as hypercapnia11, breath holding, or acetazolamide2. Lu et al.12,13 demonstrated that a simple gas delivery system coupled with MRI- Blood Oxygen Level Dependent (MRI-BOLD) imaging generates an accurate whole brain CVR maps.
Disruptions to the cerebral vasculature’s blood flow, volume, and metabolic rate of oxygen produces changes in the tissue concentrations of HbO and HbR. Tissue absorption of light at the near infrared range is sensitive to changes in the concentration of hemoglobin species, such as HbO and HbR. Therefore, measuring backscattered light over time can quantify changes of HbO and HbR concentration in the outermost cortex (approximately 2 cm)15, and can be used to assess temporal hemodynamic variations16 including cerebrovascular reactivity (CVR)17.
In our research paradigm, we employ the fNIRS instrument with continuous wave function. The device is composed of 4 sources and 10 detectors, which create 16 source-detector pairs (see Figure 1). The source-detector pairs are molded together onto a silicone strap that can easily be set over the forehead and held in place with self-adherent wrap. The device measures light intensity at 730 and 850 nm and has an acquisition frequency of 2 Hz. This system was selected because it is patient-friendly, easy to wear, and collects data from the prefrontal cortex, a brain region particularly vulnerable to TBI. Fortunately, most other fNIRS systems are compatible with our CVR acquisition technique, differing only in the cortical regions measured based on the brain area of interest.
While fMRI is considered the gold standard for functional brain imaging, fNIRS technology has unique advantages for assessing CVR in comparison to fMRI. The fNIRS imaging technique provides a high temporal resolution (with a granularity of milliseconds) and can quantify changes in both HbO and HbR concentration, while fMRI only measures changes in HbR18,19,20. Moreover, fNIRS instruments are portable, economical, and easier to operate than fMRI. Finally, fNIRS technology better resolves subject motion, which is necessary given that vascular challenges like hypercapnia are often used in combination with cognitive or physical study tasks21.
In this paper, a hypercapnia challenge, combined with fNIRS technology is presented. We measured CVR values and studied the reproducibility of this method, hoping to offer a reliable alternative to fMRI CVR measures.
Protocol
The participants were recruited under an institutional review board approved protocol (ClinicalTrials.gov NCT01789164). The equipment described in the protocol is ethically approved by our institution.
1. Prepare the Materials used for the Hypercapnia Challenge (Figure 2)
- Inflate a 200 L Douglas bag (Item #1) with a pre-mixed canister of medical-grade gas which is comprised of 5% carbon dioxide, 21% oxygen, and 74% nitrogen until full.
- Insert two diaphragms (Item #3) into the two-way non-rebreathing valve (Item #4) to safeguard that the gas will only flow in one direction. Attach one port of the three-way valve (Item #2) to the Douglas bag (Item #1) via the gas delivery tube (Item #5), and the other port to the two-way non-rebreathing valve (Item #4) via a second gas delivery tube (Item #5).
- Fasten the mouthpiece (Item #6) to the connector (Item #7) and then fasten the connector to the two-way non-rebreathing valve (Item #4).
- Insert the capnograph tubing (Item #8) into the hole in the connector (Item #7).
- Attach the air-filter (Item #9) to the capnograph tubing (Item #8).
- Screw the end of the plastic air-filter (Item #9) that isn’t connected to the capnograph tubing (Item #8) into the CO2 (Item #10) monitor.
- Connect the capnograh (Item #10) to a laptop with a cable. Open the data port reader software, select the corresponding USB port and start the data reading. Turn on the capnograph. Data will automatically be displayed on the computer screen.
- Connect the fNIRS box to the computer with a USB cable. Connect the source-detector headband to the FNIRS box. Connect the power adapter to the fNIRS box and turn on the switch.
2. Procedures during the experiment
- Ask the participant to sit on a chair and to make themselves comfortable while setting up the devices. Turn the fNIRS system on.
- Place the source-detector headband on the patient’s forehead, over the underlying prefrontal cortex areas (dorsal and inferior frontal cortical areas)21.
- Check that the source-detector headband is carefully positioned above the eyebrow and in the middle of the forehead. Place the lower detector row approximately 3.5 cm above the nasion or bridge of the nose where the indentation of the upper nose meets the forehead between the eyes.
- Make sure the detectors are firmly adhered to the participant’s skin without chromophores (e.g., make-up) or hair interfering. No skin preparation is needed.
- Under “Device Setting”, set the gain for detectors between 1 and 20. A higher gain will increase the sensitivity of the light detectors. The default value is 20. Set the “LED Current” between 5 mA and 20 mA. Larger values will result in brighter light and will increase the signal level generated by the detectors. The default value is 20 mA.
- In the acquisition software, press “Start Current Experiment”. Sources will send light at 2 wavelengths and light signal intensity detected from each detector will be displayed in real time. In case of saturated (signal>4,000) or low signal (signal <1,000), adjust the contact between the source-detector headband and the skin or the parameters in step 2.3 and 2.4. The exact full procedure has been explained in Ayaz et al.22.
- Direct the participant to inhale and exhale through their mouth at their normal breathing pace. Fasten a nose clip on the participant’s nose and remind them to continue breathing normally through their mouth, and to alert someone if they feel any discomfort or have any difficulty breathing.
- Carefully insert the mouthpiece (item#6) into the participant’s mouth so that they can continue to breathe. For increased participant comfort during the procedure, ask the participant to support the non-rebreathing valve (Item #3) with their hand.
- Press the “Baseline” button in associated software. It will measure and automatically record the light signal for the fNIRS baseline for 20 seconds (s).
- Press “Record” before starting the experiment.
- At the beginning of the experiment, start the clock, press “Manual Marker” and write on a paper the time displayed by the capnograph. Every minute turn the valve connected to the gas tubing to cycle between room air and room air mixed with 5% CO2. Again, press “Manual Marker” and write on a paper the time displayed by the capnograph each time the inhaled gas mixture is changed (Figure 3).
NOTE: Manually marking the time displayed on the capnograph is essential for future synchronization between fNIRS optical signals and capnograph’s EtCO2 trace. - After 7 min, stop the fNIRS recording by clicking the “Stop” button. Allow 60 additional seconds of recording for the end-tidal CO2 (EtCO2) and save the EtCO2 data as ASCII within the data reader software.
- Notify the participant that the procedure is completed. Carefully take off the nose clip and withdraw the mouthpiece. Offer a tissue to the participant to absorb any accumulated saliva from the procedure.
3. Clean up Procedures
- Dispose of the capnograph tubing (Item #8), filter (Item #9), mouthpiece (Item #6) and nose clip.
- Cleanse the reusable equipment. Detach the two-way valve (Item #4) from the gas-delivery tube (Item #5) and the connector tube (Item #7) and extract the diaphragms (Item #3). Immerse the diaphragms (Item #3), connector tube, and two-way valve (Item #4) in a container full of a medical-grade detergent disinfectant that is phosphate-free and contains surfactants for 20 min. Dilute the detergent with distilled water in a ratio of 1:64.
- Wash items #1,4,7 with distilled water then place them on top of a clean counter with a sterile material such as a chux pad underneath. Allow them to air-dry before re-use.
- Empty the Douglas bag.
4. Data analysis
- Signal processing using fNIRS data processing software
NOTE: Signal processing is the first step of the data analysis. It is done using an fNIRS data processing software (e.g., fNIRSoft) in order to remove noise or artefact in the data due to patient movement. Only data from the acquisition software are needed for this analysis.- In data processing software, click on “Load File” to select and then upload the acquired fNIRS data.
- Click on “Refine” and a pop-up window will appear. Select “Raw Data” and press “Next”.
- Click on both the median filtering and the sliding window motion artifact rejection (SMAR)23 tools to recognize and delete both motion artifact and saturated channels from the raw signal. Press “Apply”.
- Click on “Low Pass Frequency” filter to discard pulse and breathing component (Hanning filter, n=20, cutoff=0.1Hz)21,24,25,26. Press “Apply”.
- Click on “Detrend” to eliminate the slow temporal variation. Press “Apply”.
- Click on “OXI” to transform the light intensity into HbO and HbR concentrations. Click on “Save” and then select MATLAB as the save file format.
- Signal processing with MATLAB
NOTE: The second part of the analysis is done using MATLAB in order to correlate the fNIRS signal with the time shifted EtCO2. Data from the previous step (4.1.5) and data from the capnograph (EtCO2 trace, step 2.12) are needed for processing the data.- Import the EtCO2 trace from the capnograph in MATLAB as two columns (one for time and the second for EtCO2 values). Shift the EtCO2 time with pre-calibrated time to correct the delay from the sampling tubing time.
NOTE: This is the time difference between one breath to the mouthpiece and the appearance of that breath on the CO2 recording. In this set-up, it was 15 s. - Use the first time point recorded from the capnograph at the beginning of the experiment, step 2.11 as the starting point (t=0). Convert the EtCO2 into seconds.
- Import the oxy and deoxyhemoglobin data from step 4.1.3 into MATLAB.
- Calculate the physiological delay between EtCO2 (measured in the mouth) and the fNIRS signal (measured in the brain) by finding the higher correlation coefficient between these two signals at varying time shifts. (see MATLAB script attached for step 4.2.3 to step 4.2.6). The time shift with the higher correlation coefficient is considered the optimal time.
- Shift the EtCO2 time course by the optimal time (obtained in step 4.2.4). Keep the time points that have both fNIRS and EtCO2 data. The two-time series should have the same length.
- Calculate CVR values for each channel, which is the solution of the linear equation between HbO (or HbR) and EtCO2 using the Cholesky decomposition in MATLAB.
- Import the EtCO2 trace from the capnograph in MATLAB as two columns (one for time and the second for EtCO2 values). Shift the EtCO2 time with pre-calibrated time to correct the delay from the sampling tubing time.
Representative Results
fNIRS was performed with hypercapnia challenge on 15 healthy participants. Exclusion criteria were history of TBI, pre-existing disabling neurological or psychiatric disorders or pregnancy. The participants had a mean age of 37.7 ± 16 years (range 20-55) and 20% were female. As shown in a similar fMRI study28, a 60 s inhalation of 5% CO2 was accompanied by an increase in EtCO2 pressure as measured by capnography. In our study, the EtCO2 trace was accompanied by an increase of HbO and a decrease of HbR (Figure 4).
Physiologically, HbO and HbR are out of phase14. In Figure 4, which represents the fNIRS signal of one participant, we observed that the HbR signal precedes the HbO signal by 3.5 s (a precise measurement can be derived from the time shift for each signal). On an average, across all participants, it was observed that the HbO signal increases 2.3+ 2.6 s after the HbR signal decreases. This implied that the time shift for HbO and HbR were different and needed to be estimated before calculating a participant’s CVR. For this same reason, we also needed to estimate the time shift between the EtCO2 tracing and Hb-diff (the difference between HbO and HbR signals). The Hb-diff parameter gave us the strongest amplitude between the two conditions.
On an average, in our HC group, the increase of HbO appeared 2.3 + 2.6 s before the HbR decrease was noted. Because of this delay between HbO and HbR, the time shift between the EtCO2 tracing and HbO signal was not the same as the time shift between the EtCO2 tracing and HbR signal. In addition, also calculated was the time shift between the EtCO2 tracing and Hb-diff (difference between HbO and HbR signal). The Hb-diff parameter gave us the strongest amplitude between the two conditions.
After shifting the EtCO2 trace for HbO, HbR, and Hb-diff, we measured the Pearson correlation between the shifted EtCO2 traces and HbO, HbR, and Hb-diff. EtCO2 trace highly correlated with fNIRS signals (Pearson’s correlations of 0.94, -0.98 and 0.91 for HbO, HbR and Hb-diff, respectively; p<0.0001). (Figure 5).
We explored the CVR inter- and intra- variability between all 15 participants and all source/detector pairs. Averaging the CVR between the source/detector pair for each participant, we assessed the CVR from HbO, HbR, and Hb-diff (difference between HBO and HbR). On average between all participants, CVR values were 13.1 + 4.7 μM/mmHg using HbO, -14.6 + 10.2 μM/mmHg using HbR, and 12.4 + 3.7 μM/mmHg using Hb-diff (Table 1). Variability between the channels within each participant was also analyzed. On average, the intra-variability of CVR assessed with Hb-diff was lowest (30%), appeared to be the best parameter to investigate CVR using fNIRS.
Finally, we can overlay the CVR values on an anatomical template or directly on the structural image of the patient’s brain, as available for better visualization.27
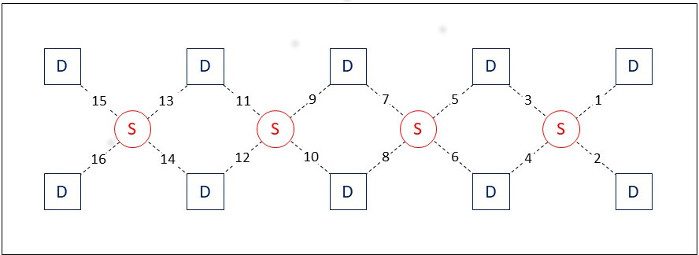
Figure 1: Optical sensor pad schematic. It is composed of 4 sources (large red circles), and 10 detectors (small red dots), which form 16 source/detector pairs having 2.5 cm separation. The sensor pad is positioned on the volunteer’s forehead. The numbers indicate the position of the 16 source/detector pairs on the sensor. Please click here to view a larger version of this figure.
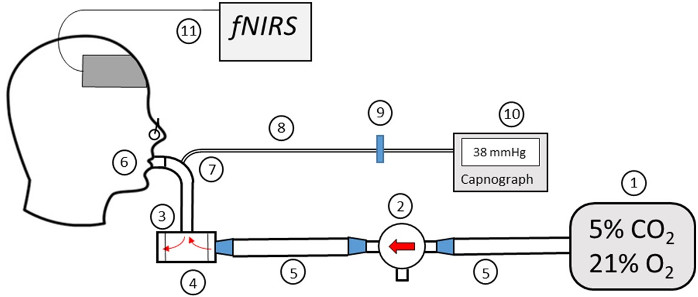
Figure 2: Diagram of the Gas Delivery System. (1) Douglas bag. (2) Three-way valve. (3) Diaphragms. (4) Two-way non-rebreathing valve. (5) Gas delivery tube. (6) Mouthpiece. (7) Connector. (8) Gas sampling tube. (9) Hydrophobic filter. (10) EtCO2 monitor. (11) fNIRS system. Please click here to view a larger version of this figure.
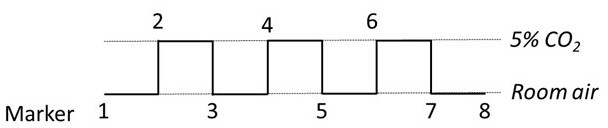
Figure 3: Timing and marker of the breathing paradigm. Every minute, the three-way valve switched between the two gases. A marker signal was sent to the fNIRS software to sort each period with the appropriate gas inhalation. Please click here to view a larger version of this figure.
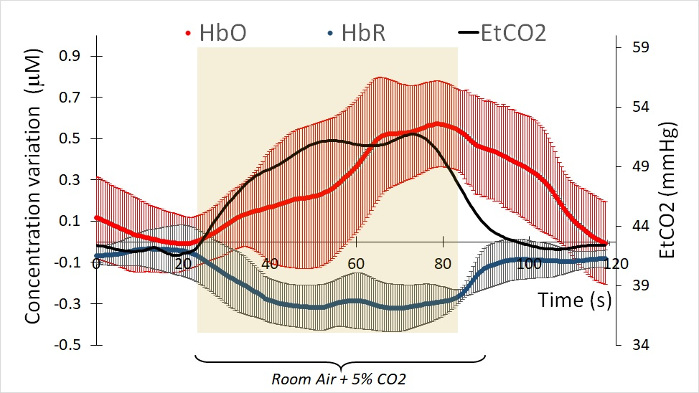
Figure 4: Example of HbO and HbR concentration measures under a 5% CO2 challenge of one participant. Each fNIRS curve is the average of 16 channels. The red curve represents the variation of HbO during 60s of room air and 60s under 5% of CO2. The blue curve represents the variation of HbR under the same conditions. The curves were time shifted in order to match the EtCO2 (black curve). Each HbO and HbR curves are the average of 3 challenges. Please click here to view a larger version of this figure.
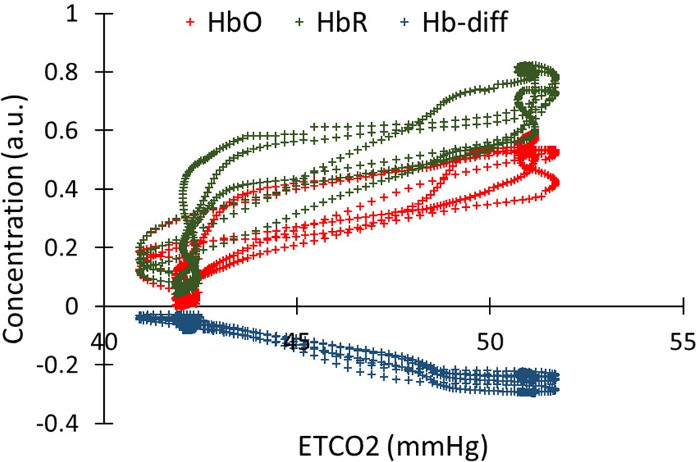
Figure 5: Correlation between the EtCO2 and the HbO, HbR, oxygenation, in one channel for one participant. HbO, HbR and oxygenation were time shifted in order to temporally match the EtCO2 trace. Pearson’s correlations is 0.94, -0.98 and 0.91 for HbO, HbR and Hb-diff, respectively (p<0.0001 ) Please click here to view a larger version of this figure.
| Mean CVR between patient | Variability between channels | |
| HbO | 13.1 +/- 4.7 | 41% |
| HbR | -14.6 +/- 10.2 | 85% |
| Hb-diff (HbO-HbR) | 12.4 +/- 3.7 | 30% |
Table 1: Inter-subject and inter-channel variability of CVR values for 15 HC. CVR variability was calculated with 3 physiological signals: HbO, HbR and oxygenation (HbO-HbR).
Discussion
We were able to measure CVR using fNIRS and a CO2 gas inhalation technique in 15 healthy volunteers. The CVR value measured is the correlation between the acquired fNIRS signal and the EtCO2. The challenge is to accurately align the temporal EtCO2 trace with the fNIRS signal, in other words, to account for the time that it takes for blood to travel from the pulmonary vascular system to the heart and then to the cerebral vasculature. The inter-channel variability is low (30%) and shows a uniform CVR value in the cortex which correlates with previous fMRI results12. Our data show the inter-participant variability in CVR among HC is lowest for the oxygenation signal but increases significantly when using HbO or HbR. For future group difference studies with HC, we recommend using CVR values with the least variability, i.e., the CVR values from the oxygenation signal.
The critical step of the technique is the same as any fNIRS experiment: the placement of the sources and light detectors on the head. A loose connection can lead to a displacement of the sensors during the experiment and artefacts in the signal. Too much artefact will corrupt the signal and disrupt the analysis.
CVR is most commonly measured in humans by 2 different techniques: Doppler ultrasound and fMRI. This third method, fNIRS, give us a precise and accurate measure of CVR under gas inhalation. Like ultrasound, it gives excellent temporal resolution, but in addition, it also provides a 2D map of CVR (cortical values). While these images are low in resolution compared to CVR measured with fMRI, fNIRS provides both measures inexpensively and non-invasively, and can be easily carried out in the outpatient clinical setting. In addition, fNIRS can measure the two components of hemoglobin (HbO and HbR), which has potential benefits for vascular research. Depending on the application, this method of measuring CVR via fNIRS can be carried out over a single area of interest or over a multi-band array for a 2D map of the brain. CVR using hypercapnia measures changes of blood flow across the brain with fNIRS, as opposed to a cognitive task which would only allow for detection of changes in a specific region of interest.
Because this procedure can be performed safely, inexpensively, and without side effects in a clinic setting, this method is well suited for both research and clinical use, especially for neurovascular diseases like vascular dementia or traumatic brain injury (TBI). In Alzheimer’s disease, early evidence of CVR deficits is detectable28. In the same way, traumatic cerebrovascular injury is one endophenotype of TBI29 in which endothelial cells are injured and lead to cerebrovascular pathology. Clinical trials targeting vascular injury can assess CVR via fMRI or fNIRS as a pharmacodynamic biomarker to provide information on proof of mechanism.
開示
The authors have nothing to disclose.
Acknowledgements
Work in the authors’ laboratory was supported by the Center for Neuroscience and Regenerative Medicine (CNRM), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, by the Military Clinical Neuroscience Center of Excellence (MCNCoE), Department of Neurology, USUHS, and by the Intramural Research Program of the National Institutes of Health. The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Materials
| Blue cuff | 22254 | Vacumed | |
| CO2-Air Gas Mixture Size 200 | R012000 2003 | Roberts Oxygen | |
| Diaphragm (Size: medium, Type: spiral) | 602021-2608 | Hans Rudolph | |
| Douglas bag (200-liters capacity) | 500942 | Harvard Apparatus | |
| Gas delivery Tube | 1011-108 | Vacumed | |
| Gas sampling Tube | T4305 | QoSINA | |
| Hydrophobic filter | 9906-00 | Philips Medical Systems | |
| Male luer | 11547 | QoSINA | |
| Mouth piece (Silicone, Model #9061) | 602076 | Hans Rudolph | |
| Nose clip (Plastic foam, Model #9014) | 201413 | Hans Rudolph | |
| Three-way valve (100% plastic) | CR1207 | Hans Rudolph | |
| Two-way non-breathing valve (22mm/ 15mm ID) | CR1480 | Hans Rudolph |
参考文献
- Amyot, F., et al. Imaging of Cerebrovascular Function in Chronic Traumatic Brain Injury. Journal of Neurotrauma. 35 (10), 1116-1123 (2017).
- Kassner, A., Roberts, T. P. Beyond perfusion: cerebral vascular reactivity and assessment of microvascular permeability. Topics in Magnetic Resonance Imaging. 15, 58-65 (2004).
- Oertel, M., et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. Journal of Neurosurgery. 103, 812-824 (2005).
- Peng, S. L., et al. Age-related changes in cerebrovascular reactivity and their relationship to cognition: A four-year longitudinal study. Neuroimage. 174, 257-262 (2018).
- Yezhuvath, U. S., et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiology of Aging. 33, 75-82 (2012).
- Baranova, A. I., et al. Cerebral vascular responsiveness after experimental traumatic brain injury: the beneficial effects of delayed hypothermia combined with superoxide dismutase administration. Journal of Neurosurgery. 109, 502-509 (2008).
- Gao, G., Oda, Y., Wei, E. P., Povlishock, J. T. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. Journal of Cerebral Blood Flow and Metabolism. 30, 628-637 (2010).
- Wei, E. P., Hamm, R. J., Baranova, A. I., Povlishock, J. T. The long-term microvascular and behavioral consequences of experimental traumatic brain injury after hypothermic intervention. Journal of Neurotrauma. 26, 527-537 (2009).
- Oda, Y., Gao, G., Wei, E. P., Povlishock, J. T., et al. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. Journal of Cerebral Blood Flow and Metabolism. 31, 1143-1154 (2011).
- Park, E., Bell, J. D., Siddiq, I. P., Baker, A. J. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism. 29, 575-584 (2009).
- Ellis, M. J., et al. Neuroimaging Assessment of Cerebrovascular Reactivity in Concussion: Current Concepts, Methodological Considerations, and Review of the Literature. Frontiers in Neurology. 7, 61 (2016).
- Lu, H., et al. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. Journal of Visualized Experiments. (94), e52306 (2014).
- Yezhuvath, U. S., Lewis-Amezcua, K., Varghese, R., Xiao, G., Lu, H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR in Biomedicine. 22, 779-786 (2009).
- Ferrari, M., Mottola, L., Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Canadian Journal of Applied Physiology. 29, 463-487 (2004).
- Firbank, M., Okada, E., Delpy, D. T. A theoretical study of the signal contribution of regions of the adult head to near-infrared spectroscopy studies of visual evoked responses. Neuroimage. 8, 69-78 (1998).
- Boas, D. A., Chen, K., Grebert, D., Franceschini, M. A. Improving the diffuse optical imaging spatial resolution of the cerebral hemodynamic response to brain activation in humans. Optics Letters. 29, 1506-1508 (2004).
- Kainerstorfer, J. M., Sassaroli, A., Hallacoglu, B., Pierro, M. L., Fantini, S. Practical steps for applying a new dynamic model to near-infrared spectroscopy measurements of hemodynamic oscillations and transient changes: implications for cerebrovascular and functional brain studies. Acadamic Radiology. 21, 185-196 (2014).
- Cui, X., Bray, S., Bryant, D. M., Glover, G. H., Reiss, A. L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 54, 2808-2821 (2011).
- Huppert, T. J., Hoge, R. D., Diamond, S. G., Franceschini, M. A., Boas, D. A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 29, 368-382 (2006).
- Sassaroli, A., de, B. F. B., Tong, Y., Renshaw, P. F., Fantini, S. Spatially weighted BOLD signal for comparison of functional magnetic resonance imaging and near-infrared imaging of the brain. Neuroimage. 33, 505-514 (2006).
- Ayaz, H., et al. Optical brain monitoring for operator training and mental workload assessment. Neuroimage. 59, 36-47 (2012).
- Ayaz, H., et al. Using MazeSuite and Functional Near Infrared Spectroscopy to Study Learning in Spatial Navigation. Journal of Visualized Experiment. (56), e3443 (2011).
- Ayaz, H., Izzetoglu, M., Shewokis, P. A., Onaral, B. Sliding-window Motion Artifact Rejection for Functional Near-Infrared Spectroscopy. Annual International Conference of IEEE Engineering in Medicine and Biology Society. , 6567-6570 (2010).
- Naseer, N. H. K. Classification of functional near-infrared spectroscopy signals corresponding to the right- and left-wrist motor imagery for development of a brain-computer interface. Neuroscience Letters. 553, 84-89 (2013).
- Kreplin, U., Fairclough, S. H. Activation of the rostromedial prefrontal cortex during the experience of positive emotion in the context of esthetic experience. An fNIRS study. Frontiers in Human Neuroscience. 7, (2013).
- Izzetoglu, M. B. S., Izzetoglu, K., Onaral, B., Pourrezaei, K. Functional brain imaging using near-infrared technology. IEEE Engineering in Medicine and Biology Magazine. 26, 38-46 (2007).
- Amyot, F., et al. Normative database of judgment of complexity task with functional near infrared spectroscopy–application for TBI. Neuroimage. 60, 879-883 (2012).
- Yezhuvath, U. S., et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiol Aging. 33 (1), 75-82 (2012).
- Kenney, K., et al. Phosphodiesterase-5 inhibition potentiates cerebrovascular reactivity in chronic traumatic brain injury. Annals Clinical Translation Neurology. 5 (4), 418-428 (2018).

.
