Measurement of Mitochondrial Respiration in Human and Mouse Skeletal Muscle Fibers by High-Resolution Respirometry
Summary
Here, we describe a comprehensive method for measuring mitochondrial oxidative phosphorylation in fresh permeabilized skeletal muscle fibers from either human or mouse muscle. This method allows for the real-time quantification of mitochondrial respiration and the assessment of fuel preference and metabolic flexibility while preserving existing mitochondrial networks and membrane integrity.
Abstract
Mitochondrial function, a cornerstone of cellular energy production, is critical for maintaining metabolic homeostasis. Its dysfunction in skeletal muscle is linked to prevalent metabolic disorders (e.g., diabetes and obesity), muscular dystrophies, and sarcopenia. While there are many techniques to evaluate mitochondrial content and morphology, the hallmark method to assess mitochondrial function is the measurement of mitochondrial oxidative phosphorylation (OXPHOS) by respirometry. Quantification of mitochondrial OXPHOS provides insight into the efficiency of mitochondrial oxidative energy production and cellular bioenergetics. A high-resolution respirometer provides highly sensitive, robust measurements of mitochondrial OXPHOS in permeabilized muscle fibers by measuring real-time changes in mitochondrial oxygen consumption rate. The use of permeabilized muscle fibers, as opposed to isolated mitochondria, preserves mitochondrial networks, maintains mitochondrial membrane integrity, and ultimately allows for more physiologically relevant measurements. This system also allows for the measurement of fuel preference and metabolic flexibility – dynamic aspects of muscle energy metabolism. Here, we provide a comprehensive guide for mitochondrial OXPHOS measurements in human and mouse skeletal muscle fibers using a high-resolution respirometer. Skeletal muscle groups are composed of different fiber types that vary in their mitochondrial fuel preference and bioenergetics. Using a high-resolution respirometer, we describe methods for evaluating both aerobic glycolytic and fatty acid substrates to assess fuel preference and metabolic flexibility in a fiber-type-dependent manner. The protocol is versatile and applicable to both human and rodent muscle fibers. The goal is to enhance the reproducibility and accuracy of mitochondrial function assessments, which will improve our understanding of an organelle important to muscle health.
Introduction
Mitochondria are the cornerstone of cellular energy production, making them indispensable for maintaining optimal cellular and organismal homeostasis. These double-membraned organelles are primarily responsible for oxidative phosphorylation. This process efficiently converts nutrients, such as sugars and fatty acids, into adenosine triphosphate (ATP), the cellular currency for energy. Beyond their role in energy metabolism, mitochondria are also key regulators of various cellular processes, including apoptosis, calcium homeostasis, and reactive oxygen species (ROS)1,2. Because of their pivotal role in maintaining cellular and organismal homeostasis, disruptions in mitochondrial function often have detrimental effects on tissue and organismal health. In skeletal muscle, mitochondrial dysfunction is associated with numerous disease states, including metabolic disorders (e.g., obesity, diabetes, and cardiovascular disease), sarcopenia, and muscular dystrophy3,4,5,6,7,8.
Mitochondrial dysfunction can primarily manifest as altered mitochondrial content, number, and morphology, as well as disrupted metabolism. Thus, achieving a comprehensive understanding of mitochondrial dysfunction requires an integrative and holistic approach. Initial characterization studies involve examining expression levels of respiratory chain protein complexes as a readout of mitochondrial content, quantifying mitochondrial DNA and markers of biogenesis as a measure of mitochondrial biogenesis, and sophisticated electron microscopy imaging to assess mitochondrial morphology9,10. Additional assessments of mitochondrial function include evaluating cellular ROS and ATP production and mitochondrial membrane potential9.
Because mitochondria are essential for cellular energy production and homeostasis, a hallmark for assessing mitochondrial function is to measure mitochondrial oxidative phosphorylation (OXPHOS). High-resolution respirometry of permeabilized muscle fibers allows for the measurement of real-time changes in mitochondrial oxygen consumption rate as a readout for the dynamic changes in mitochondrial OXPHOS respiratory chain activity9,11,12. The application of selective chemical modulators and inhibitors allows one to measure the activity of different respiratory complexes directly and sequentially. Although isolated mitochondria may be used in respirometry, the use of fresh, permeabilized muscle fibers maintains endogenous mitochondrial networks and membrane integrity – thus allowing for more physiologically relevant measurements. Additionally, because different muscle fiber types have different substrate preferences and rates of respiration, this system allows one to measure changes in fuel preference and metabolic flexibility based on fiber type13.
Here, we describe a comprehensive protocol for mitochondrial OXPHOS measurements using either human or mouse skeletal muscle fibers in a high-resolution respirometer system. Included are methods to quantify mitochondrial oxygen respiration in oxidative or glycolytic fibers using either pyruvate or palmitoyl-carnitine as a substrate. This protocol allows for the use of other fuel substrates to address specific metabolic questions pertaining to defects in substrate utilization and fuel preference.
Protocol
All mouse procedures were approved by the Institutional Animal Care and Use Committee of Washington University. Mice of any sex, age, and weight may be used for these experiments and will be dependent on the nature of the experimental question one seeks to address. Mice used here are adult (12-16 weeks old), male wild-type C57BL/6 mice. All human procedures were approved by the Institutional Review Board of Washington University. Study subjects consented to data usage, and representative human subject data included within this protocol are from a published study14. Data here is from non-diabetic post-menopausal (55-75 years old) females. Details for preparing reagents necessary for the assay are presented in Table 1. Information on the specific reagents, tools, and machines used in the assay are listed in the Table of Materials. A schematic overview of the protocol is presented in Figure 1.
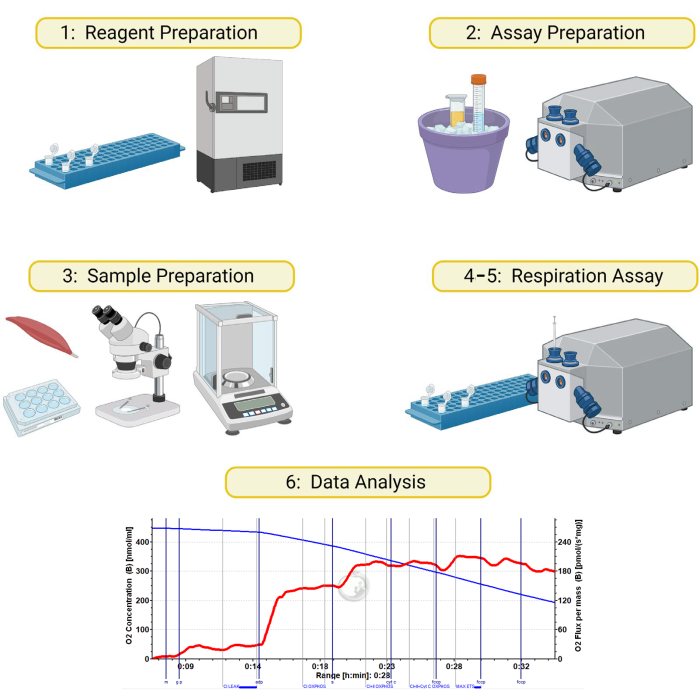
Figure 1: Schematic for high-resolution respirometry on permeabilized skeletal muscle samples. The method detailed in this manuscript is divided into 6 sections: 1) preparation of respiration buffers and reagents, 2) instrument and reagent preparation on day of assay, 3) preparation and permeabilization of muscle samples, 4) preparing sample and instrument, 5) running the respiration assay, and 6) data analysis. Created with BioRender.com Please click here to view a larger version of this figure.
1. Assay preparation and instrument calibration
- On the day of the assay, remove the needed number of aliquots of each respiration compound (blebbistatin, palmitoyl-carnitine, glutamate, malate, ADP, succinate, cytochrome C, FCCP) and thaw on ice. Thaw vials of BIOPS and MiR05 on ice.
- Prepare saponin and pyruvate solutions as indicated in Table 1. Prepare a working solution of MiR05 as in Table 1. Prepare 5 mL of working solution of MiR05 per instrument (2 chambers). Scale up as needed.
- Turn on the high-resolution respirometry system and the vacuum system. Remove the stoppers, remove the 70% ethanol storage solution by vacuum, and refill the chamber with ultrapure molecular grade H2O. Remove H2O by vacuum and refill. Repeat for a total of 3 washes. After the final wash, add 2 mL of MiR05 (without creatine or blebbistatin) to each chamber.
- Open the respirometry software. After the software initiates, a pop-up box will open for instrument settings. Set chamber stir speed to 750 rpm, temperature to 37 °C, and data recording interval to 2 s. Set gain to 1 and polarization voltage to 800 mV for the oxygen sensors. Click Connect to Oxygraph to establish communication with the instrument.
- After communication is established, a dialog box will open to name and save the experimental file. Save the file with the current date and calibration. After saving the file, a pop-up dialog box will appear for experimental details. No information is needed for the calibration run, and the box can be closed.
- Record oxygen concentration for at least 30 min to allow chambers to warm up and to record signals for air calibration. This can be done while preparing muscle fibers, as detailed in step 2 below.
- At the end of the calibration period, mark a calibration region across the region where the oxygen concentration (blue line) is stable. To do this, hold down the Shift key and the Left Mouse button and drag across a region on the timeline.
- Open the calibration window by going to the Oxygraph > O2 Calibration. For Air Calibration, select the mark generated in step 1.8. Click Calibrate and Copy to Clipboard.
- Repeat steps 1.6-1.8 for the remaining chamber. Perform air calibration daily. Stop the air calibration recording and save the file by disconnecting from the instrument. After the samples are ready, proceed to step 3 to conduct the assay.
2. Harvesting and permeabilization of skeletal muscle fibers
- Mouse tissue isolation
- For each sample to be analyzed, fill one well of a 12-well culture dish with 1 mL of BIOPS. Place plate on ice to chill.
- After euthanizing the mouse by carbon dioxide inhalation followed by cervical dislocation, harvest the skeletal muscle of interest, being sure to remove all connective tissue and fat (Figure 2A). Place the muscle in one of the wells containing BIOPS. Place on ice until fiber preparation.
NOTE: Any skeletal muscle can be examined using this protocol – the muscle type examined will be dependent on the experimental question one seeks to address. In the case of skeletal muscles with mixed fiber types, such as the gastrocnemius, it is possible to separate the tissue into white and red sections to measure predominantly fast twitch (white sections) and predominantly slow twitch (red sections) fibers (Figure 2B).
- Human tissue isolation
- Collect tissue following clinical protocol14. Quickly rinse the tissue with cold, sterile PBS and place it in a conical tube containing 2 mL of BIOPS solution. Keep tissue on ice until fiber preparation.
- Fiber preparation
- Permeabilization will take place in a 6-well tissue culture plate. Add 2 mL of BIOPS solution to one set of wells and 2 mL of MiR05 to another set of wells. One set of wells will be required for each sample being assayed.
- Pack a tray with ice and place a glass Petri dish upside down on the ice. Be sure to always keep the muscle on ice.
- Transfer harvested muscle samples to the chilled Petri dish platform using forceps. Using two fine-tipped forceps and a dissecting microscope with a light source, gently clean the tissue by removing any tendons, fascia, and adipose tissue that are attached to the muscle.
- After all extraneous non-muscle tissue has been removed, gently pull the muscle fibers apart with the fine-tipped forceps until they are small bundles of translucent fibers ranging from 0.75 – 1.0 mm in length (Figure 2C-D). After separating the fibers, use the forceps to transfer the fiber bundles to a well on the 6-well plate containing BIOPS solution on ice.
- Add 20 µL of 5 mg/mL saponin solution to each BIOPS well and incubate the plate on ice while gently rocking in a cold room for 20 min.
- After saponin treatment, transfer muscle fibers to the well containing MiR05 to rinse before the assay. Incubate on ice while gently rocking in a cold room for 15 min.
- After the fiber incubation in MiR05 is complete, gather the fibers with sharp forceps and gently blot the muscle fibers dry on a task wipe. Tare a 1.5 mL plastic microcentrifuge tube on a fine balance and place 2-3 mg of fibers in the tube. Record the final fiber weight on the side of each tube. Place the tube on ice. Repeat with other samples. Immediately proceed to step 3.
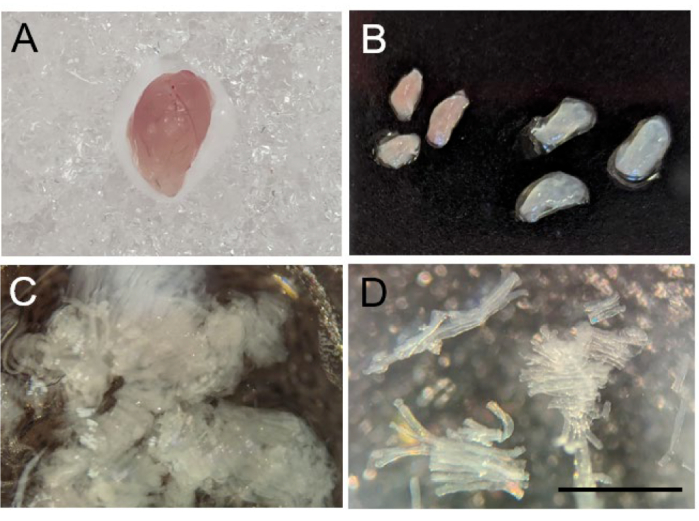
Figure 2: Separation of mouse skeletal muscle fibers. (A) Gross morphology of mouse gastrocnemius after harvest. (B) Dissection of gastrocnemius into red (left) and white (right) segments. (C) Mechanically separated muscle fibers. (D) A 10x image of successfully separated muscle fibers. Scale bar is 1 mm. Please click here to view a larger version of this figure.
3. Preparing muscle samples in the respirometer
- Vacuum out the MiR05 used for instrument calibration as detailed in step 1 above and add 2.1 mL of working MiR05 solution to each chamber.
- Initiate a new file for the experiment using the same instrument settings as detailed in step 1.5. above. Set the file name and save settings.
- Following setting the file name, the next screen dialog box will be for specific experimental details. Enter the sample information and the weight of each sample added to each chamber. Close the dialog box.
- Once in the new experimental file, set the calibration by opening the O2 Calibration window. Click Copy from file and select the file saved from step 1.9. above. Click the Calibrate and Copy to Clipboard button. Repeat the process for the remaining chamber.
- Using fine forceps, carefully transfer muscle fiber bundles into the respiration solution for the appropriate chamber. Repeat for the remaining chamber.
- Place the stoppers on the chamber and carefully semi-close the chamber by pushing the stoppers about halfway to the bottom. Once the o-rings on the stoppers engage with the chamber wall and there is resistance, use a twisting motion while pushing down to close. When the chamber is halfway closed, a small air bubble can be observed at the top of the chamber.
NOTE: Permeabilized fibers are subject to oxygen diffusion limitations under regular respiratory conditions. To circumvent this limitation, the assay is conducted under elevated oxygen concentrations11. - Fill a 10 mL plastic syringe with pure oxygen from an oxygen tank. Place the long, blunt needle supplied with the instrument on the syringe. Place the needle in the first chamber and slowly deliver approximately 1 mL of oxygen into the chamber.
- Monitor the chamber oxygen concentration in the respirometry software. When the chamber oxygen concentration reaches 350-400 nmol/mL, gently twist the stopper while pushing down to fully close the chamber. Look into the chamber and ensure that no air bubbles remain. If an air bubble is present, carefully and quickly reopen the chamber, add 100 µL of additional working MiR05 solution, and quickly close the chamber again. Repeat with the remaining chambers.
- Oxygen concentrations should be maintained above 250 nmol/mL during the assay. Add additional oxygen as needed by partially opening the chamber, adding more oxygen from the syringe into the air bubble, and carefully closing the chamber again.
- Normalize oxygen flux data to the mass of tissue used for the experiment. To adjust the graphs, change the layout to reflect O2 Flux (pmol O2 / (s x mg)). From the layout menu, select the 06 – Specific Flux per Unit Sample layout. Data will now be presented normalized to the amount of tissue in each chamber.
4. High resolution respirometry
- When the oxygen (blue line) concentration and O2 flux (red line) have stabilized following oxygen addition, begin the respiration protocol. Oxygen can be considered stable when the blue line is flat or slowly decreasing. O2 flux should also be flat and within 5 pmol O2 / s x mg.
- Add all reagents using glass syringes. It is important not to use the same syringe for substrates, inhibitors, and uncouplers. Have a separate syringe for each. After adding each compound, record oxygen flux for 1-2 min after the respiration rate stabilizes before adding the next reagent.
- Respiration measurement
- Using a 10 µL glass syringe, add 2.5 µL of 0.8 M malate to each chamber. Press F4 to mark the timeline and label the mark with M. Record stable O2 flux for 1-2 min.
- Add nutrient-specific reagents as described below.
- Aerobic Glycolytic: Using a 10 µL glass syringe, add 10 µL of 2 M glutamate and 5 µL of 2 M pyruvate to each chamber. Press F4 to mark the timeline and label the mark with G P. Record stable O2 flux for 1-2 min.
- Fatty acid: Using a 10 µL glass syringe, add 10 µL of 2 M glutamate and 10 µL of 10 mM palmitoyl-carnitine to each chamber. Press F4 to mark the timeline and label the mark with G PC. Record stable O2 flux for 1-2 min.
- Using a 25 µL glass syringe, add 20 µL of 0.5 M (with MgCl2) ADP to each chamber. Press F4 to mark the timeline and label the mark with ADP. Record stable O2 flux for 1-2 min.
- Using a 25 µL glass syringe, add 20 µL of 1 M succinate to each chamber. Press F4 to mark the timeline and label the mark with S. Record stable O2 flux for 1-2 min.
- Using a 10 µL glass syringe, add 5 µL of 4 mM Cytochrome C to each chamber. Press F4 to mark the timeline and label the mark with Cyt C. Record stable O2 flux for 1-2 min.
- Titrate in three 1 µL boluses of 1 mM FCCP using a 10 µL glass syringe. There is generally a mixing effect when adding FCCP that results in a brief decrease in O2 flux levels. Wait for the O2 flux to increase and stabilize before proceeding to the next step. Press F4 after each addition to mark the timeline and label mark with FCCP. Record stable O2 flux for 1 min after each addition.
- When the respiration assay is complete, gently remove stoppers by twisting and pulling upward. Rinse the chambers 3x with ultrapure H2O from a squirt bottle, followed by 3x with 70% ethanol from a squirt bottle. After the final ethanol rinse, fill the chambers with 70% ethanol for storage.
- Set the stoppers in the chambers until you feel resistance. Do not close the stoppers all the way. Place caps over the stoppers, save the assay file and disconnect the instrument from the Respirometry software. Turn off the instrument.
5. Data analysis
- Open the analysis file in the respirometry software. Extract data from stable oxygen flux regions obtained after injection of respiration compounds.
- To mark regions of interest, hold down the Shift key, click the Left mouse button, and drag the box across the stable O2 flux rate region for the assay stages detailed below.
- Assay stages14,15,16,17,18,19,20
- Mark the timeline after the addition of malate/glutamate/pyruvate (aerobic glycolytic protocol) or malate/glutamate/palmitoyl-carnitine (fatty acid protocol). This rate represents the Complex I State 2 respiration (LEAK(n)) rate.
- Mark the timeline after the addition of ADP. This rate represents Complex I State 3 respiration (CI OXPHOS) rate.
- Mark the timeline after addition of succinate. This rate represents Complex I+II State 3 respiration (CI+II OXPHOS) rate.
- Mark the timeline after adding Cytochrome C. This rate represents Complex I+II+Cytochrome State 3 respiration (CI+II+Cyt C OXPHOS) rate.
- Mark the timeline for the FCCP titration with the highest O2 flux rate. This rate represents Maximal Respiratory Rate (MAX ETS).
- Retrieve the data values for the marked regions on the timeline by selecting Mark > Statistics. Copy the O2 Flux rate (pmol O2/(s x mg)) for the marked chamber to a spreadsheet.
- Repeat marking and data extraction for the additional chambers.
- Calculate the respiratory control ratio (RCR) by dividing Complex I+II State 3 (following succinate addition) by Complex I State 2 (prior to ADP addition).
Representative Results
Figure 3 and Figure 4 show oxygen plots of aerobic glycolytic and fatty acid respirometry protocols, respectively, for properly prepared murine red and white gastrocnemius muscle fibers. Also shown are representative quantified results for reference. Figure 5 shows an oxygen plot of aerobic glycolytic respirometry in human muscle biopsy samples that were properly prepared. Representative quantified results are also shown. Note that for Figure 3, Figure 4, and Figure 5, addition of Cytochrome C after ADP addition does not produce an impact on oxygen flux, indicating that the outer mitochondrial membrane of the sample is intact. Figure 6 shows an oxygen plot of aerobic glycolytic respirometry where the addition of Cytochrome C after ADP results in a spike (40% increase) in oxygen flux, indicating the outer mitochondrial membrane has been damaged and thus the sample should not be used for respirometry – potential reasons for this result can be inappropriate handling or freezing/thawing of the tissue, prolong permeabilization of the tissue, and not using freshly isolated tissue.
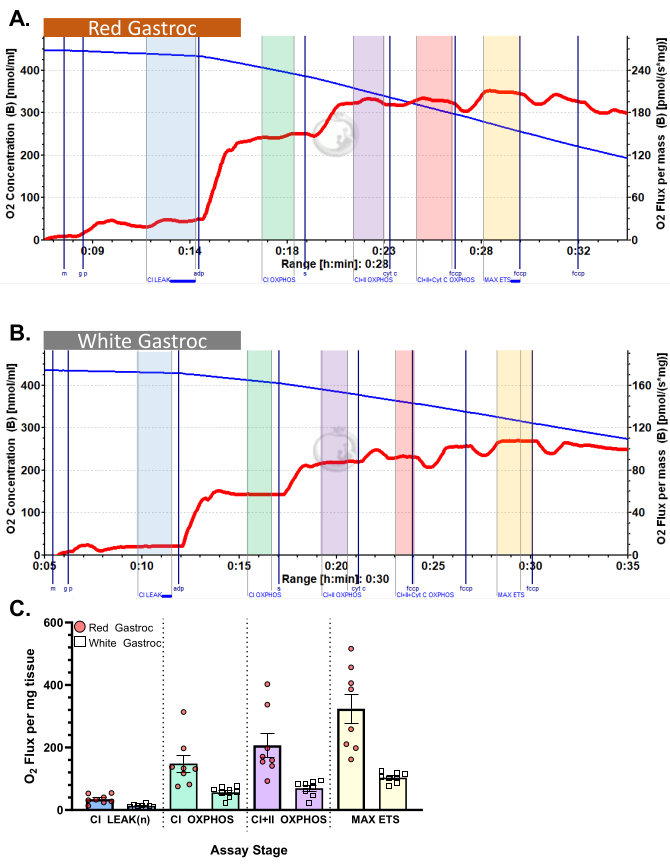
Figure 3: Oxygen consumption in mouse. The results show oxygen consumption in (A) red and (B) white gastrocnemius using pyruvate protocol. State 2 flux following the addition of malate, glutamate, and pyruvate (blue shade, CI LEAK). Significant stimulation of O2 consumption is observed after ADP administration (green shade, CI OXPHOS), with respiration driven further after the addition of succinate (purple shade, CI+II OXPHOS). Cytochrome C induced no significant increase (<15%), indicating the outer mitochondrial membrane is intact (orange shade, CI+II+Cyt C OXPHOS). Mitochondria are uncoupled following the addition of FCCP (yellow shade, MAX ETS). The blue line represents oxygen concentration in a closed chamber. The red line represents the rate of oxygen consumption (O2 flux). Compounds added: Malate (m), Glutamate (g), Pyruvate (p), Adenosine Diphosphate (ADP), Cytochrome C (cyt c), Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). (C) The bar graph reflects representative results (n=8). Data are represented as ± SEM. Please click here to view a larger version of this figure.
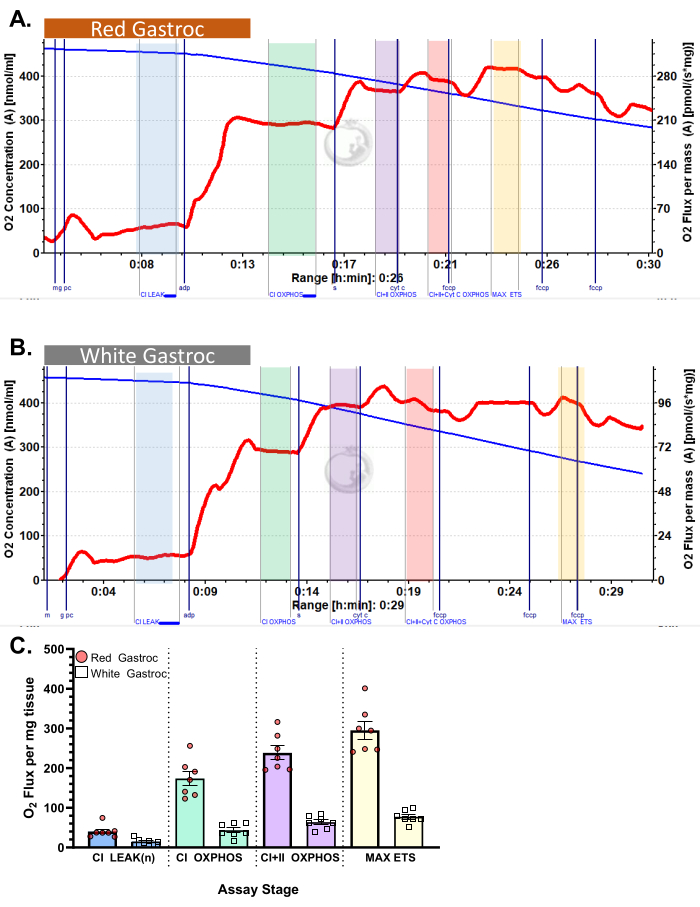
Figure 4: Oxygen consumption in mouse. The results show oxygen consumption in (A) red and (B) white gastrocnemius using palmitoyl-carnitine protocol. State 2 flux following the addition of malate, glutamate, and palmitoyl carnitine (blue shade, CI LEAK). Significant stimulation of O2 consumption is observed after ADP administration (green shade, CI OXPHOS), with respiration driven further after the addition of succinate (purple shade, CI+II OXPHOS). Cytochrome C induced no significant increase (<15%), indicating the outer mitochondrial membrane is intact (orange shade, CI+II+Cyt C OXPHOS). Mitochondria are uncoupled following the addition of FCCP (yellow shade, MAX ETS). The blue line represents oxygen concentration in a closed chamber. The red line represents the rate of oxygen consumption (O2 flux). Compounds added: Malate (m), Glutamate (g), Palmitoyl Carnitine (pc), Adenosine Diphosphate (ADP), Cytochrome C (cyt c), Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). (C) The bar graph reflects representative results (n=7). Data are represented as ± SEM. Please click here to view a larger version of this figure.
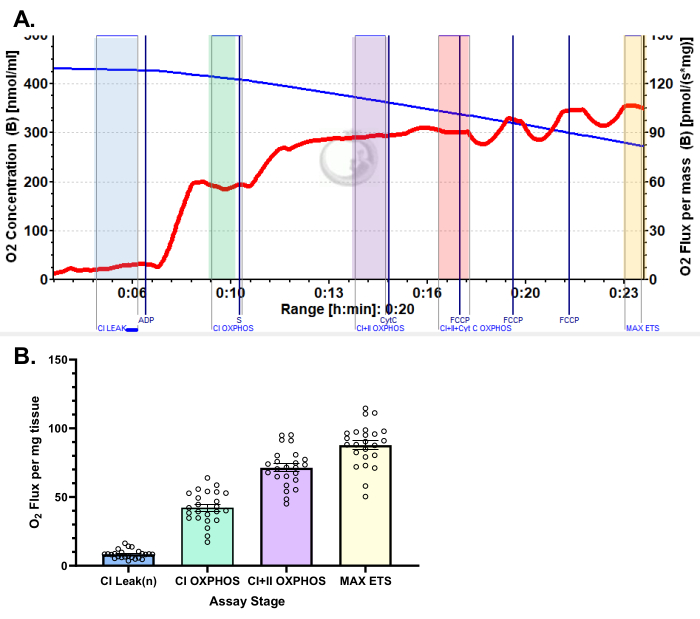
Figure 5: Representative results for oxygen consumption in human vastus lateralis using pyruvate protocol. (A) State 2 flux following the addition of malate, glutamate, and pyruvate (blue shade, CI LEAK). Significant stimulation of O2 consumption is observed after ADP administration (green shade, CI OXPHOS), with respiration driven further after the addition of succinate (purple shade, CI+II OXPHOS). Cytochrome C induced no significant increase (<15%), indicating the outer mitochondrial membrane is intact (orange shade, CI+II+Cyt C OXPHOS). Mitochondria are uncoupled following the addition of FCCP (yellow shade, MAX ETS). The blue line represents oxygen concentration in a closed chamber. The red line represents the rate of oxygen consumption (O2 flux). Compounds added: Malate (m), Glutamate (g), Pyruvate (p), Adenosine Diphosphate (ADP), Cytochrome C (cyt c), Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). (B) The bar graph reflects representative results obtained from vastus lateralis biopsies (n = 24). Data are represented as ± SEM. Please click here to view a larger version of this figure.
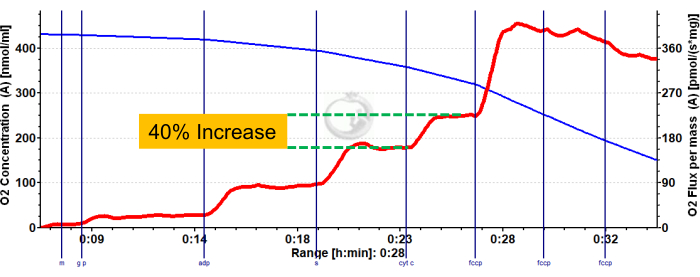
Figure 6: Representative result demonstrating compromised outer mitochondrial membrane integrity in mouse red gastrocnemius. State 2 flux following the addition of malate, glutamate, and pyruvate (blue shade, CI LEAK). Significant stimulation of O2 consumption is observed after ADP administration (green shade, CI OXPHOS), with respiration driven further after the addition of succinate (purple shade, CI+II OXPHOS). Cytochrome C induced a significant increase in O2 consumption (>15%), indicating damage to the outer mitochondrial membrane. The blue line represents oxygen concentration in a closed chamber. The red line represents the rate of oxygen consumption (O2 flux). Compounds added: Malate (m), Glutamate (g), Pyruvate (p), Adenosine Diphosphate (ADP), Cytochrome C (cyt c), Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). Please click here to view a larger version of this figure.
Table 1: Reagent preparation of respiration compounds and respiration solutions. Details for preparing reagents necessary for the assay are presented, including final stock concentrations and how to prepare and store them. Please click here to download this Table.
Discussion
This protocol provides a comprehensive and straightforward template protocol for assessing mitochondrial function in permeabilized skeletal muscle fibers for both human and mouse samples. There are several advantages to using permeabilized fibers instead of isolated mitochondria. One key advantage is that the use of permeabilized fibers requires small (2-5 mg) amounts of tissue, making this method suitable for both human muscle biopsy samples and mouse muscle. Another advantage over isolated mitochondria is that the cellular architecture remains intact, ensuring the preservation of structural and functional interactions between mitochondria and cellular components12,21,22,23.
The use of pyruvate, malate, and glutamate in our aerobic glycolytic protocol provides a comprehensive, broad-spectrum evaluation of NADH supply to Complex I24,25,26,27,28. While this comprehensive approach provides an assessment of Complex I activity under holistic and physiologically relevant metabolic conditions, the usage of pyruvate-malate or glutamate-malate may be a more appropriate experimental approach. For example, the usage of glutamate-malate may tease out differences in mitochondrial function related to amino-acid catabolism29. We encourage investigators to carefully consider the appropriate approach to use for their specific research model.
While this protocol focuses on the use of substrates to assess mitochondrial activity, the use of specific inhibitors may be necessary to achieve experimental aims. For example, rotenone can be used to inhibit Complex I12,21,30, oligomycin used to inhibit Complex V (ATP Synthase)12,21and antimycin A to block Complex III12,21 for assessment of non-mitochondrial respiration. The protocol provided above can easily be adapted to include the usage of specific inhibitors. Of significant note, one caveat regarding inhibitor usage is that these compounds are sticky and require extensive cleaning to remove from the instrument chamber. We find using a solution of 10% BSA for 60 min is sufficient for the removal of residual inhibitors.
LEAK respiration refers to the oxygen consumption rate that is independent of ATP synthesis. This rate represents the flow of protons back into the mitochondrial matrix from across the inner mitochondrial membrane. There are three accepted methods to assess oxygen consumption independent of ATP synthesis (LEAK). The first, LEAK(n), measures oxygen rate of consumption in the presence of substrates but without the addition of adenylates (ADP or ATP)31,32,33. This LEAK state represents the intrinsic leakiness of the mitochondrial membrane. The second method, LEAK(t), is measured in the presence of ATP34 and the third, LEAK(o), is measured in the presence of the ATP-synthase inhibitor oligomycin35,36,37. This protocol uses LEAK(n) for this assessment, but depending on experimental aims and models, other methods for measuring LEAK oxygen flux may be appropriate.
For this assay, MiR05 is supplemented with both creatine (3 mg/mL) and blebbistatin (10 µM). Mitochondrial ADP transport is facilitated by creatine kinase (CK), and creatine is added to the respiration solution to saturate CK activity38,39. Muscle fibers can spontaneously contract and are also sensitive to ADP-induced contraction. To assess mitochondrial respiratory activity without the influence of contraction, blebbistatin has been added to inhibit fiber contractile activity38. Additionally, studies on human muscle suggest that respiratory capacity may be influenced by the biopsy method (microbiopsy versus Bergstrom needle) and that this difference may be due to differences in obtained fiber length40,41. Shorter fibers may be more susceptible to damage during preparation, and the use of blebbistatin helps preserve function. There may be certain conditions where fiber relaxation does not fit with research aims, and, in that event, blebbistatin can be excluded from the MiR05 solution.
Permeabilization of the skeletal muscle fibers with saponin generates pores in the plasma membrane allows substrates and inhibitors to freely enter the cell. Saponin has a high affinity for cholesterol, which is rich and abundant in cellular plasma membranes, while mitochondrial membranes are cholesterol-poor42,43. It is expected that the saponin treatment used for fiber preparation in this protocol will preserve mitochondrial membrane integrity. Damage to mitochondria may also occur due to shear forces that result from the mechanical separation of the tissue into fibers. We suggest that the separation of tissue into fiber bundles be conducted quickly and with minimal handling. To assess potential mitochondrial damage, we have included titration of Cytochrome C in the respiration protocol. Cytochrome C cannot pass through an intact outer mitochondrial membrane12, therefore, any increase in O2 flux following Cytochrome C addition indicates that damage to the outer mitochondrial membrane occurred during the sample preparation process. In one of our recent studies, we found that O2 flux increased by 8%15 following Cytochrome C addition, validating that saponin usage suggested in this protocol does not elicit mitochondrial damage. We suggest that any sample demonstrating greater than a 15% increase in O2 flux after Cytochrome C is added should be excluded from analysis44. This step is included strictly as a quality control measure and not as an assessment of Complex IV activity.
While high-resolution respirometry excels in providing highly sensitive and reliable measurements of oxygen consumption, a notable limitation of the instrumentation is that only two samples can be measured simultaneously per instrument. This necessitates careful consideration when designing studies involving cohorts with multiple samples. While there may be a temptation to conduct measurements on various sample sets throughout the day, we strongly advise investigators to consider the influence of circadian rhythm on metabolism. Research on both human and rodent skeletal muscle has revealed a biological clock influence on mitochondrial function45,46. Consequently, we recommend conducting measurements over several days at the same time of day to account for these circadian fluctuations.
Lastly, to ensure reproducible and robust respirometry measurements, the respirometer must receive regular cleaning, maintenance, and calibration. Air calibration, as detailed in the protocol, should be conducted daily. We advise users to also conduct complete monthly calibration (both air and zero) of the polarographic oxygen sensors. Users should refer to the manufacturer's documentation and website for further information on this calibration method and for instructions on routine instrument maintenance.
High-resolution respirometry remains the gold standard for measuring mitochondrial respiration. The method detailed in this protocol facilitates robust assessment of mitochondrial capacity in both rodent and human skeletal muscle. This protocol has been applied to studies evaluating mitochondrial function associated with genetic mouse models15,16, in the context of chronic kidney disease19, after dietary supplement administration14,20 and exercise17,18.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by Nutrition Obesity Research Center, NIH grant P30 DK056341, and NIH grant K01 HL145326.
Materials
| 10 µL Hamilton Syringe (glass syringe) | ThermoFisher | 14-813-125 | For respiration assay titration |
| 25 µL Hamilton Syringe (glass syringe) | ThermoFisher | 14-813-133 | For respiration assay titration |
| ADP | Merck | 117105 | Respirometry Assay |
| Black Glass Dissection Dish | Scintica | DD-90-S-BLK | For sample preparation |
| Blebbistatin | Sigma | B0560 | Working MiR05 Solution |
| BSA, fatty acid free | Sigma | A6003 | MiR05 Solution |
| Calcium Carbonate | Sigma | C4830 | BIOPS Solution |
| Creatine | Sigma | 27900 | Working MiR05 Solution |
| Cytochrome C | Sigma | C7752 | Respirometry Assay |
| DatLab | Oroboros Instruments | N/A | Respirometry Software |
| Dithiothreitol (DTT) | Sigma | D0632 | BIOPS Solution |
| D-Sucrose | Sigma | 84097 | MiR05 Solution |
| EGTA | Sigma | E4378 | BIOPS & MiR05 Solution |
| FCCP | Sigma | C2920 | Respirometry Assay |
| Glutamate | Sigma | G1626 | Respirometry Assay |
| HEPES | Sigma | H7523 | MiR05 Solution |
| Imidazole | Sigma | 56750 | BIOPS Solution |
| KH2PO4 | Sigma | P5379 | MiR05 Solution |
| Lactobionic acid | Sigma | 153516 | MiR05 Solution |
| Malate | Sigma | M1000 | Respirometry Assay |
| MES hydrate | Sigma | M8250 | BIOPS Solution |
| MgCl2 – 6 H2O | Sigma | M2670 | BIOPS & MiR05 Solution |
| Oroboros Oxygraph-2K (O2K) System | Oroboros Instruments | 10203-03 | High resolution respirometer |
| Palmitoyl-Carnitine | Sigma | P4509 | Respirometry Assay |
| Potassium Hydroxide | Sigma | P1767 | BIOPS Solution |
| Precision Tweezers | Fisher | 17-467-168 | For sample preparation |
| Saponin | Sigma | S2149 | For Fiber Permeabilization |
| Sodium ATP | Sigma | A2383 | BIOPS Solution |
| Sodium Phosphocreatine | Sigma | P7936 | BIOPS Solution |
| Sodium Pyruvate | Sigma | P2256 | Respirometry Assay |
| Succinate | Sigma | S2378 | Respirometry Assay |
| Taurine | Sigma | T0625 | BIOPS & MiR05 Solution |
Riferimenti
- Rizzuto, R., De Stefani, D., Raffaello, A., Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 13 (9), 566-578 (2012).
- Ruegsegger, G. N., Creo, A. L., Cortes, T. M., Dasari, S., Nair, K. S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Invest. 128 (9), 3671-3681 (2018).
- Simoneau, J. A., Kelley, D. E. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 83 (1), 166-171 (1997).
- Ryan, T. E., et al. Extensive skeletal muscle cell mitochondriopathy distinguishes critical limb ischemia patients from claudicants. JCI Insight. 3 (21), 123235 (2018).
- Sullivan, M. J., Green, H. J., Cobb, F. R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 81 (2), 518-527 (1990).
- Giebelstein, J., et al. The proteomic signature of insulin-resistant human skeletal muscle reveals increased glycolytic and decreased mitochondrial enzymes. Diabetologia. 55 (4), 1114-1127 (2012).
- Tezze, C., et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25 (6), 1374-1389 (2017).
- Hughes, M. C., et al. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H(2) O(2) emission during impaired oxidative phosphorylation. J Cachexia Sarcopenia Muscle. 10 (2), 643-661 (2019).
- Yin, Y., Shen, H. Common methods in mitochondrial research (Review). Int J Mol Med. 50 (4), 5182 (2022).
- Vue, Z., et al. 3D reconstruction of murine mitochondria reveals changes in structure during aging linked to the MICOS complex. Aging Cell. 22 (12), e14009 (2023).
- Doerrier, C., et al. High-resolution fluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol. 1782, 31-70 (2018).
- Pesta, D., Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 810, 25-58 (2012).
- Picard, M., Hepple, R. T., Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. 302 (4), C629-C641 (2012).
- Yoshino, M., et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 372 (6547), 1224-1229 (2021).
- Mousa, M. G., et al. Site-1 protease inhibits mitochondrial respiration by controlling the TGF-beta target gene Mss51. Cell Rep. 42 (4), 112336 (2023).
- Moon, S. H., et al. Genetic deletion of skeletal muscle iPLA(2)gamma results in mitochondrial dysfunction, muscle atrophy and alterations in whole-body energy metabolism. iScience. 26 (6), 106895 (2023).
- Bittel, A. J., et al. A single bout of premeal resistance exercise improves postprandial glucose metabolism in obese men with prediabetes. Med Sci Sports Exerc. 53 (4), 694-703 (2021).
- Bittel, A. J., et al. A single bout of resistance exercise improves postprandial lipid metabolism in overweight/obese men with prediabetes. Diabetologia. 63 (3), 611-623 (2020).
- Bittel, D. C., Bittel, A. J., Varadhachary, A. S., Pietka, T., Sinacore, D. R. Deficits in the skeletal muscle transcriptome and mitochondrial coupling in progressive diabetes-induced CKD relate to functional decline. Diabetes. 70 (5), 1130-1144 (2021).
- Mills, K. F., et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24 (6), 795-806 (2016).
- Djafarzadeh, S., Jakob, S. M. High-resolution respirometry to assess mitochondrial function in permeabilized and intact cells. J Vis Exp. (120), e54985 (2017).
- Lemieux, H., Semsroth, S., Antretter, H., Hofer, D., Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol. 43 (12), 1729-1738 (2011).
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 41 (10), 1837-1845 (2009).
- Appelman, B., et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 15 (1), 17 (2024).
- O’Rourke, A. R., et al. Impaired muscle relaxation and mitochondrial fission associated with genetic ablation of cytoplasmic actin isoforms. FEBS J. 285 (3), 481-500 (2018).
- Inigo, M. R., et al. Estrogen receptor-alpha in female skeletal muscle is not required for regulation of muscle insulin sensitivity and mitochondrial regulation. Mol Metab. 34, 1-15 (2020).
- Musci, R. V., et al. Phytochemical compound PB125 attenuates skeletal muscle mitochondrial dysfunction and impaired proteostasis in a model of musculoskeletal decline. J Physiol. 601 (11), 2189-2216 (2023).
- Englund, D. A., et al. p21 induces a senescence program and skeletal muscle dysfunction. Mol Metab. 67, 101652 (2023).
- Zhang, K., et al. Mitochondrial supercomplex assembly regulates metabolic features and glutamine dependency in mammalian cells. Theranostics. 13 (10), 3165-3187 (2023).
- Davis, M. S., Barrett, M. R. High-resolution fluoro-respirometry of equine skeletal muscle. J Vis Exp. (192), e65075 (2023).
- Schytz, C. T., et al. Skeletal muscle mitochondria demonstrate similar respiration per cristae surface area independent of training status and sex in healthy humans. J Physiol. 602 (1), 129-151 (2024).
- Hingst, J. R., et al. Inducible deletion of skeletal muscle AMPKalpha reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise. Mol Metab. 40, 101028 (2020).
- Krumschnabel, G., Eigentler, A., Fasching, M., Gnaiger, E. Use of safranin for the assessment of mitochondrial membrane potential by high-resolution respirometry and fluorometry. Methods Enzymol. 542, 163-181 (2014).
- Gnaiger, E., Mendez, G., Hand, S. C. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A. 97 (20), 11080-11085 (2000).
- Basse, A. L., et al. Nampt controls skeletal muscle development by maintaining Ca(2+) homeostasis and mitochondrial integrity. Mol Metab. 53, 101271 (2021).
- Flensted-Jensen, M., et al. Combined changes in temperature and pH mimicking exercise result in decreased efficiency in muscle mitochondria. J Appl Physiol. 136 (1985), 79-88 (2024).
- Porter, C., et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 309 (3), E224-E232 (2015).
- Perry, C. G., et al. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 437 (2), 215-222 (2011).
- Veksler, V. I., et al. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 270 (34), 19921-19929 (1995).
- Isner-Horobeti, M. E., et al. Microbiopsies versus Bergstrom needle for skeletal muscle sampling: impact on maximal mitochondrial respiration rate. Eur J Appl Physiol. 114 (5), 885-889 (2014).
- Hughes, M. C., et al. Mitochondrial bioenergetics and fiber type assessments in microbiopsy vs. Bergstrom percutaneous sampling of human skeletal muscle. Front Physiol. 18 (6), 360 (2015).
- Kuznetsov, A. V., et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 3 (6), 965-976 (2008).
- Elustondo, P., Martin, L. A., Karten, B. Mitochondrial cholesterol import. Biochim Biophys Acta Mol Cell Biol Lipids. 1862 (1), 90-101 (2017).
- Ramos, P. M., Li, C., Elzo, M. A., Wohlgemuth, S. E., Scheffler, T. L. Mitochondrial oxygen consumption in early postmortem permeabilized skeletal muscle fibers is influenced by cattle breed. J Anim Sci. 98 (3), 044 (2020).
- van Moorsel, D., et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 5 (8), 635-645 (2016).
- de Goede, P., et al. Time-restricted feeding during the inactive phase abolishes the daily rhythm in mitochondrial respiration in rat skeletal muscle. FASEB J. 36 (2), e22133 (2022).

.
