Preclinical Positron Emission Tomography with Body Conforming Animal Molds for Cloud-Based Automated Image Analysis in Mice
Summary
This protocol describes the procedure for performing in vivo PET imaging on mice using Body Conforming Animal Molds (BCAMs) in the G8 PET/CT scanner. Technical details on mouse preparation, including proper tumor implantation, optimal positioning, BCAM-assisted PET/CT image acquisition, and data analysis, are provided.
Abstract
Positron Emission Tomography (PET) is a molecular imaging modality that can be used to investigate a multitude of pharmacological questions, such as biomarker modulation, receptor occupancy, and biodistribution of compounds of interest. In biodistribution studies, experimental subjects are often longitudinally imaged after receiving the test article. The images are then analyzed to derive the compound's distribution profile in various organs at different timepoints. This constitutes a crucial step in drug development to understand the distribution and potentially binding profile of an investigative compound. Standard/manual methods of PET imaging-based biodistribution analyses, however, are labor-intensive and time-consuming and are often associated with high inter-operator variability. Further, it is challenging to keep the animals' positions consistent across different timepoints. To address these shortcomings, a series of mouse Body Conforming Animal Molds (BCAMs) were used to enable rigid and consistent positioning of animals during PET/CT imaging acquisition. Further, a Software-as-a-Service (SaaS) platform consisting of a cloud-based Organ Probability Map (OPM) and an artificial intelligence-powered segmentation tool were employed to enable reliable and automated quantitation of in vivo PET imaging data. The workflow presented here includes (1) prepping mice for imaging with the BCAMs, including the proper implantation of subcutaneous tumors to be compatible with the molds, (2) acquiring PET/CT images with BCAMs using the G8 scanner, and 3) performing automated organ segmentation and biodistribution analysis using the cloud-based SaaS. [18F]FDG was used as an exemplar tracer here, but other biomarkers and/or radio-labeled compounds can be readily adapted into the workflow. This procedure can be executed accurately and effectively with minimal training, and the automated PET data analysis yielded satisfactory results consistent with the manual method.
Introduction
In vivo molecular imaging technologies are important tools in drug development to facilitate the assessment of all three pillars of pharmacology – biodistribution, target engagement, and modulation of pharmacodynamics1,2,3. Positron emission Tomography (PET), with its clinical translatability and high sensitivity, is among the most commonly used molecular imaging modalities in both the clinic and the preclinical research and development space. The blooming field of preclinical PET imaging and radiotheranostics is valuable for the development of both tumor-intrinsic therapeutics, targeted therapeutics, as well as novel Immuno-Oncology (IO) therapies. The continual emergence and development of new Immuno-PET radiotracers have the potential to influence specific tailored IO drug regimens and identify responders versus non-responders4,5,6. Meanwhile, metabolic-specific radiotracers, such as [18F]FDG and [18F]FLT, can monitor a tumor's response to therapy and can respectively shed light on whether a tumor is actively proliferating or consuming glucose7, or if tumor cells are undergoing cell cycle arrest by ways of inhibition8,9.
Non-invasive in vivo imaging presents multiple advantages compared to traditional, non-imaging pharmacological experiments. For example, imaging allows systemic, holistic evaluation of a drug's distribution and/or pharmacological effect in the entire subject, which enables not only the monitoring of efficacy at the target site, but also the detection of unexpected off-site activities. Due to its non-invasive nature, the same set of animals can be assessed longitudinally across different timepoints and/or with different biomarkers to address distinct biological questions10. Not only does this significantly reduce the number of animals needed for the study, but it also provides an orthogonal control across timepoints and, therefore, helps to improve correlations and reduce individual variabilities. Despite such promising development of imaging biomarkers, the need to evaluate investigative compounds in vivo with PET imaging-based biodistribution studies, however, is met with a lack of efficient automated processes for analysis. PET biodistribution studies generate valuable information on a compound's kinetics, tissue accumulation, binding, and metabolism. Obtaining highly reproducible quantitative data is critical for the development of pharmaceutical therapies.
To address these unmet needs in automated PET image analysis, InVivo Analytics has designed a set of mouse Body Conforming Animal Molds (BCAMs)11. These are 3D-printed plastic molds that hold a mouse's head, torso (and internal organs), tail, and limbs in rigid, pre-defined positions. Different-sized molds can be selected based on the animal's body weight, and cut-outs can be created in specific regions, such as the shoulder or lower flank, to allow subcutaneous tumors to protrude out. PET/CT images acquired with these BCAMs are highly uniform and can be analyzed in batches by the company's cloud-based automated segmentation platform11. This article presents an automated in vivo biodistribution analysis workflow that is powered by physically acquiring images with BCAMs and performing image segmentation and analysis by the SaaS.
Protocol
All procedures performed on animals followed regulations and established guidelines and were reviewed and approved by Pfizer's Institutional Animal Care and Use Committee or through an ethical review process. PET imaging study was performed following all institutional radiation safety protocols. Female C57BL/6 mice, 10-12 weeks old, with body weights ranging from 18-26 g, were used in this study. The details of the reagents and equipment used are listed in the Table of Materials.
1. Prepping and staging of animals
NOTE: This step involves staging the animals for PET imaging with BCAM template-guided tumor implantation.
- Prior to cell implantation, shave the fur of the mice in the desired region of tumor growth.
- Set up the anesthesia station and attach a BCAM mold of the appropriate size to the pre-clip with the top flipped up 90 degrees. This will serve as the tumor implantation base (Figure 1A).
- Anesthetize animals in a suitable induction box with 2.0% isoflurane and 98% oxygen at a flow rate of 0.5-1.5 L/min.
NOTE: All anesthesia systems are variable.- Use a calibrated system and test before use. Place an anesthetized mouse on the base and extend its limbs (Figure 1B).
- Gently place a BCAM tumor injection template mold (Figure 1C) on the dorsal side of the mouse (Figure 1D).
- Carefully create a temporary circular reference mark at the desired cut-out location (e.g., shoulder or flank, left or right) with a marker (Figure 1D). Remove the template mold.
- Prepare the cells for injection by drawing up the desired volume into a syringe. Attach a 27 G needle to the syringe and fill the dead volume space. A volume of 0.1-0.2 mL per inoculation is recommended.
- Lift skin with fingers or forceps using the temporary marks from step 1.5 as a reference to create a tent, slowly insert the needle, and inject cells subcutaneously in the desired location. Discard the needle in a biohazard sharps container.
- Monitor the tumor growth. The average tumor volume at enrollment in this example was 200-300 mm3.
NOTE: Avoid using metal components such as ear tags or microchips to prevent CT imaging artifacts.
2. Drawing radioactive doses
- Following all institutional radiation safety protocols, carefully draw up a target dose of [18F]FDG in the range of approximately 70-80 µCi into a sterile syringe suitable for intravenous injection.
NOTE: The 70-80 µCi range is optimal to fit the dynamic range of the Sofie G8 PET scanner. Other imagers may have different dynamic ranges that require a different target dose. - Carefully measure the radioactive dose in a dose calibrator by placing the syringe into the well chamber and lowering the dipper. Pay attention to selecting the appropriate isotope or calibration number prior to measurement.
- Take a stable reading. Record date and time. Adjust the dose if needed.
- (Optional) Use sterile saline to bring the final dose volume to 0.1-0.2 mL.
NOTE: The desired injection volume may vary. - Dose is ready to be administered.
3. Intravenous dose administration
- Following institutional IACUC and Radiation safety protocols, prepare the mouse tail for IV injection by dilating the tail vein. In this study, the tail vein was dilated by placing the animal cage or induction chamber on a slide warmer set at 42 °C.
NOTE: It may take up to 10 min of warming for the tail vein to fully dilate. - Before injection, record mouse weight. This will determine which weight-specific BCAM to use for PET/CT imaging.
- Once ready, transfer the mouse into a suitable restrainer (e.g., a Tail vein injection platform was used in the present study) and locate the tail vein.
- Carefully handle the radioactive syringe and IV inject the dose into a conscious mouse. Record the injection time.
- Carefully set the syringe aside and clean excess blood drops off the tail with gauze. Remove the mouse from the restrainer and place it back in the appropriate housing.
- Carefully place the syringe back in the dose calibrator chamber and record any residual readings for proper dose calculations. Record the date and time of the residual activity reading.
- Once the injections are complete, take caution and place cohorts of mice behind lead enclosures and/or away from personnel, following institution-safe radiation practices. Note all materials used are subject to minor radioactive contamination. Monitor all areas with a calibrated Geiger counter and clean per radiation safety guidelines.
4. Anesthetizing and positioning the animals for PET imaging
- At an appropriate time (e.g., 10-15 min before the end of the tracer uptake period), turn on all anesthesia equipment and animal warming instruments required for both anesthesia induction and PET imaging. Anesthesia can be set at 2.0%-2.5% isoflurane in 98% oxygen (settings may vary with mouse strain and disease model).
- Confirm all adapters and valves are switched on and the gas flow rate is 0.5-1.0 L/min. Specific flow rates may vary among anesthesia systems and imaging systems.
- Place appropriate animal(s) in an induction box and wait until the subject reaches the correct depth of anesthesia.
- Apply eye lubricant ointment to each eye.
- Transfer mouse into an appropriately sized BCAM based on body weight.
- Gently pick up the mouse by the nape of the neck, insert the tail into the tail slot, and wrap it under the BCAM. Place the tail on the tail platform and tape to secure the position. Check that the spine is straight. Gently close the BCAM (Figure 2A,B).
- Carefully position all four limbs to rest on each of the BCAM paw platforms. Use tape as needed to secure paws to each platform (Figure 2B).
- Gently snap the BCAM into the imaging bed shuttle by inserting the front end first (Figure 2C). The shuttle should already be connected to the G8 loading dock with anesthesia flowing (Figure 2D). Push down on the back of the BCAM, and a click will indicate that it is secured to the G8 shuttle.
5. Image acquisition
- Open the G8 PET acquisition software. Study folders, scan parameters, and subject IDs can be created beforehand.
- Enter study details into the software: mouse weight, date/time and amount of the initial dose drawn, date/time of injection, and date/time and amount of residual activity reading.
- Insert the shuttle with the mouse into the scanner opening. A blue indicator light will indicate proper docking to the system.
- Select the appropriate PET/CT parameters. Acquire imaging data.
NOTE: In this study, a 7-min static 18F PET scan was run, followed by a 70-s default CT scan, and a maximum-likelihood expectation-maximization 3D reconstruction algorithm (default and only option in the G8 scanner) was applied to reconstruct the data12. - Upon acquisition completion, the shuttle will return to its initial starting position. Remove the shuttle with the mouse and insert it into the dock. Press on the back of the BCAM vertical tab to remove it from the G8 shuttle, and gently pull up and remove the BCAM with the mouse.
- Press on the two retaining tabs to flip up and open the top of the BCAM.
- Gently remove the mouse. Ensure the mouse has fully recovered from anesthesia and is bright, alert, and responsive (BAR) before returning to its home cage.
- Continue the procedure at set time intervals to scan the entire cohort/group of mice.
6. Uploading images to the SaaS
- Login to app.invivo.ax and create a project.
- Click on the red upload tab at the top right of the window. Select an imaging system and reporter used in the imaging study (i.e., [18F]FDG). Select the folder with imaging data.
- Navigate to the annotation tab within the project folder. Click on Annotate to add relevant scan information (subject name, sex, BCAM size, subject weight, cohort name, timepoint, and injected dose value). Most metadata will auto-populate.
7. Automated data analysis
- Within the project folder, all scans should populate. Click on scans to navigate from project, group, and individual subject levels. Select Analyze at the top right corner.
- At the top right of the analysis ribbon window, under Project, select the plus symbol and select Organ Probability Map ROI. The Organ Probability Map (OPM) is a statistic mouse organ atlas encompassing multiple pre-defined organs and tissues. Select individual organs of interest based on research needs.
NOTE: In this example, manual segmentation and analysis were done using VivoQuant, using the spline tool, followed by interpolation within the 3D ROI operator. Automated analysis results were compared to the manual segmentation results as a benchmark.
Representative Results
Once uploaded, the PET data can be viewed on the SaaS website as a panel of coronal, sagittal, and transverse images overlayed with the selected organ ROIs that are dictated by the OPM (Figure 3A). Not only can the software load individual images, but multiple mice from a group or a whole study can be viewed at once as a single, collapsed image. Within this analysis window, there are options to customize ROI colors, scale bar intensity, and other common parameters. Once the ideal analytical metrics are selected, the SaaS generates a downloadable biodistribution bar graph of the selected organs (Figure 3B).
To evaluate the performance of the OPM-based SaaS platform, the results from the automated analysis were compared to those that were derived by an experienced imaging analyst as the gold standard. Fit linear regression was performed on the [18F]FDG uptake values from brain, heart, kidney (right), and spleen. Of note, the platform offers a comprehensive list of organs for users to select from, including the skeleton, brain, bladder, heart, kidneys (right and left), liver, spleen, lungs, and quadricep muscles (right and left). The four above organs were selected in this study for illustration purposes only. Further, due to the variable size, shape, and depth/location of implanted tumors, the Tumor is not included as an organ in the OPM, and therefore excluded from this comparative analysis. As shown in Figure 4, the automated analysis and manual analysis yielded overall consistent results, with the brain showing the highest correlation (r2 = 0.8466). This is expected due to the large size of the organ and the highly constant position of the brain within the skull. The right kidney and the heart also showed reasonable r2 values of 0.6874 and 0.4849, respectively. In contrast, the correlation analysis for the spleen generated an r2 value of 0.3922, which is the lowest among the tested organs. This is not surprising as the spleen is nested in the pancreas and adipose tissues that lack defined morphology and presume the same soft-tissue density on CT. The spleen is, therefore, difficult to segment manually, and the OPM may present a more precise means to demarcate this organ (i.e., outperforming the gold standard). The slope and Y-intercept of these correlation analyses are also shown in Figure 4. Consistent with the r2 values, the brain had a slope closest to 1 (i.e., a perfect correlation between OPM and manual results) and the smallest Y-intercept (i.e., minimum systemic bias), whereas the other organs showed greater and variable deviation from a perfect correlation.

Figure 1: BCAM and template-guided tumor implantation. (A) The pre-clip (1) is attached to the bottom piece of a BCAM mold (2) with a connected anesthesia line (3). This serves as the tumor implantation base. (B) A mouse with the target implant site shaved (in this instance, the right shoulder) is placed on the base under anesthesia prior to cell injection. (C) Tumor implantation template (4) with pre-defined cut-outs to guide the placement of tumor cell inoculation. (D) Making a temporary reference mark prior to cell injection with the template mold placed on the dorsal side of the mouse. Please click here to view a larger version of this figure.
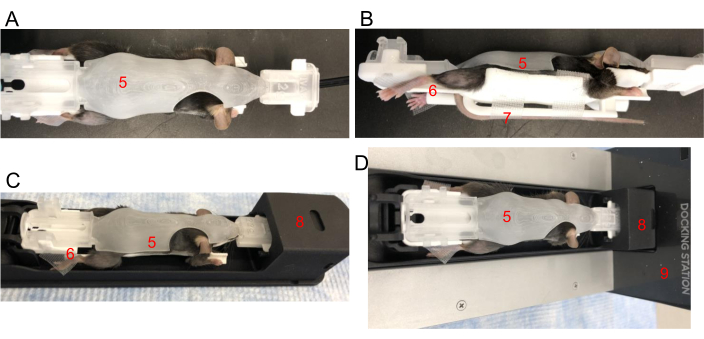
Figure 2: Positioning a mouse in a BCAM. (A) Top view – Place a mouse snuggly in an appropriately sized BCAM (5). (B) Side view – Tape is used to secure hind paws to the platforms (6). The tail is secured with tape to the tail platform (7) directly underneath the mouse. (C) Mouse, in the BCAM, is inserted into the imaging shuttle (8). (D) Mouse, BCAM, and shuttle are connected to an anesthesia-running docking station (9). Please click here to view a larger version of this figure.
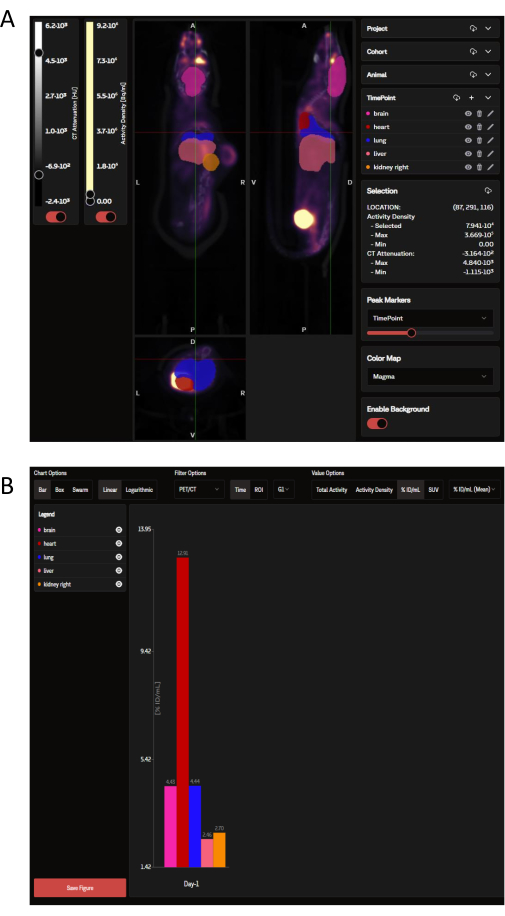
Figure 3: Representative images and data output as viewed and analyzed by the SaaS tool, respectively. (A) Representative images as viewed on the SaaS website. Coronal, sagittal, and transverse views of the PET/CT image overlayed with organ ROIs selected from the Organ Probability Map (OPM). Pink: brain; red: heart; dark blue: lung; salmon: liver; orange: kidney (right). Tumor is not defined by the OPM. (B) A representative downloadable graph generated from the automated analysis. A list of the selected organs can be seen in the upper left panel. Data here is reported in %ID/mL. Please click here to view a larger version of this figure.
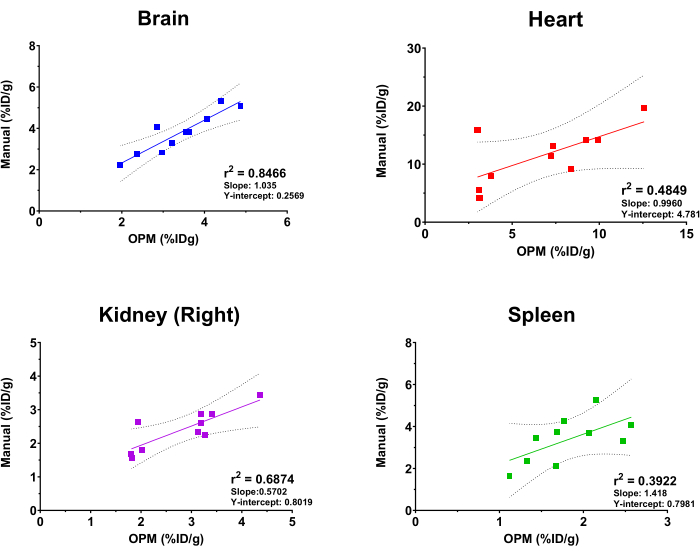
Figure 4: Linear regression analysis comparing organ [18F]FDG uptake values derived by manual analysis and the OPM-based automation workflow. Ten PET/CT scans of female C57BL/6 mice were used. r2 values, 95% confidence lines, slope, and Y-intercepts are displayed on the graph for each indicated organ. Please click here to view a larger version of this figure.
Discussion
The present report describes the procedure of performing mouse in vivo PET imaging with the BCAM molds and analyzing data with the automated SaaS tool. Step-by-step instructions on mouse prepping, imaging acquisition, and data processing are provided. This automation workflow also yielded data that was consistent with manual analysis. A few key technical considerations are highlighted below.
Although a Tumor is not one of the organs defined by the OPM, a critical step in this protocol is the proper implantation of subcutaneous tumors to ultimately ensure optimal alignment of all the mouse organs within the body cavity and BCAM. The OPM can only produce accurate data when a mouse’s spine is relatively straight and the internal organs are neutrally positioned. Excessively large or misplaced tumors could impact the mouse’s posture and press into its body cavity, thereby shifting the internal organs. This shift, in turn, will skew the resulting quantitative analysis from the OPM algorithm. Others have reported the impact of an unstraight spine on the alignment of internal organs using various imaging modalities and segmentation algorithms13,14. Therefore, it is crucial to use the template to guide tumor implantation and monitor the tumor growth closely to ensure the tumors are both properly positioned and in the ideal size range.
Another area of possible deviation is the selection of a properly sized BCAM. BCAMs are created in 2 g integrals, with a range of 18-26 g. However, since mice with different body compositions (e.g., more muscle versus more fat) may have the same body weight but different physical sizes, it is important to choose the most fitting BCAM while using their body weight as a general reference. Level up or down by trial and error, and keep in mind that proper alignment is critical. Choosing a BCAM that is too large will cause the mouse’s internal organs to ‘sink’ closer to the bottom of the bed, which will particularly impact the accuracy of analysis for the dorsal organs such as the kidneys and spine. In contrast, placing a mouse in BCAM that is too small could press on their body cavities, skew organ positioning, and potentially cause difficulty in their breathing. Using tape to ensure limbs are secured on paw platforms will help with setup and positioning.
Although this reported workflow enables standardized and automated PET image analysis, it is worthwhile to point out that the current OPM is constructed based on the immunocompetent C57BL/6 mouse strain and healthy mice. Other strains and health conditions of mice may present different anatomic features that require additional adjustment, optimization, and validation of the algorithm. For example, immune-deficient strains such as NSG or NCG have smaller spleens. Similarly, mice bearing orthotopic tumor implants may have different sizes, shapes, and locations of internal organs depending on their disease status. Another limitation of this workflow is the overall throughput during the acquisition phase of an experiment. This is mainly due to the additional time required to set up a mouse properly in the BCAM. Training and practice could increase the operator’s efficiency in proper placement. The addition of a second docking station can also increase overall throughput, where one dock can serve for BCAM setup, and the other can serve for removing a mouse to a recovery cage. Nonetheless, even with the increased time that is required for BCAM setup prior to scanning, the investment in time pays off by saving time on the data analysis end. For example, in the present study, it took less than 10 min for the OPM to analyze ten images (with 4 ROIs), whereas it took 3-4 h for a highly experienced analyst to manually segment and analyze the same set of images and ROIs. An inexperienced analyst would require even more time for manual analysis. The SaaS platform allows for the analysis of PET scans in large batches, where full in vivo biodistribution analysis can be completed within minutes following data upload. This is an extremely helpful feature when handling large amounts of data from multiple groups and/or across several timepoints, as is discussed below.
Manual segmentation of organs for a single subject can be time-consuming, and it usually requires significant upfront training of the operator. Even with adequate training, manual analyses inevitably face inter-operator variabilities, which can skew quantitative results15,16. In contrast, automated segmentation and data analysis can accurately and efficiently determine a radiotracer’s overall biodistribution and eliminate variabilities that are rooted in human operations. Such benefits of automation have been seen both in clinical image analysis and in the preclinical space. For example, Sluis et al. demonstrated that a single scan required 4 h to segment the organs of interest compared to 30 min by AI cloud-based methods17. Another study from Nazari et al. also reported that algorithm-based analysis of the liver and kidneys demonstrated accurate results within 7.0% when compared to two human medical physicists18. The data in Figure 4 also clearly showcased the reliability of the SaaS-based PET imaging analysis workflow, and that automated analysis yielded consistent results when compared to manual analysis.
In summary, this article illustrates the workflow of using BCAM molds to facilitate standardized preclinical PET/CT image acquisition as well as SaaS-enabled automated PET data analysis. It is demonstrated that this platform technology is relatively straightforward to employ, and the quality of automation-generated data is consistent with manual analysis but boasts significant time-saving benefits. Therefore, this workflow can save researchers hundreds of hours in data analysis time and help standardize and reduce inter-operator variability. This procedure can aid in the development of a multitude of drug compounds across modalities and indications. Particularly, large molecule modalities such as therapeutic antibodies, bi-specific antibody immune cell engagers, antibody-drug-conjugates, and even nanoparticles can be readily labeled with radiometals (e.g., Zr-89, Cu-64) to enable PET imaging-mediated biodistribution assessment19,20. Similarly, this platform technology can help determine the dosimetry of novel radio therapeutics in mice21. The application of this procedure will be a great value-add to drug development.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Special thanks to Pfizer La Jolla Comparative Medicine Research and Technical staff.
Materials
| [18F]FDG | PETNet Solutions, Culver City, CA | NA | Radiotracer used for PET imaging |
| 27 G needle | Becton, Dickinson and Company, San Diego, CA | 305136 | Used for cell injection |
| app.invivo.ax/login | Invivo Analytics, Seattle, WA | NA | Software as a Service webpage |
| Body Conforming Animal Molds | Invivo Analytics, Seattle, WA | NA | BCAMs with weight range of 18 – 26 g |
| Dose Calibrator; CRC-55tw | Capintec, Florham Park, NJ | CRC-55tw | Measurement of radioactive doses |
| G8 PET/CT scanner | Sofie Biosciences/Xodus Imaging, Torrence, CA | NA | Benchtop scanner with loading dock |
| G8-Docking station | Sofie Biosciences/Xodus Imaging, Torrence, CA | NA | Docking station for staging |
| Implantation Mold Template | Invivo Analytics, Seattle, WA | NA | Template for optimal tumor implantation |
| Lubricant eye ointment | BAUSCH & LOMB, Ontario, Canada | Soothe Nighttime | Eye lube for mice |
| Modified G8 shuttle | Invivo Analytics, Seattle, WA | NA | Shuttle for BCAM accommodation |
| Mouse anesthesia Induction box | Patterson Scientific, Waukesha, WI | 78933388 | Used for anesthetizing a mouse |
| PRE-Clip | Invivo Analytics, Seattle, WA | NA | BCAM adapter for anesthesia connection |
| Premiere Slide warmer | LabScientific, Danvers, MA | XH-2001 | Adjustable temp warmer used for tail vein dilation and anesthesia induction |
| Syringe (1 mL) | Becton, Dickinson and Company, San Diego, CA | 309659 | Used for cell injection |
| Tail Vein injection platform | Braintree Scientific, Pembroke, MA | IL-300 | Restrainer used for administering intravenous injections. |
| Vivoqaunt Software | Invicro, Needham, MA | NA | Software used for manual segmentation |
Riferimenti
- Huang, R., Wang, M., Zhu, Y., S Conti, P., Chen, K. Development of pet probes for cancer imaging. Curr Top Med Chem. 15 (8), 795-819 (2015).
- Schwenck, J., et al. Advances in pet imaging of cancer. Nat Rev Cancer. 23 (7), 474-490 (2023).
- Matthews, P. M., Rabiner, E. A., Passchier, J., Gunn, R. N. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 73 (2), 175-186 (2012).
- Tavaré, R., et al. An effective immuno-pet imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 76 (1), 73-82 (2016).
- Maresca, K. P., et al. Preclinical evaluation of 89ZR-DF-IAB22M2C PET as an imaging biomarker for the development of the gucy2c-cd3 bispecific pf-07062119 as a t-cell engaging therapy. Mol Imaging Biol. 23 (6), 941-951 (2021).
- Nisnboym, M., et al. Immuno-pet imaging of cd69 visualizes t-cell activation and predicts survival following immunotherapy in murine glioblastoma. Cancer Res Commun. 3 (7), 1173-1188 (2023).
- Lee, C., et al. Efficacy and imaging-enabled pharmacodynamic profiling of Kras g12c inhibitors in xenograft and genetically engineered mouse models of cancer. Mol Cancer Ther. 22 (7), 891-900 (2023).
- Bollineni, V., Kramer, G., Jansma, E. P., Liu, Y., Oyen, W. J. A systematic review on [18f] flt-pet uptake as a measure of treatment response in cancer patients. Eur J Cancer. 55, 81-97 (2016).
- Everitt, S., et al. Imaging cellular proliferation during chemo-radiotherapy: A pilot study of serial 18f-flt positron emission tomography/computed tomography imaging for non-non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 75 (4), 1098-1104 (2009).
- Rashidian, M., et al. Predicting the response to CTLA-4 blockade by longitudinal non-invasive monitoring of cd8 t cells. J Exp Med. 214 (8), 2243-2255 (2017).
- Klose, A. D., Paragas, N. Automated quantification of bioluminescence images. Nat Commun. 9 (1), 4262 (2018).
- Gu, Z., et al. Performance Evaluation of G8, a high-sensitivity benchtop preclinical PET/CT Tomograph. J Nucl Med. 60 (1), 142-149 (2019).
- Bongratz, F., Rickmann, A. -. M., Wachinger, C. Abdominal organ segmentation via deep diffeomorphic mesh deformations. Sci Rep. 13 (1), 18270 (2023).
- Wang, H., Stout, D. B., Chatziioannou, A. F. Mouse atlas registration with non-tomographic imaging modalities-a pilot study based on simulation. Mol Imaging Biol. 14, 408-419 (2012).
- Montgomery, M. K., et al. Mouse lung automated segmentation tool for quantifying lung tumors after micro-computed tomography. PLoS One. 16 (6), e0252950 (2021).
- Montgomery, M. K., et al. Applying deep learning to segmentation of murine lung tumors in preclinical micro-computed tomography. Transl. Oncol. 40, 101833 (2024).
- Van Sluis, J., et al. Manual versus artificial intelligence-based segmentation as a pre-processing step in whole-body pet dosimetry calculations. Mol Imaging Biol. 25 (2), 435-441 (2023).
- Nazari, M., et al. Automated and robust organ segmentation for 3D-based internal dose calculation. EJNMMI Res. 11, 1-13 (2021).
- Goel, S., England, C. G., Chen, F., Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv Drug Deliv Rev. 113, 157-176 (2017).
- Campbell, D. O., et al. Preclinical evaluation of an anti-nectin-4 immunopet reagent in tumor-bearing mice and biodistribution studies in cynomolgus monkeys. Mol Imaging Biol. 18, 768-775 (2016).
- Bednarz, B., et al. Murine-specific internal dosimetry for preclinical investigations of imaging and therapeutic agents. Health Phys. 114 (4), 450-459 (2018).

.
