Optimizing Tubulin Yield from Porcine Brain Tissue
Summary
This protocol describes a technique for the high-yield isolation of tubulin from the porcine brain optimized for small-scale instrumentation. The isolation procedures are complemented by procedures for determining tubulin polymerization activity in vitro using co-sedimentation assays and transmission electron microscopy.
Abstract
Neural tissue from any source is an excellent material for tubulin isolation, as the dendrites and axons of neurons are rich in microtubules. Here, we present a procedure for extracting tubulin that can be employed, with minor modifications, for neural tissue from multiple sources. In the presented protocol, a new clarification step of the crude lysate has been introduced, which led to a significant reduction in the initial amount of insoluble debris before the first polymerization step occurred. This additional step allowed the processing of additional tissue while using the same instrumentation and, thus, increasing the relative volume of the processed homogenate. The newly introduced step has no significant effect on the quality of purified tubulin, as confirmed by in vitro activity assays and transmission electron microscopy. The described procedure contains all crucial steps, including tissue collection, transport, tissue homogenization, tubulin isolation cycles, and final polishing by ion exchange chromatography using FPLC and subsequent activity measurement assays. The homogeneity of purified tubulin was more than 97%, as confirmed by MS/MS analysis utilizing electrospray ionization and MALDI-TOF.
Introduction
Microtubules, hollow protein filaments (24 nm in diameter) formed by alpha- and beta-tubulin heterodimers, are involved in various essential cellular processes. They participate in forming intracellular structures, motility, cell division, cell differentiation, cellular transport, shape maintenance, and secretion1. The cellular functions of microtubules can be affected by direct or indirect interactions with microtubule-associated proteins (MAPs) and other proteins or via complex post-translational modifications defined in the tubulin code2.
Tubulin fibers arise from dynamic non-covalent interactions among alpha- and beta-subunits in the nucleation-elongation mechanism. Short microtubules are formed, and the subsequent growth of tubulin fibers is achieved by reversible elongation at both ends, forming cylinders consisting of tubulin heterodimers arranged in parallel protofilaments2. Dynamic instability refers to the fact that assembled microtubules are often not in equilibrium with their subunits but can undergo phase transition between an extended period of growth and shrinkage while maintaining a steady state1,2.
The dynamic instability of tubulin fibers is principally utilized in many tubulin separation and purification procedures using cycles of high-temperature polymerization and low-temperature depolymerization in the environment of high-purity glycerol, DMSO, GTP/ATP, Mg2+, or other chemical agents (such as taxol or polycations)3. Most of the separation procedures4 are followed by protein chromatography5,6,7,8,9, which ensures the separation of tubulin-associated proteins with nucleoside diphosphokinase and ATPase activity5. Similar results can be obtained by utilizing high-salt buffers10. Multiple sources, including neural11,12,13,14 and non-neural15 tissues, fishes16 (both fresh-water and marine), yeasts, or recombinant variants17 overexpressed in different production strains11,12,13,14, and other sources9,18 were used for fractionation and purification.
The presented protocol utilizes precipitation and protein chromatography to isolate tubulin from the porcine brain into high homogeneity (Figure 1). The main advantage is a relatively high yield achieved with instrumentation available in laboratories equipped for routine molecular biology experiments.
Protocol
The composition of all solutions and instrumentation is described in the Table of Materials. All solutions were prepared using FPLC-grade chemicals and filtered through a 0.22 µm filter prior to use. Personal protective equipment, e.g., laboratory coat, gloves, and safety glasses, were used during all operations. All instruments were clean and free of traces of detergents. During the procedures, appropriate animal care guidelines (as approved by the institution) were followed. All biological material, including brain tissue, was bought from a slaughterhouse as raw material. No live animals were used at any step of the protocol.
1. Activation of a phospho-cellulose column for fast protein liquid chromatography
- Add 700 mL of 50% ethanol to 15 g of dry phospho-cellulose resin and mix it gently in the beaker with a spoon.
- Pour the suspension into a suitable chromatographic column of appropriate volume (700 mL). Close the column with pistons equipped with frits. Leave at least 10% of empty head space.
NOTE: Other vessels can be used, but the FPLC column, in combination with a peristaltic pump, significantly reduces the time necessary for resin activation and resin losses. - Incubate on a rocker-shaker at 60 rpm for 30 min.
- Let the resin settle and remove the excess of 50% ethanol using the peristaltic pump with the flow set to 3 mL/min. Do not let the resin dry.
- Remove the top piston and add 300 mL of 50% ethanol. Close the column, briefly shake it, and remove the excess ethanol via a peristaltic pump.
- Remove the remaining ethanol. Add 300 mL of ultra-pure water into the column, close the column with the piston, and tilt until the resin is resuspended. Remove excess liquid using a peristaltic pump. Repeat this step three times.
- Open the column at the top, add 500 mL of 0.5 M HCl, close the column, and resuspend the resin by gentle tilting. Incubate on a rocker-shaker at 60 rpm for 30 min.
- Remove excess liquid using a peristaltic pump. Add 300 mL of 0.5 M HCl, resuspend the resin, and remove the excess liquid.
- Remove the remaining HCl. Add 300 mL of ultra-pure water into the column, close the column with the piston, and tilt until the resin resuspends. Remove excess liquid using a peristaltic pump. Repeat this step three times.
- Pour the 700 mL of MES buffer (25 mM MES, 0.1 mM EGTA, 0.5 mM MgCl2) into the column and resuspend the resin by tilting. Pour the suspension into the beaker, adjust pH to 6.1 with 1 M NaOH, and let incubate for 2 h under gentle agitation.
- Let the resin settle, decant the liquid, add 300 mL of MES buffer, pH 6.4, and incubate overnight at 4 °C.
- Decant the excess liquid, add 96% ethanol to 20% final concentration, and store at 4 °C until use.
2. Phospho-cellulose column packing
- Pour 12 mL of activated resin into the clean chromatographic column with a closed bottom plug. Let the resin settle. The volume of settled resin is about 5 mL. Remove the resin upper layer (approximately 2-3 mm) where lighter broken matrix fragments might deposit.
- Remove the bottom plug and let the storage solution drain freely, drop by drop. Do not let the resin dry.
- Close the column and connect it to the FPLC system. Wash the column with PEM buffer (100 mM PIPES pH 6.9, 1 mM EGTA, 1 mM MgSO4) at 1 mL/min (max. pressure 1 bar) for 30 min, adjust the piston height, turn the column upside down and wash the column under the same conditions for the next 30 min in the reverse flow.
- Store the column filled with activated phospho-cellulose at 4 °C for several days.
3. Tubulin separation and purification
NOTE: Tubulin is highly prone to degradation, and it is crucial to proceed swiftly. All solutions, instruments, and equipment should be prepared, chilled, or heated up, if necessary, in advance. The procedures are sensitive to shifts in recommended temperatures. The brain tissue should be processed as soon after dissection as possible, considering the amount of biological waste produced during purification. The procedure was optimized for the rotor with six 75 mL ultracentrifugation cuvettes. The amount of processed tissue can be increased or decreased according to the available ultracentrifuge.
- Tissue transport
- Put 500 g of freshly dissected porcine brains (6-8 pieces) into a 3 L transport vessel and pour the ice-cold Transport buffer (4.1 mM MES pH 7, 320 mM Saccharose, 1 mM EGTA) until fully immersed. Let stand on ice for 5 min and change the Transport buffer for a fresh one to facilitate heat dissipation. Transport swiftly on ice for further processing.
- Tissue homogenization
NOTE: Tissue homogenization is carried out on ice in a cold room. All instruments should be prechilled to prevent spontaneous polymerization. The primary source of tubulin is grey matter, and the rest is removed in the following steps.- Pour 100 mL of Extraction buffer (4.1 mM MES pH 7, 520 mM Saccharose, 1 mM EGTA, 1 mM ATP, and 0.1 mM GTP) into a 1 L plastic beaker and determine the weight W1 (g) including the vessel. ATP and GTP are added just prior to the use.
- Take the porcine brain out of the transport vessel and remove the whole cerebellum, fat, big chunks of white matter, meninges, and blood vessels with fingers by applying modest force. Scissors or tweezers can be used as well. Place porcine brains striped of unwanted tissue into the beaker with 100 mL of cold Extraction buffer. Proceed until all tissue is processed.
- Weigh the beaker with processed brain tissue and determine W2 (g) weight.
- Add Extraction buffer according to the formula, Extraction buffer weight = W2 – W1 (e.g., for 400 g of tissue, 300 g of additional Extraction buffer is added).
- Transfer the brain tissue with Extraction buffer into the prechilled kitchen blender and process in 4-8 short, three-second pulses.
- Process the partially homogenized tissue with a high-speed dispersion homogenizer (18 000 rpm) for 1 min. Leave the suspension on ice for 3-5 min, occasionally mixing with a spoon. Repeat five times or until complete homogenization.
- Pour into prechilled ultracentrifugation tubes and spin for 10 min, at 4 °C and 50,000 x g. Keep the supernatant and discard the pellet. Repeat until 430 mL of clarified extract is collected.
- Pour the clarified extract into prechilled ultracentrifugation tubes (70 mL each) and spin at 4 °C and 75,000 x g for 60 min. Collect the supernatant and determine the volume S1.
NOTE: If only one rotor exists, heat it in a warm water bath for the next centrifugation step (37 °C).
- First polymerization
- Add 10x MEM buffer (1 M MES pH 6.8, 10 mM EGTA, 10 mM MgCl2) according to formula V = S1/9 mL (for 585 mL of supernatant, add 65 mL of 10x MEM buffer). Mix well and add glycerol to 3.5 M and GTP to 0.1 mM final concentration. Pour the suspension into ultracentrifugation tubes (the volume of supernatant exceeds the volume of tubes; the rest is discarded).
- Incubate the tubes with the supernatant in a water bath for 45 min at 37 °C.
- Spin the tubes for 90 min at 37 °C and 75,000 x g. Measure the volume of the supernatant (S2) to determine the volume of MEM buffer, add it to the pellets in step 3.3.4, and then discard the supernatant. Keep the pellet where the polymerized tubulin is contained.
- Prepare 0.5 x S2 volume of ice-cold 1x MEM buffer. Add an equal amount of 1x MEM buffer into each ultracentrifuge tube with the pellet. Resuspend pellets in the added 1x MEM buffer, transfer suspensions into the graduated cylinder, and determine the total volume.
- Add GTP to 1 mM final concentration. Transfer the suspension into a Dounce glass homogenizer and homogenize the solution every 10 min or so for 45 min on ice. The piston needs to be carefully operated as it can break easily.
NOTE: In the meantime, chill the rotor in water with ice.
- Add GTP to 1 mM final concentration. Transfer the suspension into a Dounce glass homogenizer and homogenize the solution every 10 min or so for 45 min on ice. The piston needs to be carefully operated as it can break easily.
- Pour the homogenized solution into prechilled ultracentrifugation tubes and spin at 75,000 x g and 4 °C for 60 min. After centrifugation, determine the supernatant volume (S3).
- Second polymerization
- Mix the supernatant with the 0.35 x S3 mL of glycerol and add GTP to 1 mM final concentration. Pour the supernatant into ultracentrifugation tubes and incubate for 45 min at 37 °C.
NOTE: In the meantime, warm up the rotor to 37 °C in the water bath. - Centrifuge the polymerized supernatant from step 3.4.1 for 60 min at 75,000 x g and 37 °C. Determine the volume of the supernatant (S4).
NOTE: This is an optional stopping point. The pellet with tubulin can be shock-frozen in liquid nitrogen and stored at -80 °C. - Let the frozen pellets slowly thaw on ice for 30 min. Prepare 0.25 x S4 mL of PIPES buffer (500 mM PIPES pH 6.9, 1 mM EGTA, 1 mM MgSO4, 1 mM DTT, 0.1 mM ATP), divide evenly into tubes with pellets, resuspend the pellets with a spoon, and transfer the solution into a Dounce glass homogenizer. Homogenize each 10 min on ice for 45 min.
NOTE: In the meantime, chill the rotor in water with ice. - Pour the solution with depolymerized tubulin into ultracentrifugation tubes and spin for 60 min at 4 °C and 75,000 x g.
- Mix the supernatant with the 0.35 x S3 mL of glycerol and add GTP to 1 mM final concentration. Pour the supernatant into ultracentrifugation tubes and incubate for 45 min at 37 °C.
- Third polymerization
- Determine the volume of supernatant (S5) and add S5/9 mL of DMSO. Pour the suspension into ultracentrifugation tubes and incubate for 20 min at 37 °C to polymerize.
NOTE: In the meantime, warm up the rotor to 30 °C in the water bath. - Centrifuge the polymerized tubulin for 60 min at 75,000 x g and 30 °C. Determine the volume of supernatant (S6) and keep the pellets.
- Dissolve the pellets in 0.25 x S6 mL of PEM buffer (100 mM PIPES pH 6.9, 1 mM EGTA, 1 mM MgSO4, 1 mM DTT, 0.1 mM ATP). Use a Dounce glass homogenizer to enhance solubilization. Keep adding PEM buffer until the pellets are dissolved completely (no opalescent particles are visible anymore).
- Determine the volume of supernatant (S5) and add S5/9 mL of DMSO. Pour the suspension into ultracentrifugation tubes and incubate for 20 min at 37 °C to polymerize.
- Ion exchange chromatography purification
NOTE: The reverse ion exchange chromatography is employed to remove microtubulin-associated proteins. Tubulin flows freely through the column at the given pH, and contaminating proteins are retained on the phospho-cellulose resin. All steps are performed at temperatures close to 4° C or on ice. Do not clarify the tubulin extract before FPLC purification, either by filtration or centrifugation. - Equilibrate FPLC and the column with the PEM buffer (100 mM PIPES pH 6.9, 1 mM EGTA, 1 mM MgSO4, 1 mM DTT, 0.1 mM ATP). Use at least 10 column volumes for equilibration. The pump flow should not exceed the flow used for column packing (1 mL/min).
- Load the tubulin suspension slowly (0.5 mL/min) onto the column and collect the unbound protein flowing through the resin.
- Add 10 µL of 100 mM GTP to each 1 mL fraction containing the tubulin protein.
- Freeze fractions containing tubulin in liquid nitrogen and immediately transfer them into the container with liquid nitrogen. Tubulin can be stored for several years under these conditions. The stability decreases rapidly over several months if the tubulin is stored at – 80 °C.
Representative Results
During the separation and purification steps, samples for SDS-PAGE electrophoresis were taken and subsequently analyzed using Coomassie-blue staining (Figure 2). 20 µL of each sample was mixed with 10 µL of Laemmli sample buffer (188 mM Tris-HCl pH 6.8, 3% SDS (w/w), 30% glycerol (v/v), 0.01 bromophenol blue (w/w), 15% β-mercaptoethanol) and incubated at 95 °C for 15 min. 4 µL of each sample was loaded on 12.5% acrylamide SDS gel and separated under a constant current of 30 mA per gel in reducing and denaturing conditions.
The results confirmed an incremental increase in the relative tubulin concentration accompanied by a reduction in contaminating proteins. Moreover, there was no significant loss of tubulin in clarified lysate in the first centrifugation (step 3.2.7) compared to omitting this step (Figure 2A,B).
The protein concentration was determined using three independent methods: BCA assay, Bradford protein assay, and SDS-PAGE gel densitometry analysis19 (Figure 3). The overall yield of tubulin using the procedure described was 123 mg of purified tubulin from 250 g of neural tissue. During the measurements, it is necessary to take into account that the high DTT concentration in the storage buffer has a significant impact on the BCA assay. Both the PEM buffer and PBS buffer, with the addition of DTT, increase the background absorbance by about 0.900 A595, which significantly reduces the capacity of the BCA assay (Figure 3A). The negative effect of DTT is detectable even after ten times dilution with pure water (data not shown). The Bradford assay did not seem to be affected by the storage buffer (Figure 3B), as confirmed by densitometry analysis.
The purity of tubulin preparation was verified by mass spectrometry analysis at two independent facilities (VRI Brno, Czech Republic; CEITEC MU Brno, Czech Republic) using electrospray ionization and MALDI-TOF. Both analyses confirmed the presence of porcine tubulins alpha and beta in several isoforms. The overall purity was more than 97.07% (PSMs 1065), with the most prevalent impurities originating from Homo sapiens Keratin Type II (PSMs 246, 2.24% of impurities) which were introduced most probably during the tubulin isolation and sample preparation for MS/MS analysis. Other impurities composed of 322 PSMs and only Serum albumin, Actin gamma, and Trypsinogen originating from Sus scrofa were identified with one peptide resolution (0.0069%).
In subsequent experiments, the preservation of polymerization capacity after snap freezing and storage in liquid nitrogen was verified. The 10 mg/mL aliquot was removed from liquid nitrogen and slowly thawed on ice. Samples of different concentrations (10 mg/mL, 8 mg/mL, 6 mg/mL, 4 mg/mL, and 2 mg/mL) were prepared by diluting the aliquots in PEM buffer containing DTT, ATP, and GTP for the self-assembly experiment. One dilution series was incubated at 37 °C for 60 min, and the second one was incubated on ice for 60 min. Both series were centrifuged for 60 min at 21,000 x g and the corresponding temperature (4 °C or 37 °C). 30 µL of supernatant was removed and used for SDS-PAGE. The remaining supernatant was carefully removed using a pipette and discarded. Pellets were briefly washed by adding 100 µM of PEM buffer, followed by immediate removal using a pipette. Subsequently, the pellets were resuspended in 50 µL of 1x concentrated SDS-loading buffer so that the relative concentration of pellet and supernatant is preserved. 10 µL of SDS-loading buffer was added to each supernatant. All samples were analyzed using the SDS-PAGE and Coomassie Staining (Figure 4). The volume of each sample loaded to SDS-PAGE was adjusted according to the starting concentration, so the difference in precipitation due to the concentration is more obvious. The self-assembly test of tubulin in PEM buffer confirmed the ability to form tubulin fibers in a temperature-dependent manner.
The MAP2c driven tubulin20,21 assembly assay verifying the ability of tubulin to interact with other proteins was performed22. The serial dilutions of MAP2c were prepared where 100 µL of 1 mg/mL tubulin aliquots were mixed with MAP2c to the final concentration ranging from 0 µM to 8 µM. All samples were prepared on ice by diluting in freshly prepared PEM buffer with 1 mM DTT and 1 mM GTP. The tubulin was incubated for 15 min at 37 °C with different concentrations of MAP2c, and centrifuged for 60 min at 21,000 x g and 37 °C. The final wash of the pellet was performed twice with 100 µL of PEM buffer. The experiment confirmed the capacity of the prepared tubulin to undergo MAP2c-driven polymerization, as only the sample without MAP2c did not form a pellet (Figure 5).
Transmission electron microscopy was further used to confirm the presence of tubulin filaments from co-polymerization experiments. Suspensions of purified tubulin precipitated with MAP2c were prepared for transmission electron microscopy using negative staining. The samples were adsorbed onto Formvar-coated, carbon-stabilized copper grids. The grids were then negatively stained with 2% NH4MoO4 and examined under an electron microscope at 18,000x magnification and an accelerating voltage of 80 kV. The presence of homogenous microtubules with a clear filamentous structure and appropriate size showed the ability to form microtubules in native conformation (Figure 6).
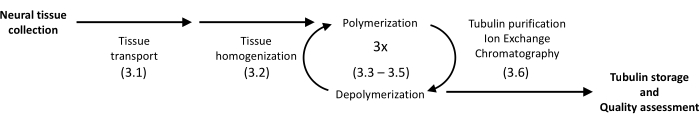
Figure 1: A schematic diagram of tubulin separation and purification. The number in parentheses refers to the protocol step. Please click here to view a larger version of this figure.
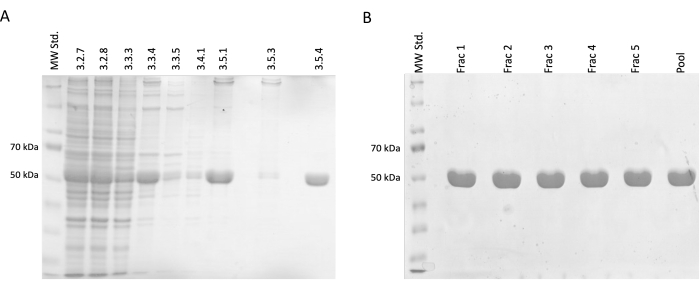
Figure 2: Tubulin separation and purification. (A) The SDS-PAGE analysis of samples taken during the separation of tubulin utilizing temperature-driven polymerization (3 µL per line). The decrease in impurities with a stable increase in relative tubulin abundance (approximately 50 kDa) is visible. The numbers above each line correspond to the step number in the protocol. (B) The SDS-PAGE analysis of fractions obtained after protein chromatography on phospho-cellulose resin (4 µL per line). Please click here to view a larger version of this figure.
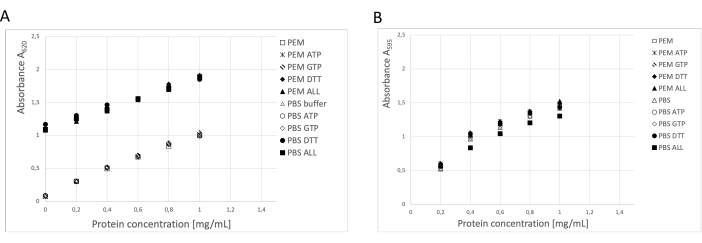
Figure 3: Protein concentration. The dilution series of bovine serum albumin of up to 1 mg/mL in PEM buffer or PBS was compared with BSA dilution series containing 0.1 ATP, 1 mM GTP, 1 mM DTT separately or in combination (0.1 mM ATP, 1 mM GTP, and 1 mM DTT) in the PEM or PBS buffer. (A) When the BCA assay was used, there was a significant shift in the background of samples containing DTT (solid symbols). (B) This effect was not detected when concentrations were measured in the Bradford assay. Please click here to view a larger version of this figure.
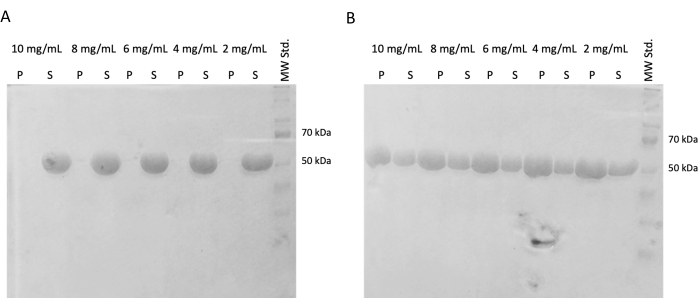
Figure 4: Tubulin self-assembly assay. The SDS-PAGE analysis of the self-assembly assay confirmed the capacity of stored tubulin incubated at either (A) 4 °C or (B) 37 °C to polymerize in a temperature-dependent manner in a broad spectrum of concentrations. (P – pellet, S – supernatant; the respective concentration of tubulin is denoted above each line pair; the amount of each sample loaded was 10 µg). Please click here to view a larger version of this figure.
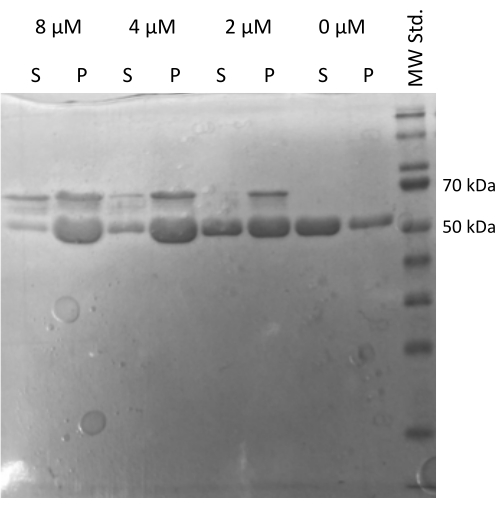
Figure 5: Tubulin co-sedimentation assay. The SDS-PAGE analysis of MAP2c-assisted tubulin assembly confirmed the capacity of stored tubulin to undergo polymerization driven by interaction with microtubule-associated protein in a concentration-dependent manner. The molar concentration of MAP2c is indicated above each line. (P – pellet, S – supernatant). Please click here to view a larger version of this figure.
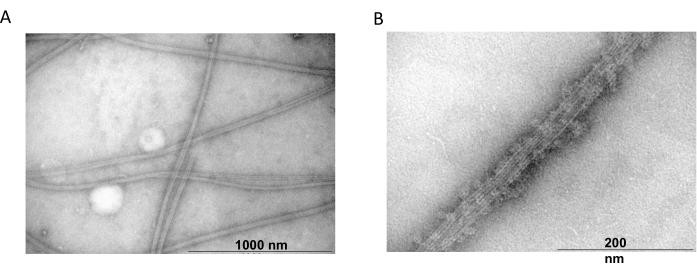
Figure 6: Transmission electron microscopy. The TEM microphotographs showed that tubulin assembles (A) into microtubules of appropriate diameter (B) consisting of homogenous tubulin filaments decorated with MAP2c proteins. The bar corresponds to 1000 nm (A) and 200 nm (B). Please click here to view a larger version of this figure.
Discussion
The neural tissue from any source is an excellent material for tubulin isolation as the dendrites and axons of neurons are rich in microtubules (up to 40%)23. Brain tissue can be relatively easily obtained in sufficient quantities. The main disadvantage might be an unspecified level of post-translational modifications, which can impact subsequent experiments24,25. Otherwise, the only concern is the rapid degradation of starting material, especially in warm conditions. We employed several transfer-buffer exchanges to enhance heat dissipation prior to the transportation and swift transport for the processing, which begins as soon as possible. The fresh tissue is dissected in conditions preventing heating and homogenized in a buffer containing GTP and glycerol, which further stabilizes tubulin26.
In the presented protocol, the clarification step (step 3.2.7) of the crude lysate has been introduced. Prolonged centrifugation before the first polymerization is generally not recommended due to tubulin susceptibility to irreversible modifications and proteases. On the other hand, the present experiments demonstrated that a short spin in high G-force reduces the debris and thus increases the relative volume of the processed homogenate without significantly impacting tubulin quality.
The exact protein concentration determination is essential for further experiments, mainly when interactions with tubulin-binding proteins or inhibitors are studied. During the investigations, we encountered significant differences in the results of protein concentration assays. The main reason was the presence of DTT, GTP, and ATP at high concentrations interfering with the assays. The BCA assay was shifted to the upper limits due to the high amount of DTT in the storage buffer, decreasing assay capacity. Moreover, the similar absorbance maxima of proteins and ATP or GTP caused inconsistencies in protein concentration determination using the absorbance at 280 nm. The same issue was noticeable in readouts from the FPLC UV detector. The most reliable assay, with stable results, was the Bradford protein assay, where no influence of buffer compounds was observed. Nevertheless, it is essential to prepare the protein standard dilution series in the storage buffer.
The ability of purified tubulin to create appropriate arrangements is a prerequisite for subsequent experiments. Tubulin is, by nature, highly prone to degradation even in the glycerol-GTP-rich environment, resulting in a reduced capacity to form homogenous filaments. It is critical to follow the purification process and experimentally verify the ability of stored tubulin to polymerize into stable regular filaments. Several independent methods verifying tubulin state have been introduced in vivo and in vitro. Among the most prominent ones, the circular dichroism spectroscopy27, surface plasmon resonance assay28, thermal shift assay29, polymerization inhibition assay30, immunofluorescence staining31,32, coprecipitation22,33 and transmission electron microscope analysis32 can be mentioned. The polymerization assay and coprecipitation of tubulin used in this protocol are easy to perform. They can be swiftly evaluated by either the pellets occurring at the bottom of the tube or using SDS-PAGE. On the other hand, the precipitated tubulin can be in the form of aggregates. More sophisticated methods, such as transmission electron microscopy, must be included to confirm the presence of microtubule fibers for quality control.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The study was supported by Technology Agency of the Czech Republic (project nr. TN02000017 – National Centre for Biotechnology in Veterinary Medicine – NaCeBiVet).
Materials
| 1.5 mL tubes | — | — | Common material |
| 10 mL tubes | — | — | Common material |
| 50 mL tubes | — | — | Common material |
| ATP | ROTH | HN35.2 | Analytical grade |
| DMSO | ROTH | A994.2 | Analytical grade |
| Dounce glass homogenizer | P-LAB | H244043 | Homogenizer |
| DTT | ROTH | 6908.1 | Analytical grade |
| EGTA | ROTH | 3054.3 | Analytical grade |
| Ethanol | PENTA | 70390-11001 | Analytical grade |
| Glycerol | ROTH | 6967.2 | Analytical grade |
| Graduated beakers | — | — | Common equipment |
| Graduated cilinders | — | — | Common equipment |
| GTP | MERCK | 36051-31-7 | Very high quality |
| HCl | PENTA | 19360-11000 | Analytical grade |
| Izolated box for tissue transport | — | — | Common equipment |
| Kitchen blender | Waring | 7011HB | Glass or plastic vessel |
| Liquid nitrogen | — | — | Common material |
| MES | ROTH | 6066.4 | Analytical grade |
| MgCl2 | MERCK | 814733 | Analytical grade |
| MgSO4 | PENTA | 43180-31000 | Analytical grade |
| NaOH | PENTA | 15650-11000 | Analytical grade |
| Optima XPN100 | Beckman Coulter | A94469 | Ultracentrifuge |
| Phosphocellulose column | VWR | GENO786-1291 | Empty column |
| Phosphocellulose resin | Creative – Biomart, inc | Phosphate-001C | Ion exchange resin |
| PIPES | ROTH | 9156.2 | Analytical grade |
| Saccharose | PENTA | 24970-31000 | Analytical grade |
| Scales | — | — | Common equipment |
| Scissors | — | — | Common equipment |
| Spoons | — | — | Plastic or glass |
| Ti45 rotor | Beckman Coulter | 339160 | Rotor for Ultracentrifuge |
| Tweezers | — | — | Common equipment |
| Ultra turrax IKA T18 basic | IKA | 356 1000 | Laboratory dispenser |
| Water bath 37 °C | — | — | Stirred |
Riferimenti
- Verhey, K. J., Gaertig, J. The tubulin code. Cell Cycle. 6 (17), 2152-2160 (2007).
- Murphy, D. B., Hiebsch, R. R. Purification of microtubule protein from beef brain and comparison of the assembly requirements for neuronal microtubules isolated from beef and hog. Anal Biochem. 96 (1), 225-235 (1979).
- Borisy, G. G., Marcum, J. M., Olmsted, J. B., Murphy, D. B., Johnson, K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann NY Acad Sci. 253, 107-132 (1975).
- Weisenberg, R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 177 (4054), 1104-1105 (1972).
- Roychowdhury, S., Gaskin, F. Separation of assembly-competent tubulin from brain microtubule protein preparations using a fast-performance liquid chromatography procedure. J Neurochem. 46 (5), 1399-1405 (1986).
- Weingarten, M. D., Suter, M. M., Littman, D. R., Kirschner, M. W. Properties of the depolymerization products of microtubules from mammalian brain. Biochimica. 13 (27), 5529-5537 (1974).
- Souphron, J., et al. Purification of tubulin with controlled post-translational modifications by polymerization-depolymerization cycles. Nat Protoc. 14 (5), 1634-1660 (2019).
- Jacobs, M., Huitorel, P. Tubulin-associated nucleoside diphosphokinase. Eur J Biochem. 99 (3), 613-622 (1979).
- Munguía, B., et al. Purification of native M. vogae and H. contortus tubulin by TOG affinity chromatography. Exp Parasitol. 182, 37-44 (2017).
- Castoldi, M., Popov, A. V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein ExpPurif. 32 (1), 83-88 (2003).
- Minoura, I., et al. Overexpression, purification, and functional analysis of recombinant human tubulin dimer. FEBS Lett. 587 (21), 3450-3455 (2013).
- Liu, C., Yao, J., Yin, J., Xue, J., Zhang, H. Recombinant α- and β-tubulin from Echinococcus granulosus: Expression, purification and polymerization. Parasite. 25, 62 (2018).
- Ti, S. -. C., Wieczorek, M., Kapoor, T. M. Purification of affinity tag-free recombinant tubulin from insect cells. STAR protoc. 1 (1), 100011 (2020).
- Bodakuntla, S., Jijumon, A. S., Janke, C., Magiera, M. M. Purification of tubulin with controlled post-translational modifications and isotypes from limited sources by polymerization-depolymerization cycles. JVisExp. (165), e61826 (2020).
- Sackett, D. L., Werbovetz, K. A., Morrissette, N. S. Isolating tubulin from nonneural sources. Methods Cell Biol. 95, 17-32 (2010).
- Modig, C., Strömberg, E., Wallin, M. Different stability of posttranslationally modified brain microtubules isolated from cold-temperate fish. Mol Cell Biochem. 130 (2), 137-147 (1994).
- Fourest-Lieuvin, A. Purification of tubulin from limited volumes of cultured cells. Protein Exp Purif. 45 (1), 183-190 (2006).
- MacRae, T. H., Gull, K. Purification and assembly in vitro of tubulin from Trypanosoma brucei. Biochem J. 265 (1), 87-93 (1990).
- Jansen, S., et al. Quantitative mapping of microtubule-associated protein 2c (MAP2c) phosphorylation and regulatory protein 14-3-3ζ-binding sites reveals key differences between MAP2c and its homolog Tau. JBiol Chem. 292 (24), 3-3 (2017).
- Melková, K., et al. Structure and functions of microtubule associated proteins Tau and MAP2c: Similarities and differences. Biomolecules. 9 (3), E105 (2019).
- Herzog, W., Weber, K. Fractionation of brain microtubule-associated proteins: Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 92 (1), 1-8 (1978).
- Castle, A. G., Crawford, N. Isolation of tubulin from pig platelets. FEBS Lett. 51 (1), 195-200 (1975).
- Wloga, D., Joachimiak, E., Fabczak, H. Tubulin post-translational modifications and microtubule dynamics. Int J Mol Sci. 18 (10), E2207 (2017).
- Lafanechère, L., Job, D. The third tubulin pool. Neurochem Res. 25 (1), 11-18 (2000).
- Arai, T., Ihara, Y., Arai, K., Kaziro, Y. Purification of tubulin from bovine brain and its interaction with guanine nucleotides. J Biochem. 77 (3), 647-658 (1975).
- Mahaddalkar, T., et al. Structural investigations into the binding mode of a novel noscapine analogue, 9-(4-vinylphenyl) noscapine, with tubulin by biochemical analyses and molecular dynamic simulations. J Biomol Struct Dyn. 35 (11), 2475-2484 (2017).
- Chen, H., et al. Structure-activity relationship study of novel 6-Aryl-2-benzoyl-pyridines as tubulin polymerization inhibitors with potent antiproliferative properties. J Med Chem. 63 (2), 827-846 (2020).
- Yao, D., et al. Ferulin C triggers potent PAK1 and p21-mediated anti-tumor effects in breast cancer by inhibiting Tubulin polymerization in vitro and in vivo. Pharmacol Res. 152, 104605 (2020).
- Sweetnam, P. M., et al. The role of receptor binding in drug discovery. JNat Prod. 56 (4), 441-455 (1993).
- Qi, Z. -. Y., et al. Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors. Bioorg Chem. 94, 103392 (2020).
- Sáez-Calvo, G., et al. Triazolopyrimidines are microtubule-stabilizing agents that bind the vinca inhibitor site of tubulin. Cell Chem Bio. 24 (6), 737-750 (2017).
- Bellocq, C., et al. Purification of assembly-competent tubulin from Saccharomyces cerevisiae. EurJ Biochem. 210 (1), 343-349 (1992).

