Subcellular Fractionation for the Isolation of Synaptic Components from the Murine Brain
Summary
This protocol presents a robust, detailed method to obtain highly pure synaptosomes, synaptic vesicles, and other synaptic fractions from the mouse brain. This method enables the evaluation of synaptic processes, including the biochemical analysis of protein localization and function with compartmental resolution.
Abstract
Synaptic terminals are the primary sites of neuronal communication. Synaptic dysfunction is a hallmark of many neuropsychiatric and neurological disorders. The characterization of synaptic sub-compartments by biochemical isolation is, therefore, a powerful method to elucidate the molecular bases of synaptic processes, both in health and disease. This protocol describes the isolation of synaptic terminals and synaptic sub-compartments from mouse brains by subcellular fractionation. First, sealed synaptic terminal structures, known as synaptosomes, are isolated following brain tissue homogenization. Synaptosomes are neuronal pre- and post-synaptic compartments with pinched-off and sealed membranes. These structures retain a metabolically active state and are valuable for studying synaptic structure and function. The synaptosomes are then subjected to hypotonic lysis and ultracentrifugation to obtain synaptic sub-compartments enriched for synaptic vesicles, synaptic cytosol, and synaptic plasma membrane. Fraction purity is confirmed by electron microscopy and biochemical enrichment analysis for proteins specific to sub-synaptic compartments. The presented method is a straightforward and valuable tool for studying the structural and functional characteristics of the synapse and the molecular etiology of various brain disorders.
Introduction
Synapses are the basic computational units of the brain through which neurons communicate and exert diverse and exquisitely complex functions. Synapses are, thus, fundamental to the health of the brain1; synaptic dysfunction is implicated as a source or result of many disorders2. Synapses are constituted by pre- and post-synaptic terminals, extensions of two different neurons that are closely apposed and separated by a synaptic cleft traversed by synaptic adhesion molecules. Information flows from the pre- to post-synaptic compartment in the form of chemical messengers called neurotransmitters1. The molecular processes involved in neurotransmission are active areas of research3,4,5. Understanding the pathogenic processes within synaptic terminals and the response of synapses to pathology in other neuronal sub-compartments are crucial steps to addressing disorders of the brain1,2. Several methodological advancements, predominantly applied to murine models, have advanced this pursuit6. The isolation of synaptic fractions by differential centrifugation is one such paradigm-shifting method that has enabled the detailed evaluation of synaptic processes in health and disease.
The adult human brain consists of 80-90 billion neurons7,8. Among murine species, the rat brain contains approximately ~200 million neurons, while mice have ~70 million9,10. Each neuron forms thousands of specific synaptic connections with a network of highly polarized neurons intermingled with glial cells and dense vasculature. In such complex and heterogeneous tissue, it was once unthinkable to isolate and study synapses as an independent system. In the 1960s, Victor Whittaker, Catherine Hebb, and others made this possible by isolating intact synaptic terminals using subcellular fractionation11,12,13,14. In an attempt to isolate synaptic vesicles (SVs), they homogenized brains through liquid shear force in iso-osmotic (0.32 M) sucrose followed by ultracentrifugation. They obtained pinched-off, plasma membrane-enclosed, intact nerve terminals or varicosities, which they called nerve-ending particles (NEPs)11,13. As the structural and functional characteristics of the synapse were preserved in these structures, NEPs were later termed "synaptosomes" for congruence with other subcellular organelles13,15. It is worth noting that the work of Eduardo de Robertis and colleagues, who coined the term "synaptic vesicle", overlapped with that of Whittaker and colleagues and contributed to the validation of "synaptosome" isolation and characterization16,17,18.
Synaptosomes are physiologically active structures that contain all the cellular and molecular properties required for the storage, release, and reuptake of neurotransmitters13,18. The preservation of key synaptic characteristics in vitro and freedom from non-synaptic components also contribute to the utility of this isolation method. Synaptosomes have contributed immensely to the understanding of the chemical and physiological properties of neurotransmission and are now being used to study synaptic molecular processes and their alterations in disease19,20,21,22,23. Synaptosomes are also the initial source material for isolating synaptic components such as SVs, clathrin-coated vesicles (CCVs), synaptic cytosol, synaptic plasma membrane, synaptic mitochondria, synaptic adhesion molecules, and other components of interest, which can facilitate the understanding of the molecular mechanisms of synaptic function18,19,20,24,25,26,27,28. These sub-synaptic components can be obtained by the osmotic lysis of synaptosomes and sucrose density gradient ultracentrifugation15,29. Although the original subcellular fractionation method by Whittaker's research group is known to be efficient in isolating quality synaptosomes and SVs13,30, recent optimizations enhance the purity of the subcellular fractions22,23,31,32. This article provides a highly detailed and accessible version of a classic protocol for the subcellular fractionation of murine brain tissue to isolate synaptosomes, SVs, and other sub-synaptic components.
Protocol
All experiments with mice were approved by the Institutional Animal Care and Use Committee (IACUC) at Yale University (Protocol 2021-11117) and performed in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal care and housing complied with the Guide for the Care and Use of Laboratory Animals33 and were provided by the Yale Animal Resource Center (YARC). Animals were maintained in a 12 h light/dark cycle with ad libitum access to food and water. Five to eight mice or two to four rats per genotype or condition are required for the following protocol. Fewer rats are necessary due to their larger brain volumes. Similarly, the age of the experimental animals may affect fraction yield; additional mice may be required for ages less than 2 months. Otherwise, the outlined procedures apply to both murine species and healthy adult animals of any age. The representative data presented in this study utilized wild-type (C57BL/6J) mice (age = 2 months; four males and four females per replicate) obtained from a commercial source (see Table of Materials).
1. Experimental preparation
NOTE: This protocol requires ~11 h for a single researcher to complete. It is highly recommended to complete benchtop setup (Figure 1), buffer preparation (Table 1), the precooling of centrifuges and rotors to 4 °C, and the collection and labeling of necessary materials and equipment (see Table of Materials) the day prior to protocol execution, where applicable.
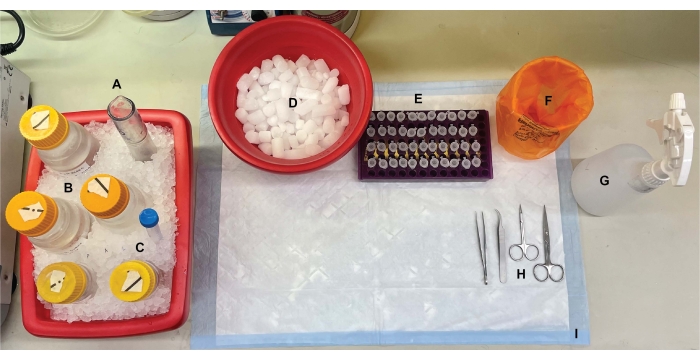
Figure 1: Benchtop setup. Prior to brain dissections, (A) Dounce glass homogenizers and (B) all buffers were chilled on ice. (C) Protease inhibitor stock solutions were thawed on ice. A second container of wet ice for centrifuge tubes, a Dewar of liquid nitrogen (not shown), and (D) a container of dry ice for short-term storage of the samples flash-frozen in liquid nitrogen were obtained. (E) Microcentrifuge tubes were pre-labeled for all samples, as four aliquots of each subcellular fraction sample per genotype or condition were collected during this procedure (time-saving tip: thoroughly label all the tubes the day before the experiment is performed). (F) An appropriate biohazard waste container, (G) 70% ethanol, (H) surgical tools, and (I) an absorbent surface pad. The required centrifuge tubes and disposables were set aside for efficient access during protocol implementation (not shown). Please click here to view a larger version of this figure.
- Prepare the benchtop for surgery and collect the scissors and forceps required for brain excision (see Table of Materials). Pre-label 1.5 mL microcentrifuge tubes for mouse tail biopsies and four tubes per collected fraction, as outlined in Figure 2.
- Obtain two containers of wet ice, one container of dry ice, and a benchtop liquid nitrogen Dewar flask.
- Thaw phenylmethylsulfonyl fluoride (PMSF), pepstatin A, aprotinin, and leupeptin stock solutions on ice (see Table of Materials). Prepare the necessary buffers (Table 1).
NOTE: Sucrose solutions can be prepared in advance and stored at 4 °C. However, protease inhibitors (thawed stocks and tablets) must be added fresh to all the buffers at the start of the experiment due to the instability of these reagents in aqueous solutions. Further, all the buffers must be prepared with detergent-free glassware and detergent-free water to enable the collection of intact synaptosomes. - Chill all the buffers and glass Dounce homogenizers (see Table of Materials) on ice. Set the centrifuges to 4 °C and chill the rotors to 4 °C.
- Add 14 mL of Buffer A (Table 1) to a Dounce homogenizer on ice.
Table 1: Composition of the subcellular fractionation buffers. Please click here to download this Table.
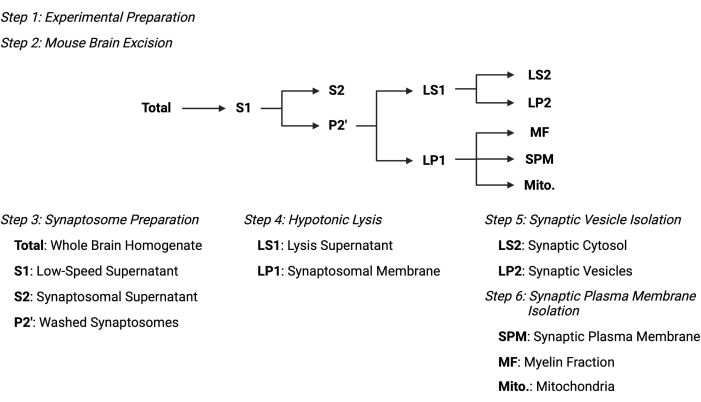
Figure 2: Overview of the subcellular fractionation protocol. Summary schematic of the subcellular fractionation steps and collected samples. Please click here to view a larger version of this figure.
2. Mouse brain excision
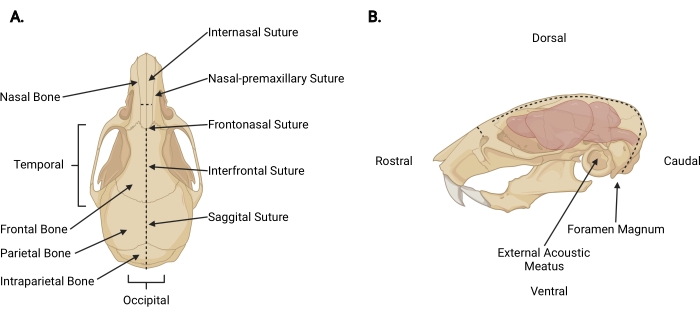
Figure 3: Craniofacial anatomy. (A) Dorsal view of a mouse skull with relevant cranial structures indicated. (B) Left lateral view of a mouse skull and brain with relevant cranial structures and anatomical directions indicated. The dashed lines represent the locations where incisions should be made. Please click here to view a larger version of this figure.
- Deeply anesthetize each mouse with 100% isofluorane in an anesthesia chamber located in a fume hood or biosafety cabinet using an open drop method34. Sacrifice each mouse by cervical spine dislocation followed swiftly by decapitation. Alternate between genotypes or experimental groups for each sacrifice and dissection12. Obtain tail biopsies after euthanasia by excising 2 mm of the distal tail tip with fine scissors. Store the tissue for genotyping.
- Spray the decapitated head with 70% ethanol to prevent hair from adhering to the tissue and surgical instruments during dissection.
- Insert fine scissors under the skin at the decapitation incision to a pericranial depth and make a midsagittal incision up to the internasal suture (Figure 3A) to retract the scalp from the skull.
- Working from the occipital area toward each temporal aspect, trim the fascia and muscle to expose the external surface of the skull beyond each external acoustic meatus (Figure 3B).
- Secure the scalp and rostral aspect of the skull with the non-dominant hand. With the other, insert fine scissors 2 mm into the caudal side of the foramen magnum, where the spinal cord is visible exiting. Make a midline incision until the scissors reach the internal surface of the intraparietal bone (Figure 3; dashed lines).
NOTE: During the initial incision, the scissors must be parallel to the spinal cord with pressure applied toward the internal surface of the skull to prevent damage to the brainstem and cerebellum. - Change the angle of the scissors so the blades run parallel with the dorsal surface of the skull. Continue advancing the midsagittal incision rostrally through the parietal and frontal bones, using the sagittal and interfrontal sutures as a guide. Use constant upward pressure to avoid damage to the cortex. Terminate the incision just beyond the internasal suture (Figure 3A).
- Make a small perpendicular incision (~3 mm) to the nasal bone, rostral to the internasal suture, by placing the scissors perpendicular to the skull with each blade positioned at a nasal-premaxillary suture and making one even cut (Figure 3; dashed lines).
NOTE: This step will increase the ease of retracting the skull and will be critical for collecting the olfactory bulb if this area is of interest. - While securing the rostral aspect, use one side of a pair of textured forceps to gently lift the skull up from the brain, then laterally and ventrally. Repeat along the midline as needed, then for the other hemisphere until the entire brain surface is exposed.
- Using curved forceps or a fine spatula, gently lift the rostral side of the brain. Cut the optic and cranial nerves to complete the excision from the skull.
- For each condition, collect five to eight mouse brains together into the chilled glass Dounce homogenizer containing 14 mL of Buffer A (Table 1).
3. Synaptosome preparation
NOTE: The schematics of this procedure are shown in Figure 4.
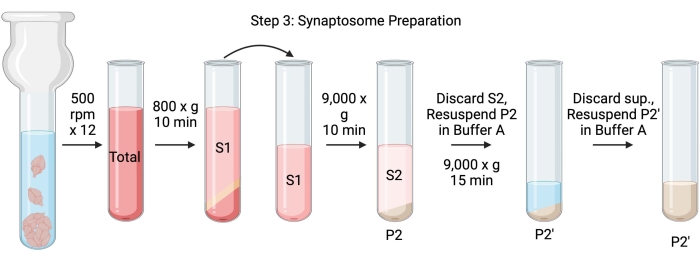
Figure 4: Synaptosome preparation. Schematic of step 3, the generation of synaptosomes (P2'). Please click here to view a larger version of this figure.
- Homogenize the brains using a glass Dounce homogenizer in 12 up-down passes at 500 rpm (total). Pause briefly at each downstroke to ensure thorough homogenization of the tissue. Homogenize preferentially in an ice bath to avoid warming and protein denaturation. Take 5 µL aliquots for protein concentration determination by the bicinchoninic acid assay (BCA, see Table of Materials). Take 100 µL of whole brain lysate aliquots for western blot (WB). For this and all subsequent samples (Figure 2), take two aliquots for BCA and two aliquots for WB. Flash-freeze all the collected aliquots in liquid nitrogen and store them at −80 °C.
- Spin the total brain homogenate in a high-speed round bottom centrifuge tube (14 mL) (see Table of Materials) at 800 x g for 10 min at 4 °C to obtain the supernatant (S1). Transfer S1 to a new centrifuge tube, leaving the pellet behind (P1), which contains intact cells and nuclei. Avoid pipetting up the fluffy, white, loose, superficial pellet. Take 2 x 5 µL of S1 for BCA and 2 x 100 µL of S1 for WB.
- Spin S1 at 9,000 x g for 15 min at 4 °C to obtain the synaptosomal supernatant (S2) and crude synaptosome pellet (P2). Take 2 x 10 µL of S2 for BCA and 2 x 500 µL of S2 for WB. Discard the supernatant after obtaining aliquots and proceed to the next step with the pellet.
- Resuspend P2 in 3 mL of ice-cold Buffer A with protease inhibitors and centrifuge at 9,000 x g for 15 min at 4 °C to obtain the supernatant (S2') and washed synaptosomes (P2'). Discard the supernatant and keep the pellet.
- Resuspend P2' in 3 mL of Buffer A. Avoid resuspending the dark red portion at the bottom of the pellet, which mainly contains mitochondria. Take 2 x 20 µL of P2' for BCA and 2 x 100 µL of P2' for WB.
NOTE: This can be achieved by gently pipette-mixing the edges and surface of the pellet to resuspend the white washed synaptosomes while directing the pipette tip away from the red center of the pellet.
4. Hypotonic lysis
NOTE: The schematics of this procedure are shown in Figure 5.
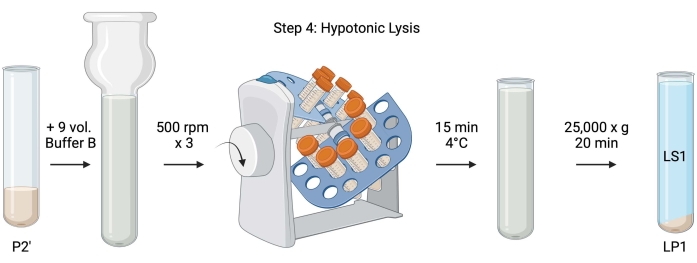
Figure 5: Hypotonic lysis. Schematic of step 4, the hypotonic lysis of synaptosomes to generate the lysis supernatant (LS1) and synaptosomal membrane fractions (LP1). Please click here to view a larger version of this figure.
- For the hypotonic lysis of washed synaptosomes, add 9 volumes of chilled Buffer B (Table 1) to resuspended P2' (~27 mL). Homogenize the synaptosomes in a glass Dounce homogenizer (three up-down passes at 500 rpm).
- Transfer the samples to 50 mL capped conical centrifuge tubes. Rotate them on a tube revolver in a 4 °C cold room for 15 min.
- Centrifuge lysed P2' at 25,000 x g for 20 min at 4 °C to obtain the lysis supernatant (LS1) and the lysis pellet containing synaptosomal membranes (LP1). Take 2 x 50 µL of LS1 for BCA and 2 x 400 µL of LS1 for WB. Transfer LS1 into a capped centrifuge tube for ultracentrifugation (see Table of Materials).
5. Synaptic vesicle isolation
NOTE: The schematics of this procedure are shown in Figure 6.
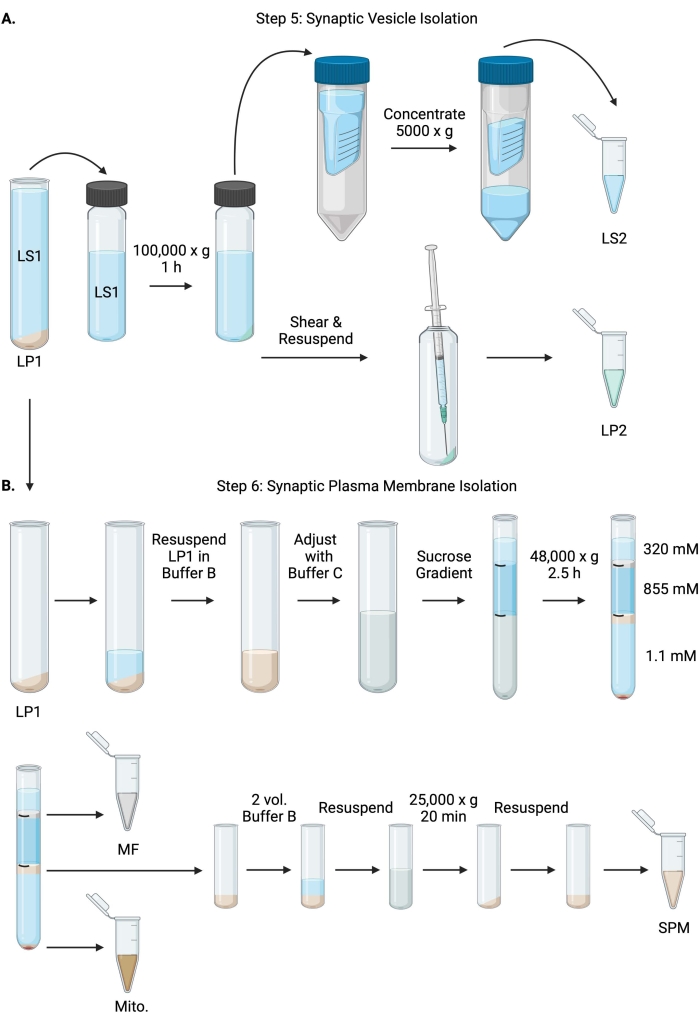
Figure 6: Synaptic vesicle isolation and synaptic plasma membrane isolation. (A) Schematic of step 5, the isolation of synaptic cytosol (LS2) and synaptic vesicle (LP2) fractions, and (B) step 6, the generation of myelin (MF), synaptic plasma membrane (SPM), and mitochondrial (Mito.) fractions following the ultracentrifugation of sucrose gradients. Please click here to view a larger version of this figure.
- Centrifuge LS1 in a fixed angle ultracentrifuge rotor (see Table of Materials) at 100,000 x g for 60 min at 4 °C to obtain synaptic cytosol supernatant (LS2) and synaptic vesicle pellet (LP2). LP2 will be small, translucent, and strongly adhered to the side of the centrifuge tube.
- Resuspend LP2 in 500 µL of Buffer A. Using a 23 G needle and a 1 mL syringe, shear LP2 with gentle trituration. Take 2 x 10 µL of LP2 for BCA and 2 x 250 µL of LP2 for WB.
- Transfer LS2 (~30 mL) to centrifugal filter units with a 10 kDa cutoff (see Table of Materials).
NOTE: If proteins smaller than 10 kDa are of interest, 4 kDa cutoff centrifugal filter units are available but will result in longer spin times. - Concentrate LS2 to approximately 0.5 mL by spinning at 5000 x g for up to 1 h at 4 °C. Take 2 x 10 µL of concentrated LS2 for BCA and 2 x 250 µL of concentrated LS2 for WB. After starting the spin, proceed directly to step 6.1.
6. Synaptic plasma membrane isolation
- Resuspend LP1 (step 4.3) in 1 mL of Buffer B (Table 1). Take 2 x 10 µL of LP1 for BCA and 2 x 50 µL of LP1 for WB. Adjust the remaining LP1 to a final volume of 7.5 mL and a final sucrose concentration of 1.1 M with Buffer B and Buffer C (Table 1).
- Transfer 7.5 mL of resuspended LP1 into a 14 mL ultracentrifuge tube (see Table of Materials). Carefully overlay LP1 with 3.75 mL of Buffer D (Table 1), and then overlay with 1.25 mL of Buffer A (or a larger volume to fill just below the top of the centrifuge tube). Avoid pipetting down the side of the tube, which will disrupt the sucrose gradient interfaces. After overlaying each sucrose fraction, mark the top of the solution with a pen. Balance the tubes for ultracentrifugation by weight, not volume, with the dropwise addition of Buffer A to within 10 mg. Centrifuge at 48,000 x g for 2.5 h at 4 °C in a swinging bucket ultracentrifuge rotor (see Table of Materials).
- Acquire images of the intact gradients following ultracentrifugation to document the distinctness of each sucrose interface and the success of fractionation.
- Carefully remove the superficial layer of 320 mM sucrose (Buffer A). Recover the myelin fraction (MF) at the 320 mM/855 mM sucrose interface in an 800 µL volume. Recover the synaptic plasma membrane (SPM) fraction at the 855 mM/1.1 M sucrose interface in a 1,000 µL volume. Pipette each fraction up from the wall of the tube in a circular manner to ensure the complete fraction is collected. Carefully aspirate off the remaining sucrose and recover the mitochondrial pellet (Mito.) by resuspending in 200 µL of Buffer B. Take 2 x 100 µL of MF for BCA and 2 x 10 µL of Mito. for BCA; divide the remainder of MF and Mito. samples in half for WB.
- Dilute the SPM fraction with 2 volumes of Buffer B (~2 mL), and then centrifuge in a fixed angle rotor in a 3.5 mL centrifuge tube (see Table of Materials) at 25,000 x g for 20 min at 4 °C. Discard the supernatant and resuspend the SPM pellet in Buffer A for a final volume of 250 µL. Take 2 x 5 µL of SPM for BCA and divide the remaining SPM in half for WB.
- Perform a BCA to determine the protein concentration of each sample, accounting for variable aliquot volume.
NOTE: For WB analysis, the suggested working protein concentration for all subcellular fractions is 2 µg/µL (or as high as achievable for LS1 and MF).
Representative Results
The presented method results in 11 brain subcellular fractions that can be subjected to further purification and various forms of downstream analysis35,36. The gold standard method to assess the quality of synaptosomes, SVs23, and other components is electron microscopy (EM) (Figure 7). Quantitative immunoblotting for proteins that are present in specific subcellular fractions can also be performed to assess markers of fraction purity (Figure 8). For example, immunoblot analysis of fractions reveals the enrichment of N-cadherin (CDH2, UniProt name) in the synaptic plasma membrane fraction (SPM), α-synuclein (SYUA) in the synaptic cytosol (LS2), synaptophysin (SYPH) in the synaptic vesicle fraction (LP2), and myelin basic protein (MBP) in the myelin fraction (MF) when compared to protein levels in the initial whole brain homogenate (Total) (Figure 8). Once fraction purity has been established (for example, note the absence of CDH2 in the LS2 fraction or the many-fold increase in SYPH in the LP2 fraction), quantitative immunoblotting can be used to determine the localization of proteins of interest or query differences in protein distribution between genotypes or treatments. Understanding the subcellular localization of synaptic proteins can enable the dissection of previously undescribed protein functions. Further, this method may elucidate trafficking defects or synaptic dysfunction in disease states, especially when paired with functional assays. For example, our team has used this method to identify a pool of enzymatically active palmitoyl protein thioesterase 1 that is enriched in the synaptic cytosol19.
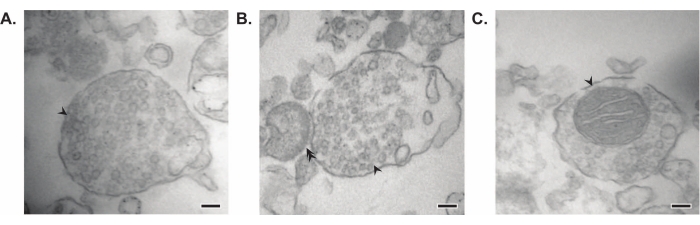
Figure 7: Electron microscopy (EM) of synaptosomes. (A) Representative EM image of a synaptosome containing synaptic vesicles (arrow). (B) Representative EM image of a synaptosome with both pre- (arrow) and post-synaptic components (double arrow). (C) Representative EM image of a synaptosome containing synaptic vesicles and a mitochondrion (arrow) (scale bars = 100 nm). Please click here to view a larger version of this figure.
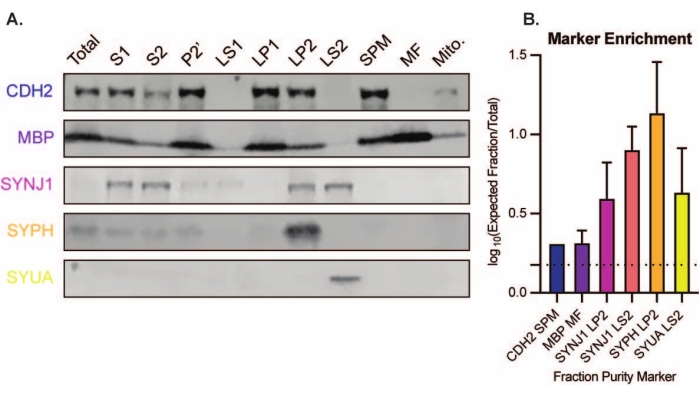
Figure 8: Immunoblot analysis of subcellular fractions. (A) Markers of subcellular fraction purity (indicated with UniProt nomenclature) are appropriately localized compared to the whole brain homogenate (total): N-cadherin (CDH2) in the synaptic plasma membrane fraction (SPM), synaptophysin 1 (SYPH) and synaptojanin 1 (SYNJ1) in the synaptic vesicle enriched fraction (LP2), α-synuclein (SYUA) in the synaptic cytosol (LS2), and myelin basic protein (MBP) in the myelin fraction (MF). (B) Immunoblot quantification analysis reveals the enrichment (fold-change from total) of fraction purity markers. Data are represented as mean ± standard deviation on a log10 scale. The dotted line indicates a 1.5-fold change (y = 0.176) (n = 3 replicate experiments with 8 wild-type mice; age = 2 months; n = 4-5 blots for SYPH, SYUA, MBP, with n = 3 plotted values previously published by Gorenberg et al.19; n = 5 for SYNJ1; n = 1 for CDH2). Please click here to view a larger version of this figure.
Discussion
In their seminal studies, Whittaker and colleagues used four morphological criteria to identify synaptosomes: (1) the structures have a sealed plasma membrane; (2) the structures contain SVs resembling those in nerve terminals and varicosities in situ in size and number; (3) the structures possess one or more small mitochondria; and (4) the presynaptic membrane is frequently adhered to a post-synaptic component11,12,13. Though the first two criteria generally apply to every isolation method, in the most recent protocols described in this article, not all resulting synaptosomes will have mitochondria and attached post-synaptic terminals. Approximately 60% of the synaptosomes will have mitochondria, and only up to 15% are estimated to have attached post-synaptic terminals37. If post-synaptic components are of particular interest, the use of an isotonic Krebs-like homogenization buffer and pressure filtration for enrichment are known to yield high concentrations of synaptosomes with post-synaptic terminals (also termed synaptoneurosomes)22,38.
The method of sacrificing the animal can impact the quality of synaptosomes and synaptic subfractions. Adult animals sacrificed using a euthanasia method that does not require anesthesia will result in the best fraction quality. Further, the brains should be freshly dissected, not frozen, and homogenized using a 1:10 ratio of homogenization buffer (weight/volume) for the most viable synaptic fractions22. The brain has a heterogeneous population of synapses that can be differentiated by the type of neurotransmitters they carry. Synaptosome formation is generally unaffected by synapse type or neurotransmitter content13. An exception is mossy fibers in the cerebellum, which are known to be disrupted in optimal conditions for obtaining synaptosomes from the rest of the brain39,40. Thus, removal of the cerebellum prior to brain homogenization is recommended if the exclusion of this region does not affect the experimental goal. If interested in isolating synaptosomes of a particular neurotransmitter character, areas of the brain that are enriched for neurons containing the neurotransmitter of interest can first be isolated. However, this approach will impose limitations on the final fraction yield, depending on the size of the region of interest (the age of animals is also, therefore, a consideration). There are immunochemical methods for the isolation of neurotransmitter-specific synaptosomes, but the viability and yield will be significantly compromised22. If assessing synaptosome metabolic viability is important, the measurement of neurotransmitter release41,42 or certain enzymatic assays43 can be employed.
Common contaminants in synaptosome preparations include microsomes, free mitochondria, SVs, and neuronal and glial membranes. Contamination can be reduced by increasing the number of washes at the P1 and P2 fractions22 and avoiding resuspension of the red mitochondrial pellet in subsequent steps. In experiments where metabolic viability and time are crucial, reducing the number of washes and using Ficoll or Percoll gradients over sucrose gradients will be helpful44,45,46. These methods also reduce contamination significantly. Whittaker's original protocol yielded high-quality SVs. Further optimization by Nagy et al.23, included in this method, produces SVs with remarkable homogeneity and purity without compromising significantly on yield36. If specific SV subtypes are of interest, such as glutamatergic (VGLUT-1-containing) or GABAergic (VGAT-1-containing) SVs, immunoisolation using specific antibodies can be performed47,48. Alternative methods are also available for isolating CCVs from synaptosomes, which, due to differential density, may not be present at the same interface as SVs obtained with this method20,49,50.
Overall, the present protocol to isolate synaptic components can be further optimized to obtain fractions with improved homogeneity and viability based on the quality and quantity of the source brain tissue and the experimental goals. For further troubleshooting details, one should refer to book chapters by Dunkley and Robinson22 and Ganzella et al.36.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We would like to thank P. Colosi for EM image preparation. This work was supported by the National Institutes of Health (R01 NS064963, SSC; R01 NS110354, SSC; R01 NS083846, SSC; R21 NS094971, SSC; T32 NS007224, SMT; T32 NS041228, SMT), the United States Department of Defense (W81XWH-17-1-0564, SSC; W81XWH-19-1-0264, VDJ), Aligning Science Across Parkinson's (ASAP) Collaborative Research Network (SSC), and the Michael J. Fox Foundation Target Advancement Program (MJFF-020160, SSC & VDJ). We created graphical illustrations using BioRender.com.
Materials
| 1 mL TB Syringe | BD | 309649 | |
| 1.5 mL Eppendorf Tubes | USA Scientific | 1415-2500 | |
| 14 mL, Open-Top Thinwall Ultra-Clear Tube | Beckman Coulter | 344060 | Compatible with SW 40 Ti |
| 23 Gauge Precision Glide Hypodermic Needle | BD | 305145 | |
| 26.3 mL, Polycarbonate Bottle with Cap Assembly | Beckman Coulter | 355618 | Compatible with Ti70 |
| 3.5 mL, Open-Top Thickwall Polypropylene Tube | Beckman Coulter | 349623 | Compatible with TLA-100.3 |
| 50 mL Falcon Tubes | Fisher Scientific | 14-432-22 | |
| Amicon Ultra-15 Centrifugal Filter Unit | Millipore Sigma | UFC901024 | |
| Aprotinin | Sigma-Aldrich | A6279 | 1 mg/mL in diH2O |
| Avanti J-26 XP Centrifuge | Beckman Coulter | B22984 | <26,000 rpm |
| Benchtop HDPE Dewar Flask | Thermo Scientific | 5028U19 | |
| C57BL/6J Mice | The Jackson Labs | 000664 | |
| Centrifuge 5810R | Eppendorf | EP022628168 | <14,000 rpm |
| complete, Mini, EDTA-free Protease Inhibitor Cocktail Tablets | Roche | 11873580001 | Add 1 tablet per 50 mL of solution |
| Curved Forceps | Fine Science Tools | 11273-20 | |
| Fine Surgical Scissors | Fine Science Tools | 8r | |
| Glas-Col Tissue Homogenizing System | Cole-Parmer | UX-04369-15 | |
| Graefe Forceps | Fine Science Tools | 11650-10 | |
| High-Speed Polycarbonate Round Bottom Centrifuge Tubes | ThermoFisher | 3117-0500 | Compatible with JA20 |
| Isofluorane | Henry Schein Animal Health | NDC 11695-6776-2 | |
| JA-20 Rotor | Beckman Coulter | 334831 | |
| Leupeptin | American Bio | AB01108 | 1 mg/mL in diH2O |
| N-[2-Hydroxyethyl] piperazine-N’-[2-ethanesulfonic acid] (HEPES) | American Bio | AB00892 | |
| Optima L-80 XP Ultracentrifuge | Beckman Coulter | <100,000 rpm | |
| Optima TLX Ultracentrifuge | Beckman Coulter | <120,000 rpm | |
| Pepstatin A | Thermo Scientific | 78436 | 1 mg/mL in DMSO |
| Phenylmethylsulfonyl fluoride (PMSF) | American Bio | AB01620 | |
| Pierce BCA Protein Assay Kit | Thermo Scientific | 23335 | For determination of protein concentration |
| Pipette Tips | |||
| Serological Pipettes | |||
| Sucrose | Sigma-Aldrich | S0389 | |
| Surgical Scissors | Fine Science Tools | 14002-12 | |
| SW 40 Ti Swinging-Bucket Rotor | Beckman Coulter | 331301 | |
| Teflon-Coated Pestle and Mortar Tissue Grinder | Thomas Scientific | 3431D94 | |
| Ti70 Rotor | Beckman Coulter | 337922 | |
| TLA-100.3 Rotor | Beckman Coulter | 349490 | |
| Tube Revolver | Dot Scientific | DTR-02VS |
Riferimenti
- Kandel, E. R., Schwartz, J. H., Jessell, T. M., Siegelbaum, S. A., Hudspeth, A. J., Education, A. J. Synaptic Transmission. Principles of Neural Science, Fifth Edition. , (2014).
- Lepeta, K., et al. Synaptopathies: synaptic dysfunction in neurological disorders – A review from students to students. Journal of Neurochemistry. 138 (6), 785-805 (2016).
- Südhof, T. C., Malenka, R. C. Understanding synapses: Past, present, and future. Neuron. 60 (3), 469-476 (2008).
- Südhof, T. C. The molecular machinery of neurotransmitter release (Nobel lecture). Angewandte Chemie International Edition. 53 (47), 12696-12717 (2014).
- Jahn, R., Boyken, J., Pfaff, D. W. Molecular Regulation of Synaptic Release. Neuroscience in the 21st Century: From Basic to Clinical. , 351-401 (2013).
- Xiong, H., Gendelman, H. E. . Current Laboratory Methods in Neuroscience Research. , (2014).
- Azevedo, F. A., et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. Journal of Comparative Neurology. 513 (5), 532-541 (2009).
- Herculano-Houzel, S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proceedings of the National Academy of Sciences of the United States of America. 109, 10661-10668 (2012).
- Herculano-Houzel, S., Lent, R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. Journal of Neuroscience. 25 (10), 2518-2521 (2005).
- Herculano-Houzel, S., Mota, B., Lent, R. Cellular scaling rules for rodent brains. Proceedings of the National Academy of Sciences of the United States of America. 103 (32), 12138-12143 (2006).
- Gray, E. G., Whittaker, V. P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. Journal of Anatomy. 96, 79-88 (1962).
- Gray, E. G., Whittaker, V. P. The isolation of synaptic vesicles from the central nervous system. Journal of Physiology. 153, 35-37 (1960).
- Whittaker, V. P. Thirty years of synaptosome research. Journal of Neurocytology. 22 (9), 735-742 (1993).
- Jahn, R., Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 490, 201-207 (2012).
- Whittaker, V. P., Michaelson, I. A., Kirkland, R. J. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes). Biochemical Journal. 90 (2), 293-303 (1964).
- De Robertis, E., Rodriguez De Lores Arnaiz, G., Pellegrino De Iraldi, A. Isolation of synaptic vesicles from nerve endings of the rat brain. Nature. 194, 794-795 (1962).
- De Robertis, E., Pellegrino De Iraldi, A., Rodriguez, G., Gomez, C. J. On the isolation of nerve endings and synaptic vesicles. The Journal of Biophysical and Biochemical Cytology. 9 (1), 229-235 (1961).
- Zimmermann, H., Whittaker, V. P., Murphy, K. M. The Discovery of the Synaptosome and Its Implications. Synaptosomes. , 9-26 (2018).
- Gorenberg, E. L., et al. Identification of substrates of palmitoyl protein thioesterase 1 highlights roles of depalmitoylation in disulfide bond formation and synaptic function. PLoS Biology. 20 (3), 3001590 (2022).
- Vidyadhara, D. J., et al. Dopamine transporter and synaptic vesicle sorting defects initiate auxilin-linked Parkinson’s disease. bioRxiv. , (2022).
- Schrimpf, S. P., et al. Proteomic analysis of synaptosomes using isotope-coded affinity tags and mass spectrometry. Proteomics. 5 (10), 2531-2541 (2005).
- Dunkley, P. R., Robinson, P. J., Murphy, K. M. Synaptosome Preparations: Which Procedure Should I Use. Synaptosomes. , 27-53 (2018).
- Nagy, A., Baker, R. R., Morris, S. J., Whittaker, V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Research. 109 (2), 285-309 (1976).
- Takamori, S., et al. Molecular anatomy of a trafficking organelle. Cell. 127 (4), 831-846 (2006).
- Wagner, J. A., Kelly, R. B. Topological organization of proteins in an intracellular secretory organelle: the synaptic vesicle. Proceedings of the National Academy of Sciences of the United States of America. 76 (8), 4126-4130 (1979).
- Jahn, R., Schiebler, W., Ouimet, C., Greengard, P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 82 (12), 4137-4141 (1985).
- Binotti, B., Jahn, R., Pérez-Lara, &. #. 1. 9. 3. ;. An overview of the synaptic vesicle lipid composition. Archives of Biochemistry and Biophysics. 709, 108966 (2021).
- Siegel, D. P., Ware, B. R. Electrokinetic properties of synaptic vesicles and synaptosomal membranes. Biophysical Journal. 30 (1), 159-172 (1980).
- Whittaker, V. P., Michaelson, I. A., Kirkland, R. J. The separation of synaptic vesicles from disrupted nervending particles. Biochemical Pharmacology. 12 (3), 300-302 (1963).
- Clementi, F., Whittaker, V. P., Sheridan, M. N. The yield of synaptosomes from the cerebral cortex of guinea pigs estimated by a polystyrene bead "tagging" procedure. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 72, 126-138 (1966).
- Carlson, S. S., Wagner, J. A., Kelly, R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochimica. 17 (7), 1188-1199 (1978).
- Huttner, W. B., Schiebler, W., Greengard, P., De Camilli, P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. Journal of Cell Biology. 96 (5), 1374-1388 (1983).
- Hawkins, P., et al. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals. 45 (1), 1-13 (2011).
- Risling, T. E., Caulkett, N. A., Florence, D. Open-drop anesthesia for small laboratory animals. Canadian Veterinary Journal. 53 (3), 299-302 (2012).
- Deutsch, C., Drown, C., Rafalowska, U., Silver, I. A. Synaptosomes from rat brain: Morphology, compartmentation, and transmembrane pH and electrical gradients. Journal of Neurochemistry. 36 (6), 2063-2072 (1981).
- Ganzella, M., Ninov, M., Riedel, D., Jahn, R. Isolation of synaptic vesicles from mammalian brain. Methods in Molecular Biology. 2417, 131-145 (2022).
- Dunkley, P. R., et al. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Research. 441 (1-2), 59-71 (1988).
- Schwartz, R. D., Skolnick, P., Hollingsworth, E. B., Paul, S. M. Barbiturate and picrotoxin-sensitive chloride efflux in rat cerebral cortical synaptoneurosomes. FEBS Letters. 175 (1), 193-196 (1984).
- Pittaluga, A., Thellung, S., Maura, G., Raiteri, M. Characterization of two central AMPA-preferring receptors having distinct location, function and pharmacology. Naunyn-Schmiedeberg’s Archives of Pharmacology. 349 (6), 555-558 (1994).
- Israël, M., Whittaker, V. P. The isolation of mossy fibre endings from the granular layer of the cerebellar cortex. Experientia. 21 (6), 325-326 (1965).
- Khvotchev, M., Lonart, G., Südhof, T. C. Role of calcium in neurotransmitter release evoked by alpha-latrotoxin or hypertonic sucrose. Neuroscienze. 101 (3), 793-802 (2000).
- Lonart, G., Janz, R., Johnson, K. M., Südhof, T. C. Mechanism of action of rab3A in mossy fiber LTP. Neuron. 21 (5), 1141-1150 (1998).
- Nicholls, D. G., Sihra, T. S. Synaptosomes possess an exocytotic pool of glutamate. Nature. 321 (6072), 772-773 (1986).
- Dunkley, P. R., Jarvie, P. E., Robinson, P. J. A rapid Percoll gradient procedure for preparation of synaptosomes. Nature Protocols. 3 (11), 1718-1728 (2008).
- Cotman, C. W., Matthews, D. A. Synaptic plasma membranes from rat brain synaptosomes: Isolation and partial characterization. Biochimica et Biophysica Acta. 249 (2), 380-394 (1971).
- Booth, R. F., Clark, J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochemical Journal. 176 (2), 365-370 (1978).
- Takamori, S., Riedel, D., Jahn, R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. Journal of Neuroscience. 20 (3), 4904-4911 (2000).
- Burger, P. M., et al. Synaptic vesicles immunoisolated from rat cerebral cortex contain high levels of glutamate. Neuron. 3 (6), 715-720 (1989).
- Blondeau, F., et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proceedings of the National Academy of Sciences of the United States of America. 101 (11), 3833-3838 (2004).
- Maycox, P. R., Link, E., Reetz, A., Morris, S. A., Jahn, R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. Journal of Cell Biology. 118 (6), 1379-1388 (1992).

