Measuring Properties of the Membrane Periodic Skeleton of the Axon Initial Segment using 3D-Structured Illumination Microscopy (3D-SIM)
Summary
The present protocol describes a method to visualize and measure actin rings and other components of the membrane periodic skeleton of the axon initial segment using cultured rat hippocampal neurons and 3D-structured illumination microscopy (3D-SIM).
Abstract
The axon initial segment (AIS) is the site at which action potentials initiate and constitutes a transport filter and diffusion barrier that contribute to the maintenance of neuronal polarity by sorting somato-dendritic cargo. A membrane periodic skeleton (MPS) comprising periodic actin rings provides a scaffold for anchoring various AIS proteins, including structural proteins and different ion channels. Although recent proteomic approaches have identified a considerable number of novel AIS components, details of the structure of the MPS and the roles of its individual components are lacking. The distance between individual actin rings in the MPS (~190 nm) necessitates the employment of super-resolution microscopy techniques to resolve the structural details of the MPS. This protocol describes a method for using cultured rat hippocampal neurons to examine the precise localization of an AIS protein in the MPS relative to sub-membranous actin rings using 3D-structured illumination microscopy (3D-SIM). In addition, an analytical approach to quantitively assess the periodicity of individual components and their position relative to actin rings is also described.
Introduction
The axon initial segment (AIS) is a short, uniquely specialized region of the proximal axon of vertebrate neurons1. The AIS comprises a transport filter and diffusion barrier essential in maintaining neuronal polarity by sorting somato-dendritic cargo2,3,4,5,6,7. In addition, the unique structure of the AIS allows it to accommodate clusters of voltage-gated ion channels that facilitate its function as the site of action potential initiation8. A highly stable structural complex underlies the unique functions of the AIS. Research within the last decade has revealed the presence of a membrane periodic skeleton (MPS) containing actin rings connected by spectrin and providing a scaffold for anchoring various AIS proteins9,10.
The distance between actin rings in the MPS (~190 nm)9,10 is under the resolution limit of conventional light microscopy. Early attempts to use electron microscopy to visualize the MPS were not successful, as the harsh preparation procedures involved failed to preserve the structure of the MPS. Thus, super-resolution microscopy techniques have proven invaluable in elucidating some of the structural details of the MPS11. However, the understanding of the AIS structural complex, the identity and functions of its components, and its spatiotemporal regulation are still incomplete. Recent proteomic studies succeeded in creating a sizeable list of proteins that localize to the AIS close to structural components of the AIS12,13. Still, details of their function and precise place in the AIS complex are lacking. Thus, super-resolution microscopy techniques serve as an essential tool to examine the accurate positions of these proteins relative to other MPS components and investigate their functions. Several light microscopy techniques can achieve resolutions higher than the diffraction limit of light, some even capable of localizing single molecules. However, many of these techniques typically require specialized fluorophores or imaging buffers, and image acquisition is often time-intensive14.
3D structured illumination microscopy (3D-SIM), owing to its ease of use and simple sample preparation requirements, requires no special reagents for imaging or sample preparation, works well with a wide array of fluorophores and samples, can be readily implemented in multiple colors, and is capable of live-cell imaging15. While the best possible resolution SIM offers (~120 nm) is low compared to many other super-resolution techniques, it is sufficient for many applications (for example, for resolving the components of the MPS in neurons). Thus, it is crucial to consider the requirement for specific applications to determine if SIM is a suitable choice or if an even higher resolution is necessary. Here, a protocol is described for using cultured hippocampal neurons and 3D-structured illumination microscopy (3D-SIM) to examine the position and organization of putative AIS proteins relative to actin rings in the MPS, as implemented in Abouelezz et al.16
Protocol
Primary hippocampal neurons used in these experiments were harvested16 from embryonic day 17 Wistar rat embryos of either sex under the ethical guidelines of the University of Helsinki and Finnish law.
1. Sample preparation
- On high-fidelity glass coverslips, allow rat hippocampal neurons to grow in sparse culture conditions (~5,000-10,000 cells/cm2) for 14 days (14 days-in-vitro).
NOTE: Using sparse cultures reduces the chances of overlapping neurites, which helps quantify the MPS. - Fix neurons using 4% paraformaldehyde for 12 min at room temperature. Wash the coverslip once in 0.2% BSA in PBS (BSA-PBS), then incubate for 10 min in a 1% solution of Triton-X in PBS at room temperature. Wash once in BSA-PBS.
- Dilute anti-ankyrin G antibodies (1:200) in BSA-PBS. Add antibody solution to the coverslip and incubate overnight at 4 °C. Optionally, add chicken anti-MAP2 antibodies (see Table of Materials) to the antibody solution to demarcate the somato-dendritic domain.
- Wash cells once in BSA-PBS, followed by 0.1% Triton-X in PBS, then do a final wash in BSA-PBS.
- Dilute fluorophore-tagged anti-mouse secondary antibodies (1:200) (see Table of Materials) in BSA-PBS, add to the coverslip, and incubate for 1 h at room temperature. Wash the cells once in BSA-PBS, once in 0.1% Triton-X in PBS, then once in PBS.
- Prepare a 1 µM solution of tagged phalloidin in PBS (see Table of Materials) and add it to the cells. Incubate for 2 h at room temperature. Wash cells once in PBS, once in 0.1% Triton-X in PBS, then once in PBS.
NOTE: AlexaFluor488-tagged phalloidin was used here, but other tags will also work. - For mounting the coverslip on a glass slide, apply a drop of mounting media on a glass slide, dip the coverslip in deionized water, and dab using a soft paper towel (to remove excess water).
- Place the coverslip on the glass slide. Incubate at room temperature for 24 h.
NOTE: No adverse effects on the sample were observed using hard-setting mounting media (refractive index 1.47 after curing).
- Place the coverslip on the glass slide. Incubate at room temperature for 24 h.
2. Imaging
- If possible, consult with the microscopy facility's personnel before imaging. Use an immersion oil calculator (see Table of Materials) to select the immersion oil suitable for the sample.
- Once the samples are ready, ensure the coverslips are clean and clear of any residue or excess mounting media. If necessary, use a cotton tip dipped in water or ethanol to clean. Place the sample in a 3D SIM-capable microscope (see Table of Materials) and find a cell to image.
- Adjust the power of the relevant laser lines and the exposure times to maximize the signal-to-noise ratio without significantly bleaching the sample.
NOTE: A good signal-to-noise ratio will show a clear grid-like appearance focused on the sample and lead to accurate high-resolution reconstructions. 488 nm and 640 nm laser lines were used to visualize F-actin and ankyrin G, respectively. - Set the upper and lower limits of the sample in the z-dimension and proceed to acquire a stack.
NOTE: z-step size of 125 nm was used here. - For successful SIM reconstruction, ensure that the microscopy software (see Table of Materials) has an optical transfer file (OTF) suitable for the used dyes.
NOTE: These are typically created and maintained by specialized personnel. In the absence of a functional OTF, open-source tools are also available to perform reconstructions based on estimates17. In this work, up-to-date OTF files were used controlled by specialized personnel. - Run the reconstruction algorithm on the stack to obtain a super-resolved reconstruction. Check the reconstructions for known artifacts and, if necessary, adjust the parameters to correct them.
NOTE: The default algorithm parameters typically give accurate results. Many of these artifacts involve some repeating patterns: striped lines along with multiple directions on one z-plane, 'ghosting' (repeated features appearing in various z-planes), or a hexagonal 'honeycomb' pattern emerging in some areas. These artifacts can often be fixed with better sample preparation, a better signal-to-noise ratio, adjusting the parameters of the reconstruction algorithm, or ensuring the refractive index of the chosen immersion oil is suitable for the mounting medium and sample. For a more detailed discussion of SIM artifacts and a freely-available tool to check for the presence of artifacts, see Ball et al.18 - Compare the SIM reconstruction to a wide-field image to observe improvements in resolution.
- When performing multi-color SIM, use the alignment algorithm to align the different channels correctly once a satisfactory reconstruction is ready.
NOTE: The alignment algorithm uses an alignment reference file generated using a microsphere bead preparation as per the instruction of the microscope manufacturer.
3. Image analysis
- Actin rings in the MPS have a distinctive periodic appearance. Using image analysis software (see Table of Materials), create a maximum intensity projection image using all the focal planes where actin rings are visible.
- On the maximum intensity projection image, draw a perpendicular line across visible adjacent rings and record the fluorescence intensity along with the software's line 'Plot Profile' function.
- To calculate the mean inter-peak distance, note the local maxima in the line profile and measure the distance between individual adjacent fluorescent intensity peaks.
NOTE: This can be easily calculated, for example, using the 'findpeaks' function in MATLAB or the (open-source) GNU Octave platform. - To evaluate the colocalization of different proteins with actin rings in the MPS, run a colocalization analysis procedure19,20,21 on SIM reconstructions of actin and the candidate protein. Manually draw a selection to define the AIS as a region of interest for the EzColocalization plugin in the software platform19 and run the analysis to calculate the Pearson's coefficient of correlation (PCC) of co-localization19,20,21. A PCC value close to 1 indicates strong colocalization.
NOTE: This protocol is summarized in Figure 1.
Representative Results
Using cultured rat hippocampal neurons and 3D-SIM, a protocol is described to visualize and measure actin rings and other components of the MPS in the AIS. Reconstructions of image stacks showed clear periodicity of actin rings (Figure 2A). In our hands, the mean inter-peak distance of actin rings in the MPS, visualized using Alexa 488-tagged phalloidin, was 190.36 ± 1.7 nm (mean ± SEM). This is in line with the previously reported average distance of ~190 nm between actin rings in the MPS. Similarly, an anti-ankyrin G antibody was used to visualize ankyrin G (Figure 2B). The colocalization of ankyrin G and F-actin was tested in the AIS using a colocalization analysis procedure to calculate the PCC of co-localization19,20,21. The PCC of colocalization of ankyrin G and F-actin fluorescence was 0.36 ± 0.03 (mean ± SEM, Figure 2B). As ankyrin G and actin rings bind βIV-spectrin at different domains, they do not show significant colocalization. These data were adapted from Abouelezz et al.16
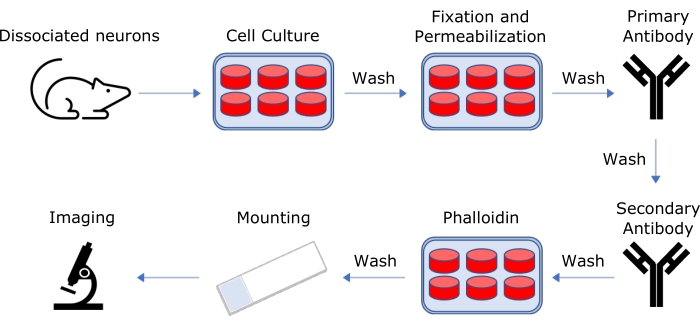
Figure 1: Schematic representation of the protocol for sample preparation for 3D-SIM imaging. Hippocampal neurons are harvested from rat embryos, dissociated, and allowed to grow on glass coverslips in culture for 14 days. Cells are then fixed and stained using tagged phalloidin and appropriate antibodies, then mounted on glass slides for 3D-SIM imaging. Please click here to view a larger version of this figure.
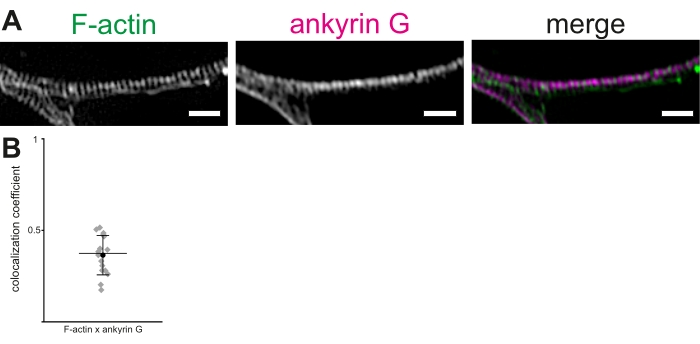
Figure 2: 3D-SIM reconstruction of the membrane periodic skeleton (MPS) in the axon initial segment (AIS). (A) F-actin (green) and ankyrin G (magenta) show a regular distribution in the AIS, visualized by 3D-SIM. Scale bar = 1 µm. (B) Pearson's coefficients of correlation (PCC) of colocalization of ankyrin G with F-actin in the MPS in the AIS. The mean PCC of ankyrin G was 0.36 (black circle). Gray diamonds represent individual cells (n = 16), mid-line represents the median, error bars represent 25th and 75th percentiles. The data is adapted from Abouelezz et al.16 Please click here to view a larger version of this figure.
Discussion
The protocol described here provides a method for visualizing and measuring MPS proteins using the super-resolution technique. As actin rings and other MPS components display a periodicity of ~190 nm9,10, conventional diffraction-limited imaging approaches cannot reveal the details of the MPS. Several microscopy setups may resolve diffraction-limited structures in super-resolution, and SIM represents a robust and uncomplicated option. Importantly, SIM is compatible with the most widely-used fluorophores, providing significant flexibility. Furthermore, SIM is effective in visualizing potentially dim structures in the MPS, such as actin rings in latrunculin-treated neurons22, as well as live neurons15.
An essential aspect for the success of this protocol is preserving the integrity of the MPS in the first place. The most critical steps to successful SIM imaging occur during sample preparation. For example, moderate fixation (4% PFA for 12 min at room temperature) and strong permeabilization (1% Triton-X for 10 min) provide the best results. It is crucial to keep in mind that harsh treatments that may be required for specific preparations may have a negative effect on the structural integrity of the MPS. Therefore, it is perhaps best to consider modifying such treatments to maximize the preservation of the MPS.
The other crucial factor to consider when preparing samples for imaging is to maximize the labeling density. Ideally, every molecule would be tagged and detected. For example, the anti-ankyrin G antibody used in this experiment is commonly used to label the AIS. It provides outstanding performance in conventional fluorescence microscopy, even when used at a dilution of 1:1000 and incubated for just 1 h at room temperature. However, to obtain good labeling density for super-resolution microscopy, it is highly effective to use 5-fold that concentration (1:200) and incubate the antibody overnight at 4 °C. While the specific requirements for each antibody or labeling technique will vary and need to be determined experimentally, it is perhaps helpful to keep this in mind.
In addition, it is essential to note that achieving a high signal-to-noise ratio lends itself well to accurate and successful SIM reconstructions. A good rule of thumb is that the grid pattern should be visible in the individual images once the SIM modality is engaged. However, this is not always possible.
Finally, it is essential to note that SIM is among the weakest super-resolution techniques in resolving power14. Thus, while it is generally sufficient for revealing periodicity and overall organization of MPS proteins, it is significantly less capable of providing details about their interactions than stochastic optical reconstruction microscopy (STORM)10. Furthermore, the technique’s utility described here is limited to the study of proteins for which a specific, well-performing antibody is available. However, this can partly be circumvented through exogenous expression of tagged proteins15.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Dr. Pirta Hotulainen is acknowledged for her critical comments, invaluable for preparing this manuscript. Dr. Rimante Minkeviciene is acknowledged for her help in preparing the neuronal cultures used for the original experiments. All imaging was performed in the Biomedicum Imaging Unit. This work was supported by the Academy of Finland (D.M., SA 266351) and Doctoral Programme Brain & Mind (A.A.)
Materials
| 24-well plates | Corning | 3524 | |
| 4% Paraformaldehyde | |||
| Alexa-488 Phalloidin | ThermoFisher | A12379 | |
| Alexa-647 anti-mouse | ThermoFisher | A31571 | |
| Anti-Ankyrin G antibody | UC Davis/NIH NeuroMab Facility, Clone 106/36 | 75-146 | |
| Anti-MAP2 antibody | Merck Millipore | AB5543 | |
| B-27 | Invitrogen | 17504044 | |
| Bovine Serum Albumin (BSA) | BioWest | P6154 | |
| Deltavision OMX SR | GE Healthcare Life Sciences | N/A | |
| Fiji software package | ImageJ | ||
| GNU Octave | GNU | ||
| High performance coverslips | Marienfeld | 117530 | |
| Immersion Oil Calculator | Cytiva Life Sciences | https://tinyurl.com/ImmersionOilCalculator | |
| L-Glutamine | VWR | ICNA1680149 | |
| MATLAB R2020a | Mathworks | ||
| Neurobasal media | Invitrogen | 21103049 | |
| OMX SR | Delta Vision OMX | ||
| Primocin | InvivoGen | ant-pm-1 | |
| ProLong Gold mounting media | Invitrogen | P10144 | |
| softWoRx Deconvolution | Cytiva Life Sciences | ||
| Superfrost Slides | Epredia | ISO 8037/1 | |
| TetraSpeck microspheres 0.1 µm | ThermoFisher | T7279 | |
| Triton-X | Sigma | X100 |
Riferimenti
- Leterrier, C. The Axon initial segment, 50 years later: A nexus for neuronal organization and function. Current Topics in Membranes. 77, 185-233 (2016).
- Leterrier, C., Dargent, B. No pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Seminars in Cell & Developmental Biology. 27, 44-51 (2014).
- Brachet, A., et al. Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. Journal of Cell Biology. 191 (2), 383-395 (2010).
- Nakada, C., et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nature Cell Biology. 5 (7), 626-632 (2003).
- Song, A. H., et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 136 (6), 1148-1160 (2009).
- Sun, X., et al. Selective filtering defect at the axon initial segment in Alzheimer’s disease mouse models. Proceedings of the National Academy of Sciences of the United States of America. 111 (39), 14271-14276 (2014).
- Winckler, B., Forscher, P., Mellman, I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 397 (6721), 698-701 (1999).
- Kole, M. H., et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nature Neuroscience. 11 (2), 178-186 (2008).
- Xu, K., Zhong, G., Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 339 (6118), 452-456 (2013).
- Leterrier, C., et al. Nanoscale architecture of the axon initial segment reveals an organized and robust scaffold. Cell Reports. 13 (12), 2781-2793 (2015).
- Leterrier, C. The axon initial segment: An updated viewpoint. The Journal of Neuroscience. 38 (9), 2135-2145 (2018).
- Hamdan, H., et al. Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nature Communications. 11 (1), 100 (2020).
- Zhou, R., et al. Proteomic and functional analyses of the periodic membrane skeleton in neurons. bioRxiv. , (2020).
- Valli, J., et al. Seeing beyond the limit: A guide to choosing the right super-resolution microscopy technique. Journal of Biological Chemistry. 297 (1), 100791 (2021).
- Wang, T., et al. Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. Journal of Cell Biology. 219 (5), 201902001 (2020).
- Abouelezz, A., Stefen, H., Segerstråle, M., Micinski, D., Minkeviciene, R., Lahti, L., Hardeman, E., Gunning, P., Hoogenraad, C., Taira, T., Fath, T., Hotulainen, P. Tropomyosin Tpm3.1 is required to maintain the structure and function of the axon initial segment. iScience. 23 (5), 101053 (2020).
- Muller, M., Monkemoller, V., Hennig, S., Hubner, W., Huser, T. Open-source image reconstruction of super-resolution structured illumination microscopy data in ImageJ. Nature Communications. 7, 10980 (2016).
- Ball, G., et al. SIMcheck: a toolbox for successful super-resolution structured illumination microscopy. Scientific Reports. 5, 15915 (2015).
- Stauffer, W., Sheng, H., Lim, H. N. EzColocalization: An ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Scientific Reports. 8 (1), 15764 (2018).
- Dunn, K. W., Kamocka, M. M., McDonald, J. H. A practical guide to evaluating colocalization in biological microscopy. American Journal of Physiology-Cell Physiology. 300 (4), 723-742 (2011).
- Lahti, L. Data analysis supplement to the research article Abouelezz, Stefen, Segerstråle, Micinski, Minkeviciene, Lahti, Hardeman, Gunning, Hoogenraad, Taira, Fath, & Hotulainen. Tropomyosin Tpm3.1 Is Required to Maintain the Structure and Function of the Axon Initial Segment. , (2021).
- Abouelezz, A., Micinski, D., Lipponen, A., Hotulainen, P. Sub-membranous actin rings in the axon initial segment are resistant to the action of latrunculin. Journal of Biological Chemistry. 400 (9), 1141-1146 (2019).

