Direct Reprogramming of Human Fibroblasts into Myoblasts to Investigate Therapies for Neuromuscular Disorders
Summary
This protocol describes the conversion of skin fibroblasts into myoblasts and their differentiation into myotubes. The cell lines are derived from patients with neuromuscular disorders and can be used to investigate pathological mechanisms and to test therapeutic strategies.
Abstract
Investigations into both the pathophysiology and therapeutic targets in muscular dystrophies have been hampered by the limited proliferative capacity of human myoblasts. Several mouse models have been created but they either do not truly represent the human physiopathology of the disease or are not representative of the broad spectrum of mutations found in humans. The immortalization of human primary myoblasts is an alternative to this limitation; however, it is still dependent on muscle biopsies, which are invasive and not easily available. In contrast, skin biopsies are easier to obtain and less invasive to patients. Fibroblasts derived from skin biopsies can be immortalized and transdifferentiated into myoblasts, providing a source of cells with excellent myogenic potential. Here, we describe a fast and direct reprogramming method of fibroblast into a myogenic lineage. Fibroblasts are transduced with two lentiviruses: hTERT to immortalize the primary culture and a tet-inducible MYOD, which upon the addition of doxycycline, induces the conversion of fibroblasts into myoblasts and then mature myotubes, which express late differentiation markers. This quick transdifferentiation protocol represents a powerful tool to investigate pathological mechanisms and to investigate innovative gene-based or pharmacological biotherapies for neuromuscular disorders.
Introduction
Cellular models obtained directly from human tissues are useful to model many human genetic disorders, with the advantage of having the original genomic context and, in many cases, reproducing the same molecular and cellular hallmarks observed in the patients. In the field of neuromuscular disorders, muscle biopsies have been a great source of human myoblasts and have helped in the elucidation of pathological mechanisms. Additionally, they are an important tool for in vivo testing of drugs and gene therapies. On one hand, the derivation of myoblasts from muscle fragments is relatively easy. On the other hand, the culture and maintenance of primary myoblasts are challenging, because of their limited proliferation rate and replicative senescence in vitro1. An alternative for these limitations is to immortalize myoblasts with the insertion of the human telomerase (hTERT) and/or cyclin-dependent kinase 4 (CDK4) genes2,3, with preservation of skeletal muscle features4. Nevertheless, the obtention of primary myoblasts is still dependent on muscle biopsy, a surgical procedure with disadvantages to the patients, which, in many cases, have their muscles in advanced degeneration. Thus, the muscle of these patients is composed of a significant proportion of fibrotic and/or adipose tissue and yields fewer muscle cells, requiring the purification of the cells previously to the immortalization.
In contrast to muscle biopsies, skin biopsies are more accessible and are less harmful to patients. Primary fibroblasts can be derived from skin fragments in vitro. Although fibroblasts are not primarily affected by mutations causing neuromuscular disorders, they can be transdifferentiated into myoblasts. This can be achieved by the insertion of the Myod gene, a myogenic regulatory transcription factor5. In this manuscript, we describe the protocol to obtain transdifferentiated myoblasts, from the establishment of fibroblasts cultures to the obtention of differentiated myotubes (a representative summary of the method is depicted in Figure 1).
Pre-clinical testing of therapeutic strategies is dependent on cellular and animal models carrying mutations similar to the mutations found in human patients. Although the development of animal models has become more feasible with the advance of gene-editing technologies such as CRISPR/Cas96, it is still challenging and costly. Thus, patient-derived cell lines are an accessible option to have models, covering the large spectrum of mutations of disease such as Duchenne muscular dystrophy (DMD). Obtention and creation of cell models are crucial to the development of personalized therapies for such pathologies.
Among personalized therapies that have been investigated, exon skipping strategies is one of the promising ones for different muscular dystrophies7,8. This strategy consists of producing a shorter but functional protein. This is performed by hiding the exon definition to the spliceosome, therefore excluding the mutated exon from the final messenger. This is a very promising technology that has been approved by the FDA for DMD. Thus, we also describe in this protocol, methods to transfect myoblasts with two different exon skipping related technologies: antisense oligonucleotides (AON) and U7snRNA-adeno-associated virus (AAV). AON transfection is a good tool for the initial screening of several sequences designed to promote exon skipping9. However, the activity of AONs is transient. To obtain a sustained expression of antisense sequences, we also explored small nuclear RNAs (snRNAs) combined with AAV, allowing nuclear localization and inclusion in the splicing machinery10. U7 is an snRNA involved in the processing of histone mRNA that can be engineered to bind proteins that will redirect it to the spliceosome and deliver antisense sequences11. The use of modified U7 snRNAs in combination with AAV vectors overcomes limitations of AONs resulting in a continued expression of the AONs and better transduction of tissues of interest12. We use cells derived from DMD patients for this protocol to illustrate the exon-skipping strategy.
Protocol
All experiments and biopsies were carried out following the ethical rules of the institutions involved under the approval of the Nationwide Children's Hospital Institutional Review Board.
1. Initiation of dermal fibroblasts culture
- Establishment of fibroblasts culture
- Aliquot 10 mL of fibroblast medium (Table 1) in 15 mL conical tubes. The skin biopsy should be placed and transported in this medium. The biopsy can be stored at 4 °C until it is processed, preferentially on the same day.
NOTE: Use the skin biopsy within 24-36 hours to avoid potential growth of contamination. - Aspirate the media from the tube and rinse the biopsy with 10 mL 1X PBS (room temperature) three times. After the third wash, leave the PBS in the tube.
- Pour out the PBS and the skin onto a 10 cm2 dish.
- Using sterile scalpels, cut the biopsy into as small as possible fragments.
- Using a pipette, transfer an individual skin fragment and drop it into a clean 10 cm2 dish. Place 10 to 12 fragments per dish.
- Aspirate the excess of PBS from around each fragment. Be careful to not aspirate the fragment.
- Cover the dishes partially with the lid and allow the skin fragments to dry for 5-20 min. Do not allow the fragments to dry excessively.
- Once the fragments are dry, tilt the dish at 45 degrees and slowly add 12 mL of fibroblast medium to the corner. Lower down the dish, carefully distributing the media so the fragments do not lift by the media.
- Place the dishes into the incubator (37 °C, 5% CO2). Replace the media in 5-7 days, and once a week after.
- Observe the fibroblasts emerging from the fragment (Figure 2) and, once confluent, passage the cells into 75 cm2 flasks. Remove the medium, rinse the cells with PBS and add 1 mL 0.25 % trypsin. Incubate at 37 °C for 5 min or until all cells are lifted. Add 10 mL fibroblast growth medium to inhibit the trypsin and transfer the cells to a new flask.
NOTE: For passage number nomenclature, P1 is established when the first fibroblasts that emerged from the skin biopsy are transferred to a new flask for proliferation.
- Aliquot 10 mL of fibroblast medium (Table 1) in 15 mL conical tubes. The skin biopsy should be placed and transported in this medium. The biopsy can be stored at 4 °C until it is processed, preferentially on the same day.
- Cryopreservation of primary fibroblast lines
- Once the 75 cm2 flask is confluent, rinse it with 10 mL 1X PBS and aspirate PBS.
- Add 3 mL of 0.25 % trypsin to the cell surface. Place the flask in the incubator for 5 min. Check the flasks under the microscope to see if the cells are lifted. If not, place the flask back in the incubator for an additional 5 min.
- Once the cells are detached, add 7 mL of fibroblast media to the flask and pipette up and down to resuspend the cells. Collect the cells into a 50 mL conical tube.
- Prepare 100 µL aliquot of trypan blue, and remove 100 µL from the sample being cryopreserved, mix with the trypan blue. Load the mix on the hemocytometer to count. Count the cells in four different fields of the hemocytometer under the microscope. To calculate the total number of cells, use the formula: (counted cells/100) * volume of culture.
- Spin the conical tubes at 300 x g for 10 minutes at room temperature or 4 °C.
- Aspirate off the medium and resuspend the cells in the adequate volume of freezing medium: 1 mL per each 1 million cells/vial. Pipette up and down to homogenize and distribute 1 mL to each labeled cryovial.
- Place the vials into the freezing box, and allow the vials to freeze at a rate of 1 °C/min at -80 °C freezer overnight.
- The following day transfer the vials to a liquid nitrogen tank or -150 °C freezer.
2. Establishment of FibroMyoblasts (FM) cell line
- Seed primary fibroblasts at approximately 30% of confluency in two wells of a 12-well plate (2 x 104 cells/well) in order to have about 50% of confluency the next day.
- For lentiviral transduction, add 2 to 5 x 109 vg (viral genome particles) of hTERT-puromycin lentivirus in 400 µL of fibroblast medium. To the second well, add just 400 µL of fibroblast medium. Add 1 mL of media the following day.
NOTE: Plasmids for lentivirus production were obtained from the group that published the Chaouch et al, 2009 paper. They are also described individually in Aure et al, 200713 for hTERT plasmid and Barde et al, 200614 for the Tet-on system utilized for the design of MyoD plasmid. They were obtained thanks to a Material Transfer Agreement with Genethon, France (please contact Dr. Vincent Mouly to obtain these plasmids – vincent.mouly@upmc.fr). Briefly, the hTERT consists of hTERT variant 1 driven by a CMV promoter while the puromycin is driven by a PGK promoter. The MyoD plasmid contains a MyoD variant 1 driven by a CMV promoter under the control of the repressor rtTA2. This plasmid also contains the hygromycin selection expressed thanks to the SV40 promoter.
Lentiviruses were produced using regular lentiviral production (see Wang and McManus JoVe protocol15). Briefly, MDL-helper, Rev-Helper, SVS-G-helper were transfected via calcium chloride precipitation also of either hTERT or MyoD plasmids. After 48 h, the supernatant was collected, and then for additional three days. All supernatant was then concentrated by ultracentrifugation. The pellet was then resuspended into Tris-HCL+NaCl+EDTA buffer. Titer estimation was evaluated by standard lentivirus qPCR assay. - One or two days later, transfer the cells into a 6-well plate and grow them until reaching 60-70% confluence.
- Supplement the fibroblast medium with 1 µg/mL of puromycin and add 2 mL to each well.
- Keep the cells under selection until all cells in the control well are dead (up to 12 days), changing media every 2-3 days. Passage the cells from the 6-well plate into two 10 cm2 dishes for further proliferation.
- Freeze vials of fibroblasts after selection. Label as F(hTer).
- Seed hTERT-expressing fibroblasts (F(hTer)) at about 30% confluence in fibroblast medium, in two wells of a 12-well plate, to have about 50% confluence the next day.
- For lentivirus transduction, mix 2 to 5 x 109 vg of MyoD-hygromycinB lentivirus in 400 µl of fibroblast medium and add to respective wells; to the third well add 400 µl fibroblast medium. Add 1 mL of medium the next day.
- One or two days later transfer the cells into a 6-well plate and grow until 60-70% confluence.
- Supplement the fibroblast growth medium with hygromycin B (400 µg/mL) and add 2 mL to each well.
- Keep the cells under selection until all cells in the control well are dead (up to 12 days), changing media every 2-3 days.
- Freeze vials of fibroblasts after selection. Label as FM followed by the cell identification number/name.
3. Transdifferentiation protocol
- Seed transduced FM onto 10 cm2 dishes with 30-40% confluence. In a 12-well plate, seed 6 x 104 cells (this is dependent on the individual cell line).
NOTE: For immunostaining, seed cells onto glass coverslips or chamber slides coated with Matrigel. Dilute Matrigel at 1:10 in DMEM medium, add a volume enough to cover the surface, and let the slides sit at room temperature for one hour. Aspirate off right before seeding the cells. - For myoblasts induction, when the fibroblasts reached 70% confluence (Figure 3A), rinse the cell surface with PBS and add fresh myoblast media supplemented with fresh 8 µg/mL doxycycline.
NOTE: The success of differentiation is compromised past 80% confluence. - After two to three days later, cells are 90-95% confluent and their morphology will have changed (Figure 3B). Rinse the cell surface with PBS and add fresh differentiation media supplemented with fresh 8 µg/mL doxycycline.
- Continue to change media every 2-3 days without passaging until myotubes are established (confirm via morphology) (Figure 3C).
- Seven to ten days after starting myotube differentiation, cells should be fully differentiated and may start to detach or die. Before this happens, harvest myotubes for further analysis.
NOTE: The time course of myotube formation depends on the cell line. Mutations in muscle-related proteins may interfere in the myogenic potential. When myotubes start to appear bright and look white at the borders it is a signal they are starting to detach (Figure 4). - To harvest myotubes, collect media and transfer it to a 50 mL conical tube. The medium may contain myotubes that have detached.
- Rinse the myotubes with 5 mL PBS and transfer PBS to the 50 mL tube.
- Add 3 mL of 0.25 % trypsin to the cell surface. Place the dish in the incubator for 5 min. Check the dish under the microscope to see if the cells are lifted. If not, place it back in the incubator for an additional 5 min.
- Once the cells are detached, add 7 mL of fibroblast media to the dish and pipette up and down to resuspend the cells. Collect the cells to the 50 mL conical tube.
- Centrifuge at 1,200 x g for 7 min at 4 °C.
- Carefully aspirate off the liquid, without disturbing the pellet. Store the pellets at -80 °C until further processing.
4. Immunostaining of differentiated myotubes
NOTE: For immunostaining, grow the cells in glass coverslips or chamber slides as noted above.
- Once myotubes are fully differentiated, aspirate off media and carefully rinse the slides with PBS. Aspirate PBS off.
- Add fresh 4% PFA (500 µL per well of a 12-well plate) and incubate at room temperature for 10 min. Aspirate PFA off.
- Rinse with 1 mL PBS.
- Incubate with 0.2 M glycine at room temperature, for 10 min. Aspirate glycine off.
- Permeabilize with PBS 0.5% TritonX-100 (300 µL/well of a 12-well plate), for 10 min with gentle agitation.
- Block with 300 µL/well of blocking solution, for 10 min with gentle agitation.
- Incubate with primary antibody diluted 1:50 in 300 µL of blocking solution, for 2 hours at room temperature, with gentle shaking.
- Rinse three times with 1 mL/well of PBS for 5 min, with gentle shaking.
- Incubate with secondary antibody diluted 1:500 in 300 µL of blocking solution, for 1 hour, at room temperature, with gentle shaking. Cover the plate with aluminum foil.
- Rinse three times with 1 mL/well of PBS for 5 min, with gentle shaking.
- Incubate with DAPI diluted in PBS for 10 minutes. Rinse three times with 1 mL/well of PBS.
- Add a drop of mounting medium to a glass slide. Remove the coverslip with forceps and place it face down on the drop of mounting medium.
- Invert slide onto a paper towel and gently press to remove bubbles and excess of mounting medium.
- Seal the slides with nail polish and store at 4 °C until imaging.
5. Antisense oligonucleotide transfection
NOTE: The protocol below is for transfection of a 6-well plate. Adjust volumes accordingly for smaller or bigger plates. The transfection is done in 100% confluent myoblasts when the cells are ready for the differentiation step.
- Aspirate the myoblast growth media and rinse the cells with 1 mL PBS.
- Add 500 µL/well of OptiMEM media and incubate at 37 °C for 1 hour.
- Dilute the antisense oligonucleotide (AON) in 100 µL of OptiMEM to the desired final concentration (i.e. 50 nM, 100 nM, 200 nM, 500 nM). Incubate at room temperature for 5 min.
NOTE: This protocol is optimized for 2'omethyl-phosphorothioate AONs. - Mix the lipofectamine with OptiMEM (final volume of 100 µL) to give a final ratio of 1:1 (µg DNA: µL lipofectamine). Incubate at room temperature for 5 min.
- Combine the diluted lipofectamine with the diluted AON. Mix gently by pipetting and incubate for 20 min at room temperature to allow complex formation. Avoid air bubbles.
- Add 200 µL of lipofectamine and AON mix to respective wells. Incubate the cells overnight at 37 °C, 5% CO2.
- The following day remove the transfection mix and add 2 mL of warm differentiation media supplemented with doxycycline.
- Collect cells at least three days later for RNA extraction or seven to 21 days in case of protein analysis.
NOTE: The days of differentiation necessary to detect RNA and/or protein expression may vary accordingly to the gene of interest or the cell line. In the case of DMD, it's possible to detect its mRNA within three days. Dystrophin protein detection requires at least seven days. This will vary depending on the cell line. High concentrations of AON and transfection reagent can impact the transdifferentiation.
6. AAV1-U7 transduction
NOTE: This protocol was optimized for 6-well plates. Adjust the volumes proportionally to the culture surface area. The transduction is done in 100% confluent myoblasts when the cells are ready for the differentiation step. AAV1 is the AAV serotype with the best transduction capacity of cultured myoblasts.
- Aspirate off the myoblast growth medium and rinse the cells with 1 mL PBS.
- Dilute 0.5-1 x 1011 viral particles of AAV1-U7 in 700 µL of warm differentiation media supplemented with doxycycline.
NOTE: We use qPCR to determine the viral concentration. The amount of virus to be used may vary depending on the quantification method and should be determined previously using a reporter assay. - Add the viral mix to the well by dropping it homogenously.
- The following day, add 1.3 mL of warm differentiation media supplemented with doxycycline.
- Collect the cells at least three days later for RNA extraction or seven to 21 days in case of protein analysis.
7. RNA extraction
NOTE: All material used during this step should be RNase free.
- Add 500 µL of TRIzol per pellet and pipet up and down several times to ensure that cells are homogenously lysed.
- Transfer the cell lysate in a 1.5 mL tube and incubated for 5 min at room temperature.
- Add 100 µL of chloroform and shake manually for 15 s. Incubate for 5 min at room temperature.
- Centrifuge at 12,000 x g for 15 min at 4 °C. Collect the aqueous phase (upper one) and transfer it to a new 1.5 mL tube.
- For 1 volume of the aqueous phase, add 1 volume of ethanol 100% and mix by pipetting.
NOTE: We recommend column purification and concentration. - Transfer the sample to a Zymo-Spin IC column in a collection tube and centrifuge at 12,000 x g for 30 s. Discard the flow-through.
- For in-column DNase I digestion, pre-wash the column with 400 µL RNA Wash Buffer. Centrifuge at 12,000 x g for 30 s. Discard the flow-through.
- Prepare 40 µL of DNase reaction mix per sample. Mix 5 µL DNase I with 35 µL DNA Digestion Buffer.
- Add the mix directly to the column matrix. Incubate at room temperature for 15 min.
- Add 400 µL RNA Prep Buffer to the column and centrifuge at 12,000 x g for 30 s. Discard the flow-through.
- Add 700 µL RNA Wash Buffer to the column and centrifuge at 12,000 x g for 30 s. Discard the flow-through.
- Add 400 µL RNA Wash Buffer to the column and centrifuge at 12,000 x g for 2 min to ensure complete removal of the wash buffer. Transfer the column carefully into an RNase-free 1.5mL tube.
- Add 15 µL nuclease-free water directly to the column matrix. Incubate for 5 min and centrifuge at 12,000 x g for 1 minute.
NOTE: Collect the eluted RNA and apply it again to the column to increase yield. Centrifuge at 12,000 x g for 1 minute. - Place samples on ice and quantify the samples in a Nanodrop.
- Store samples at -80 °C.
8. RT-PCR analysis
NOTE: In this step, we present a suggestion of reagents to detect the expression of dystrophin mRNA, but it can be easily adapted to other reagents of choice.
- Reverse transcription
- Thaw all the reagents and keep them on ice.
- Prepare a mix with 4 µL of 5x Reaction Buffer, 2 µL of dNTP Mix (10 mM), 1 µL of RiboLock RNase Inhibitor, and 1 µL of RevertAid RT.
- Mix the tube gently and centrifuge briefly.
- In 0.2 mL PCR tubes, add the adequate volume of RNA in order to have 1 µg per reaction. Add nuclease-free water q.s.p 12 µL. Include one tube without the reverse transcriptase as a negative control and one tube with nuclease-free water instead of RNA.
- Distribute 8 µL of reaction mix per tube. The total volume is 20 µL.
- Place tubes in a thermocycler and incubate for 5 min at 25 °C followed by 60 min at 42 °C. Stop the reaction by heating at 70 °C for 5 minutes.
- Place the tubes on ice or at -20 °C for longer storage.
- PCR
NOTE: Design primers at exons junctions preferably.- Vortex reagents and spin down before use.
- Prepare a master mix using 0.5 µL forward primer (25 µM), 0.5 µL reverse primer (25 µM), 12.5 µL 2x PCR Master Mix, and 8.5 µL of nuclease-free water per sample.
- Aliquot 22 µL of master mix into a tube for each sample.
- Add 3 µL of cDNA (150 ng) to its respective PCR tube. Add 3 µL of nuclease-free water to the PCR negative control tube.
- Vortex and spin down the PCR tubes.
- Incubate the tubes in a thermocycler at 95 °C for 3 min, 95 °C for 30 s, (Tm-5) °C for 30 s, 72 °C for (1 min/kb) 34 times, 72 °C for 5 min.
NOTE: The optimal annealing temperature may be determined empirically. For the suggested master mix, subtract 5 °C from the primer melting temperature. - Load 12 µL of the PCR reaction on an agarose gel and freeze the samples at -20 °C.
9. Detection of dystrophin expression by Western Blotting
NOTE: This protocol is optimized for dystrophin, a large membrane protein. Specific conditions may be needed for different proteins.
- Protein extraction
- After 7-21 days of differentiation, collect cells with 5 mL of PBS with 100 µL 0.5 M EDTA, and 50 µL protease inhibitors. Incubate at 37 °C until cells detach. Centrifuge at 1,200 x g for 5 min at 4 °C. Snap freeze the pellet by dipping the tube in liquid nitrogen. Store the pellet at -80 °C or proceed to the lysis step.
- Prepare lysis buffer by adding 1% of digitonin, 1% protease inhibitor, 10% phosphatase inhibitor, and base buffer to total volume (60 µL per cell pellet).
- Add 60 µL of lysis buffer to the cell pellet, on ice. Sonicate for 5 s. Let sit on ice for 8 s. Repeat sonication and rest steps twice.
- Incubate samples on ice for 30 min.
- Centrifuge at 14,000 x g for 20 min at 4 °C.
- Transfer the supernatant to clean tubes.
- Quantify samples by bicinchoninic acid (BCA) assay, following manufacturer instructions.
- Mix the protein solution with the appropriate volume of Laemmli buffer. Make aliquots of 100 µg. If necessary, adjust the volume to 25 µl with base lysis buffer. Store samples at -80 °C.
- Western blotting
- Thaw samples on ice.
- Denature the samples at 100 °C for 5 min, then cool them down in ice, spin down.
- Dilute the 20X Tris-acetate SDS running buffer in 200 mL dH2O and add 500 µL antioxidant.
- Prepare the 3-8% Tris-acetate polyacrylamide gel by removing the comb and rinsing with dH2O. Assemble the gel in the electrophoresis apparatus. Fill the inner chamber with running buffer.
- Load 5 µL of protein ladder and 25 µL of sample in the gel. Fill the outer chamber with running buffer.
- Run at 80 V for 1 h at 4 °C. Then, at 120 V for 2 h at 4 °C.
- Prepare 3 L of 1X transfer buffer with 150 mL of 20X methanol, 150 mL of 20X transfer buffer, and 2,700 mL of dH2O. Cool it down to 4 °C.
- Cut 4 pieces of filter paper and one piece of nitrocellulose membrane. Soak the paper filter and membrane in a tray with transfer buffer.
- Gently remove the gel from the case and assemble it in the transfer apparatus with filter paper, membrane and sponges. The gel is placed on the negative side and the membrane on the positive side.
- Run transfer at 300 mA, stirring, at 4 °C, overnight.
- Block the membrane in 10 mL of blocking buffer for 1 h with gentle agitation, at room temperature.
- Prepare primary antibody solution with 10 mL of blocking buffer and 50 µL of dystrophin antibody (1:200).
- Discard the blocking buffer and add the primary antibody solution. Incubate with gentle agitation for at least 2 h at room temperature or overnight at 4 °C.
- Rinse the membrane three times with 0.1% Tween PBS, for 5 min with gentle agitation.
- Prepare secondary antibody solution using 10 mL of blocking solution, 2 µL of anti-rabbit antibody (1:5000), and 20 µL of 0.2% Tween.
- Add the secondary antibody solution to the membrane. Incubate for 1 h with gentle agitation, covered with aluminum foil to protect from light.
- Discard the antibody solution and rinse the membrane 3 times with 0.1% Tween PBS, for 5 min with gentle agitation, protected from light.
- Exposure and image the membrane on an imaging device.
- Stain the membrane for total protein with Revert 700 Total Protein stain, following manufacturer instructions.
NOTE: Dystrophin detection by western blotting depends on the age/mutation of the patient and the cell's ability to fuse and stay attached enough time to accumulate enough dystrophin.
Representative Results
This protocol shows how to establish human skin-derived fibroblast cultures and convert them into myoblasts and then into differentiated myotubes. This type of cell line is extremely useful for the study of neuromuscular disorders and in vitro testing of potential therapies.
A schematic representation of the fibroblast conversion is shown in Figure 1. Figure 2A shows a fragment of skin and the fibroblasts emerging from it. The fibroblasts should be passed to a new dish when confluence is reached (Figure 2B). Figure 3A shows the ideal confluence of fibroblasts before changing to myoblast growth medium supplemented with doxycycline. The cells should be around 70% confluent because they still proliferate during the conversion process. If cells are above 80% confluent, the differentiation may be compromised. The conversion into myoblasts takes two to four days, and it is confirmed by observation of the morphology. The cells become elongated and parallelly oriented, as shown in Figure 3B. After the addition of the differentiation medium, the myoblasts stop dividing and start to fuse to form multinucleated myotubes (Figure 3C). When the myotubes borders look white and bright, they are about to detach (Figure 4). At this point, collect or fix the cells.
The differentiation success will vary between different cell lines/mutations. Immunostaining of muscle proteins expressed by mature myotubes confirms the myogenic potential of converted fibroblasts (Figure 5). RNA-Seq analysis comparing FM myotubes and skeletal muscle showed high-level expression of transcripts from the embryonic (MYH3) and neonatal (MYH8) myosin chain genes and good overall transcriptome-wide correlation with muscle (Figure 6). Transcripts for the giant sarcomeric proteins titin (TTN), nebulin (NEB), and obscurin (OBSCN) are also expressed by FM myotubes, indicating upregulation of these large transcripts involved in myofibrillogenesis. Thus, FM cells have a muscle-specific expression profile, demonstrating that they are a useful and reliable surrogate for muscle-derived cell lines.
To illustrate exon skipping, we used this protocol in one of the most frequent exon duplications in the DMD gene. Duplication of exon 2 leads to disruption of the DMD reading frame, thus the restoration of the reading frame following exon skipping should lead to the expression of the full-length dystrophin. However, it is also possible that skipping of exon 2 is very efficient resulting in an out-of-frame transcript. Nevertheless, in this case, skipping of both copies of exon 2 induces the utilization of an alternative internal ribosome entry site (IRES) present in exon 5, thereby producing functional N-truncated dystrophin that was identified in patients still ambulant in their 70s12. Figure 7A shows representative results of RT-PCR of FM cells with exon 2 duplication. FM cells were treated either with AON or AAV1-U7 carrying an antisense sequence to skip exon 2. In Figure 7B, an immunoblot shows the detection of the N-truncated dystrophin in FM cells treated with AAV1-U7. In vitro treatment of FM cells serves as proof of concept for exon-skipping strategies.
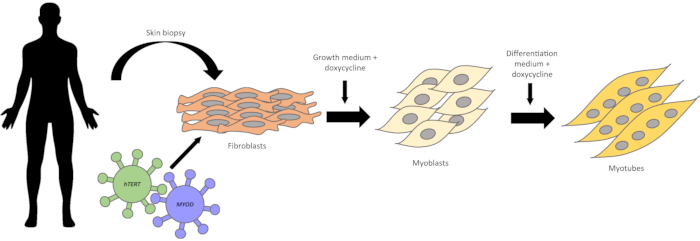
Figure 1: Schematic representation of fibroblasts conversion into myogenic cells. A skin biopsy is obtained from human subjects. Skin fragments are placed on culture dishes. Within one week, fibroblasts start to emerge. Fibroblasts are first transduced with the hTERT gene, and then with the Myod gene, using lentiviral vectors. After antibiotic selection of infected cells, the conversion into myoblasts is induced by the addition of doxycycline to the myoblast growth medium. Within two to four days, the cells become elongated and parallelly oriented. After switching to differentiation medium, the myoblast fuse with each other and form multinucleated myotubes. Please click here to view a larger version of this figure.
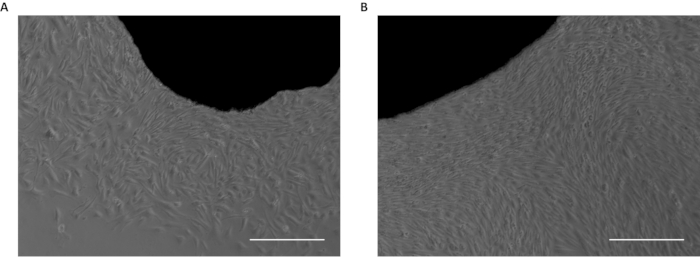
Figure 2: Skin biopsy fragments in culture. (A) First fibroblasts emerging from skin fragment. (B) Confluent fibroblasts emerged from the skin fragment. Scale bar: 50 µm. Please click here to view a larger version of this figure.
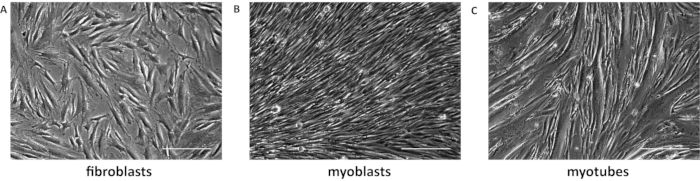
Figure 3: Fibroblasts transdifferentiation. (A) Representative image of 70% confluent fibroblasts. (B) Converted myoblasts have elongated morphology and are parallelly organized. (C) Myotubes were differentiated for 7 days. Scale bar: 50 µm. Please click here to view a larger version of this figure.

Figure 4: Representative image of detaching myotubes. The arrows indicate the white and bright edges of myotubes starting to detach. Scale bar: 50 µm. Please click here to view a larger version of this figure.
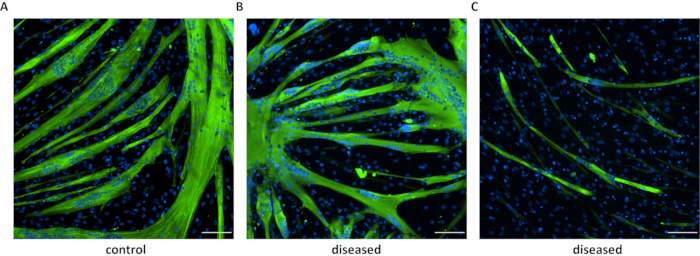
Figure 5: Immunofluorescence of differentiated myotubes. Immunostaining of myosin heavy chain in myotubes derived from a healthy (A) individual and patients with neuromuscular disorders (B and C). In B are shown cells from myotonic dystrophy type 1 (DM1) carrying 230 CTG repeats, and in C are DM1 cells with 900 CTG repeats. Scale bar: 100 µm. Please click here to view a larger version of this figure.
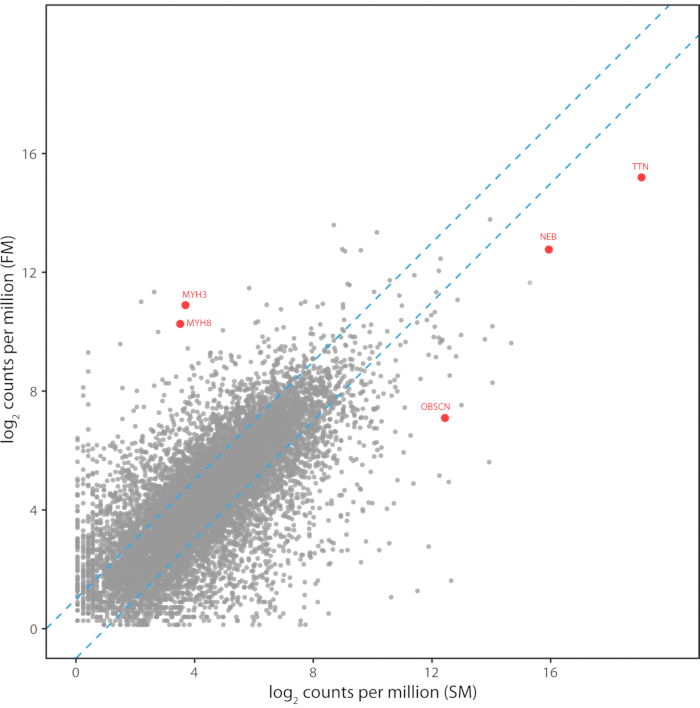
Figure 6: Transcriptome pattern of FM myotubes compared to skeletal muscle. Transcriptome pattern of FM myotubes compared to skeletal muscle. The read counts per million mapped reads for 12,134 transcripts are shown for Illumina RNA-Seq libraries prepared from FM myotubes and a human skeletal muscle biopsy. Transcript levels between the two libraries had a Pearson correlation of 0.71 and a Spearman rank correlation of 0.73. Transcripts for the developmental myosin heavy chains and the large sarcomeric proteins are highlighted in red. Please click here to view a larger version of this figure.
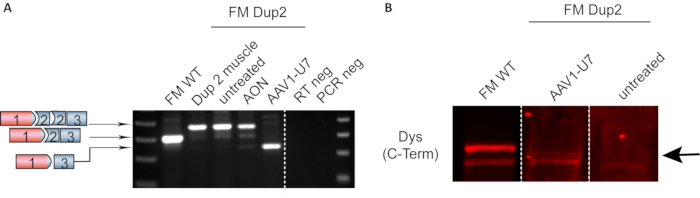
Figure 7: Representative RT-PCR and Western blot showing DMD exon skipping in FM cells. (A) Expression DMD by RT-PCR. Fibroblasts from a patient harboring a duplication of DMD exon 2 were converted into FM cells. RNA extracted from muscle biopsy was used as the control, showing that FM untreated cells express the same duplicated transcript. FM cells treated with AON have a partial skipping of exon 2 duplication, while AAV1-U7 treated cells showed a predominance of transcripts with exon 2 duplication skipped. (B) Representative immunoblot of FM cells treated with AAV1-U7. Smaller N-truncated dystrophin was detected 14 days after treatment (indicated by the arrow). Data previously published in Wein et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nature Medicine. 2014. 2020 Springer Nature Limited. Please click here to view a larger version of this figure.
| Fibroblast growth medium | DMEM with 20% FBS, 1% antibiotic-antimicotic |
| Freezing medium | 10% DMSO, 90% fibroblast medium |
| Doxycycline stock solution 1000X | 8 mg of doxycycline in 1 mL ultra-pure water. Filter in 0.22 µm syringe filter. Aliquot in PCR tubes. Store at -20 °C, protected from light. |
| Myoblast medium | Skeletal muscle cell growth medium (see list above) with supplements, 8 µg/mL doxycycline. For example: 100 µL of 1000X stock solution in 100 mL. |
| Differentiation medium | Skeletal muscle cell differentiation medium with supplements (see list above), 8 µg/mL doxycycline. For example: 100 µL of 1000X stock solution in 100 mL. |
| Blocking solution for IF staining | 10% goat serum (or serum of animal in which secondary antibody was raised) in 1X PBS |
| Base buffer for protein extraction | NaCl 150 mM, Tris 50 mM, 0.05 % NP-40. Adjust pH to 7.4. Store at 4 °C. |
Table 1: Medium recipes
Discussion
To obtain FM cell lines with good quality, some steps are critical. The sooner the skin biopsy is processed, the greater the chances are to obtain healthy fibroblasts. The passage number of fibroblasts cultures is also important. Primary cells have limited proliferative capacity and after many passages, they enter in replicative senescence. Thus, it is better to have a stock of fibroblasts at a low passage number and transform cells at the earliest passage as possible.
Another important step is also to have viral production that has maximum purity and accurate quantification. For example, viral genome quantification using qPCR provides reasonable measurements, but quantification by ddPCR (digital droplet PCR) is more accurate.
In addition, the adequate confluence of fibroblasts for myoblast conversion is critical. If the cells are below 70% or above 80% confluent, the myogenic differentiation may be impaired. If cells are too confluent, there will be the superposition of layers of myotubes, which interfere with staining and imaging. The concentration of doxycycline is crucial for correct activation and sustained expression of the Myod gene. It is very critical to always add the doxycycline to the medium right before doing media changes, as it degrades quickly after diluted in medium and stored at 4 °C. The stock should be stored at -20 °C at a concentration of 1000X and protected from light. Do not re-freeze thawed aliquots. It is very important to follow these details to ensure reproducible experiments and discriminate an impaired differentiation due to a genetic mutation from technical issues. Nevertheless, depending on the mutation or the type of disease, a good differentiation may not be possible. To ensure trustful results, it is very important to replicate experiments at similar passage numbers.
In our experience, the differentiation capacity persists at least up to passage 25-27, especially in wild-type controls. The same may be valid for some diseased cell lines, but it depends on the cell line. Some DMD cell lines still retain the myogenic potential above P20. In opposition, a myotonic dystrophy type 1 (DM1) cell line presented reduced myogenicity after P8. However, in the case of DM1, this is not surprising as it has been demonstrated that mutations in DM1 indirectly play a role in muscle differentiation16. The retention of the myogenic differentiation capacity should be addressed individually, but generally, most of the cell lines retain it up to P20-25.
In summary, the conversion of fibroblasts into myoblasts is a powerful tool to study and test therapeutic strategies for neuromuscular disorders. It facilitates access to human cell models by avoiding the complicated obtention of muscle biopsies and reduce the inconvenience of a muscle biopsy for the patients.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Vincent Mouly for sharing his knowledge in the past regarding the model. This work has been supported by the US National Institutes of Health National Institute of Neurological Disorders and Stroke (R01 NS043264 (K.M.F., and R.B.W.)), the US National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (P50 AR070604-01 (K.M.F., K.M., R.N., and N.W.). N.W. has received fellowship support from the Ohio State University/Nationwide Children's Hospital Muscle Group and the Philippe Foundation. This work was also supported by internal discretionary funds and part of the exon 2 skipping work has been supported also by CureDuchenne (K.M.F.) and Association Francaise Contre Les Myopathies. IRB number: IRB #: IRB10-00358/ CR00005138 and IBCSC#: IBS00000123.
Materials
| 100 mm dish | Corning | 430167 | |
| 0.25% Trypsin-EDTA, phenol red | Thermo Fisher | 2500056 | |
| 10X Phosphate buffered saline (PBS) | Fisher Scientific | BP3994 | |
| 12-well plate | Corning | 3513 | |
| 20X Transfer buffer | Thermo Fisher | NP00061 | |
| 20X Tris-acetate SDS running buffer | Thermo Fisher | LA0041 | |
| 3-8% Tris-Acetate gel | Thermo Fisher | EA0378BOX | |
| 75 cm2 flask | Corning | 430641U | |
| Antibiotic-Antimicotic 100X | Thermo Fisher | 15240062 | |
| Anti-myosin heavy chain, sarcomere antibody | Developmental Studies Hybridome Bank | MF20 supernatant | Dilution 1:50 |
| Antioxidant | Thermo Fisher | NP0005 | |
| BCA Protein Assay | Thermo Fisher | 23227 | |
| Chloroform | Sigma-Aldrich | C2432 | |
| DAPI | Thermo Fisher | D3571 | Dilution 1:1000 |
| Digitonin | Millipore Sigma | 300410250MG | |
| Dimethyl sulfoxide | Sigma-Aldrich | D2438 | |
| DMEM, High glucose, GlutaMAX supplement, Pyruvate | Thermo Fisher | 10569044 | |
| DNAse I set (250U) | Zymo Research Corporation | E1010 | |
| Doxycycline Hydrochloride | Fisher Scientific | BP2653-5 | |
| Dup2 human primers | Fw_5' GCTGCTGAAGTTTGTTGG TTTCTC 3' |
Rv_5' CTTTTGGCAGTTTTTGCC CTGT 3' |
|
| Dystrophin antibody | Abcam | ab15277 | Dilution 1:200 |
| Fetal bovine serum | Thermo Fisher | 16000 | |
| Glycine | Sigma-Aldrich | G8898 | |
| Goat anti-mouse, Alexa Fluor 488 | Thermo Fisher | A11001 | Dilution 1:1000 |
| Halt Protease inhibitor cocktail 100X | Thermo Fisher | 78430 | |
| Hemocytometer | Hausser Scientific | 3100 | |
| Hygromycin B | Thermo Fisher | 10687010 | |
| IRDye 680RD goat anti-Rabbit IgG (H+L) | Li-Cor | 926-68071 | Dilution 1:5000 |
| Lab-Tek II CC2 chamber slide system | Thermo Fisher | 15852 | |
| Laemmli | Bioworld | 105700201 | |
| Lipofectamine 3000 Transfection Reagent | Thermo Fisher | L3000008 | |
| Matrigel GFR membrane matrix | Corning | 354230 | |
| Methanol | Fisher Scientific | A412P-4 | |
| Mr. Frosty Freezing Container | Thermo Fisher | 51000001 | |
| Nitrocellulose membrane 0.45 µm | GE Healthcare Life Sciences | 10600002 | |
| Normal Goat serum control | Thermo Fisher | 10000C | |
| Odyssey Blocking Buffer (PBS) | Li-Cor | 927-40003 | Blocking buffer for Western blot |
| Opti-MEM I Reduced Serum Medium | Thermo Fisher | 11058021 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| PCR master mix | Thermo Fisher | K0172 | |
| Phosphatase inhibitor | Thermo Fisher | A32957 | |
| Precision Plus Protein Dual Color Standards | Bio Rad | 1610374 | |
| Puromycin | Thermo Fisher | A1113803 | |
| Revert 700 Total Protein Stain for Western Blot Normalization | Li-Cor | 926-11021 | |
| RevertAid kit | Thermo Fisher | K1691 | |
| RNA Clean & Concentrator-25 | Zymo Research Corporation | R1018 | |
| Scalpels | Aspen Surgical | 372611 | |
| Skeletal Muscle Cell Differentiation medium | Promocell | C23061 | |
| Skeletal Muscle Cell Growth medium | Promocell | C23060 | |
| Triton X-100 | Acros Organics | 215682500 | |
| TRIzol reagent | Thermo Fisher | 15596026 | |
| Tween 20 | Fisher Scientific | BP337500 | |
| Ultra low temperature freezer | Thermo Scientific | 7402 | |
| UltraPure 0.5M EDTA, pH 8.0 | Thermo Fisher | 15575020 | |
| Vectashield antifade mounting medium | Vector Labs | H1000 |
Riferimenti
- Bigot, A., et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biology of the Cell. 100 (3), 189-199 (2008).
- Mamchaoui, K., et al. Immortalized pathological human myoblasts: Towards a universal tool for the study of neuromuscular disorders. Skeletal Muscle. 1 (34), (2011).
- Pantic, B., et al. Reliable and versatile immortal muscle cell models from healthy and myotonic dystrophy type 1 primary human myoblasts. Experimental Cell Research. 342 (1), 39-51 (2016).
- Thorley, M., et al. Skeletal muscle characteristics are preserved in hTERT/cdk4 human myogenic cell lines. Skeletal Muscle. 6 (1), 43 (2016).
- Chaouch, S., et al. Immortalized skin fibroblasts expressing conditional MyoD as a renewable and reliable source of converted human muscle cells to assess therapeutic strategies for muscular dystrophies: Validation of an exon-skipping approach to restore dystrophin in duchen. Human Gene Therapy. 20 (7), 784-790 (2009).
- Zarei, A., Razban, V., Hosseini, S. E., Tabei, S. M. B. Creating cell and animal models of human disease by genome editing using CRISPR/Cas9. The Journal of Gene Medicine. 21 (4), 3082 (2019).
- Echevarría, L., Aupy, P., Goyenvalle, A. Exon-skipping advances for Duchenne muscular dystrophy. Human molecular genetics. 27 (2), 163-172 (2018).
- Hwang, J., Yokota, T. Recent advancements in exon-skipping therapies using antisense oligonucleotides and genome editing for the treatment of various muscular dystrophies. Expert reviews in molecular medicine. 21, 5 (2019).
- Wein, N., et al. Efficient Skipping of Single Exon Duplications in DMD Patient-Derived Cell Lines Using an Antisense Oligonucleotide Approach. Journal of Neuromuscular Diseases. 4 (3), 199-207 (2017).
- De Angelis, F. G., et al. Chimeric snRNA molecules carrying antisense sequences against the splice junctions of exon 51 of the dystrophin pre-mRNA induce exon skipping and restoration of a dystrophin synthesis in Δ48-50 DMD cells. Proceedings of the National Academy of Sciences of the United States of America. 99 (14), 9456-9461 (2002).
- Gorman, L., Suter, D., Emerick, V., Schümperli, D., Kole, R. Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proceedings of the National Academy of Sciences of the United States of America. 95 (9), 4929-4934 (1998).
- Wein, N., et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nature Medicine. 20 (9), 992-1000 (2014).
- Auré, K., Mamchaoui, K., Frachon, P., Butler-Browne, G. S., Lombès, A., Mouly, V. Impact on oxidative phosphorylation of immortalization with the telomerase gene. Neuromuscular Disorders. 17 (5), 368-375 (2007).
- Barde, I., et al. Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector. Molecular Therapy. 13 (2), 382-390 (2006).
- Wang, X., McManus, M. Lentivirus production. Journal of Visualized Experiments. (32), e1499 (2009).
- Amack, J. D., Mahadevan, M. S. Myogenic defects in myotonic dystrophy. Biologia dello sviluppo. 265 (2), 294-301 (2004).

