Combining Imaging and Electrophysiology to Visualize and Record Spreading Depolarizations in Mice
Summary
This protocol demonstrates the combination of imaging and electrophysiology to reliably detect spreading depolarizations in adult mice following a mild traumatic brain injury.
Abstract
Spreading Depolarizations (SDs) are massive events in the brain that often go undetected due to their slow propagation through gray matter. Because SD detection can be elusive, it is optimally confirmed using multiple methods. This protocol describes methods for combining imaging and electrophysiology to detect SDs in a manner that most laboratories can reliably and easily adopt. SDs occur following traumatic brain injuries, stroke, subarachnoid hemorrhages, ischemia, and migraine aura. Historically, SDs have been recorded using DC amplifiers, which can resolve the slow extracellular shift and the depression in high-frequency activity. However, DC amplifiers are nearly impossible to use for chronic in vivo recordings. This protocol employs a common AC amplifier for in vivo electrophysiology recordings to confirm high-frequency depression, along with non-invasive imaging necessary to detect the propagating wave of SD. These methods can be reliably adopted and/or modified for most experimental approaches to confirm the presence or absence of SDs following brain injury.
Introduction
Spreading depolarizations (SDs) are associated with various neurological conditions, including traumatic brain injury (TBI), stroke, subarachnoid hemorrhages, ischemia, and migraine aura1. SDs have been implicated in the progression of tissue damage in strokes and TBIs2. The exact mechanism underlying tissue loss progression remains unclear3,4. However, SDs occur when the metabolic demands of neurons are not met with sufficient oxygen and glucose, as the function of the ATP-dependent sodium-potassium pump becomes inadequate to maintain ion gradients. To understand the role of SDs in tissue loss, precise techniques are needed to measure and study them, which is crucial for advancing scientific knowledge. SDs are significant neurological events that can often go undetected due to experimental design and/or instrumentation limitations.
The role of spreading depolarizations (SDs) in injury progression is currently being elucidated, but their role in mild traumatic brain injuries (mTBIs) remains unclear. Recent studies using the techniques described in this manuscript have shown that SDs are initiated in closed-skull mTBI mouse models, even when there is no overt tissue damage or bleeding5,6. These studies suggest that SDs may contribute to acute neurological impairments and neuroinflammation. Additionally, the presence of mTBI-induced SDs has been closely associated with acute behavioral deficits6,7. Therefore, it is likely that SDs are initiated in all TBI models, but their role remains difficult to ascertain without confirmation. Confirming the presence of SDs is crucial for accurately assessing the outcomes of mTBIs.
Spreading depolarizations (SDs) are identified by a significant drop in extracellular potential, known as the "DC shift," and by a depression of high-frequency activity8. The "DC shift" typically takes seconds to resolve (~10 s)9. To capture the "DC shift," a DC amplifier and silver wire electrodes are required. However, in vivo electrophysiology is usually performed with AC amplifiers, which are designed to filter out low-frequency events to reduce motion artifacts. Unfortunately, this low-frequency filtering also removes the "DC shift." Additionally, silver is toxic and unsuitable for chronic recordings. Commonly used AC amplifiers can detect the depression of high-frequency activity, but without the accompanying "DC shift," confirming the presence of an SD is challenging.
Spreading depolarizations (SDs) can also be detected using various imaging techniques. Most commonly, SDs are imaged with laser speckle contrast imaging, which measures cerebral blood flow through the intact skull10. SDs significantly influence cerebral vasculature and blood flow. In most species other than mice, SDs induce a propagating wave of hyperperfusion that travels with the leading edge of the SD11. SDs can also be imaged using calcium or glutamate fluorescent indicators12, though this requires loading tissue with dyes or genetically encoded fluorescent indicators12,13. Interestingly, SDs can be observed by the naked eye due to intrinsic optical signals (iOS) associated with the SD14. iOS are complex signals composed of autofluorescent molecules like NAD(P)H and FADH15,16, tissue swelling17, and potential changes in cerebral blood flow. Despite their complexity, iOS signals are reliable and can be captured in wild-type mice18. This protocol describes the method for imaging iOS because this technique can be implemented in any lab without the need for specialized viruses or transgenic animals.
The overall goal of this method is to experimentally confirm the presence or absence of a spreading depolarization (SD) following a mild traumatic brain injury (mTBI) using simultaneous imaging and electrophysiology. In vivo electrophysiology, facilitated by advanced amplifiers, is a commonly used technique. However, these amplifiers are unable to detect the characteristic "DC shift." Therefore, imaging techniques, as described in this protocol, are employed to confidently confirm the presence or absence of SDs. The advantage of combining imaging and electrophysiology is that imaging can detect the propagation of the SD, while electrophysiology can identify the associated high-frequency depression.
Overall, this method is suitable for investigating the presence of spreading depolarizations (SDs) following a traumatic brain injury (TBI). Its application could enhance understanding of cortical network function after TBI and SDs. This protocol can be adapted from a closed skull impact model to other models, such as controlled cortical impact, fluid percussion, or weight drop. Implementing this protocol across multiple laboratories could elucidate the role of SDs across varying TBI severities. Any established TBI model can be used, followed by the iOS imaging and electrophysiology procedures described in this protocol.
Protocol
All procedures were performed in accordance with the Institute's Animal Care and Use Committee (IACUC) at the University of New Mexico. C57BL/6J mice were obtained from a commercial source. Equal numbers of male and female mice were used within the age range of 8-14 weeks. All mice weighed approximately 20 g. Details of the animals, reagents, and equipment used in this study are listed in the Table of Materials. Supplementary File 1 contains the list of abbreviations.
1. Preparing the skull for imaging and electrophysiology
- Anesthetize the animal in an isoflurane induction box with 2%-3% isoflurane in 100% oxygen, ensuring proper anesthesia depth with no flinching from a paw pinch.
- Place the animal into a stereotaxic frame and maintain anesthesia with 2% isoflurane.
- Monitor and maintain animal's body temperature with a heating pad that has body temperature feedback.
- Shave the hair above the skull where the incision will be, down the midline from the ocular fissure to lambda.
- Sterilize the skin with antiseptic and disinfectant scrub and solution, finish with 70% ethanol, and repeat 3 times to ensure a sterile surgical site.
- Using a #10 scalpel blade, make an incision down midline from rostral to caudal, immediately followed by the application of cyanoacrylate glue to the incision. Spread the skin laterally to expose the skull.
- Add another drop or two of cyanoacrylate glue over the skull to add additional protection to the skull.
NOTE: Glue application is time-sensitive as the skull will begin to go opaque when exposed to the air for longer than 10 s. This will obscure the visualization of the SD through imaging methods. - While the glue is drying, use sterile surgical scissors to increase the incision size over the cerebellum. Once the glue is set, begin the implantation of skull screw electrodes.
- Sterilize skull screws using 70% ethanol prior to implantation.
- Place the skull screws in a region distant from the SD initiation site to maintain an open visual window.
NOTE: For chronic recordings in the primary visual cortex, three skull screws are used. The first screw is placed approximately at y = -6.00 mm from the bregma, in the cerebellum, serving as a reference electrode. The recording electrodes are positioned at y = -2.79 mm from bregma and x = ±2.5 mm from the midline, within the left and right hemispheres. - With a 0.8 mm size drill bit, drill pilot holes through the skull. To ensure no damage to the underlying tissue, -0.5 mm in z is ideal. Use #00-90 screws that are pre-soldered to gold pins with stainless steel wire.
- Turn the screws down into the skull.1.5-2 full turns will get the screw through the skull and in contact with the cortical surface.
- Wind excess wire around the screw, allowing some slack in the wire for headstage placement later.
- Using the same procedures, place the other skull screws.
- Once all skull screws are in place, plug all the gold pins into the desired headstage connector. Hold the connector in place using the stereotaxic arm and hold the connector pins close above the screw heads while ensuring the wires are not touching.
- Seal the entire area with cyanoacrylate glue, making sure not to disrupt the imaging area in front of the skull screws.
- Build up dental cement around the base of the gold pins, ensuring that the gold pins from the skull screws are not connected to the gold pins in the head stage.
- Allow the dental cement to cure before removing the headstage connector.
- Remove the animal from the stereotaxic frame, give 0.01 mg/kg buprenorphine via intraperitoneal injection, and place it back into the home cage to recover. Follow standard postoperative care and keep animals on a heating pad while recovering from anesthesia.
NOTE: After surgery, animals were single-housed and monitored for pain daily. Give additional buprenorphine to manage pain 24 h post-op based on pain assessment.
- Remove any objects that may catch on the electrodes, including housing and enrichment. Use standard bedding with ad libium access to food and water.
NOTE: Allow for a two-day recovery period between surgery and recording.
2. Detecting spreading depolarizations with intrinsic optical signal (iOS) imaging and AC- electrophysiology
- Anesthetize the animals with 2% isoflurane in 100% oxygen.
NOTE: The imaging part of this procedure should take ~5 min, and the amount of isoflurane exposure should be consistent. - Place the animal into a stereotaxic frame. Through the nose cone, maintain 1%-2% isoflurane in oxygen.
NOTE: It is critical to maintain proper depth of anesthesia, as isoflurane is known to reduce SDs. Maintaining 60-70 breaths/min is recommended throughout the experiment. - Place the animal beneath the camera and ensure the camera is focused on the cortical surface by adjusting the focus ring.
- Apply mineral oil to the imaging area to increase transparency.
- Turn on the computers, LED panel board, and electrophysiology equipment.
NOTE: LED panel board works best when positioned in front of the animal to illuminate the imaging window while overhead lights are off. The light was set to ~2,400 lux (95% power) with a cool temperature setting of 4000K. - Load setting for iOS using Micro-Manager19,20,21,22.
- Press Live to view the mouse under the camera and center the animal's skull under the camera. Close the "Live" window.
- Press Multi-D Acq.
- In the Multi-Dimensional Acquisition window, select the desired time points.
NOTE: A count of 750 images at an interval of 250 ms is recommended. - Save images to the desired file path and name prefix.
- Connect the gold pins from the animal to the headstage connector and then to the headstage.
- Load setting for Intan software.
- Click on Choose file format icon, and in pop-up window, select One File Per Signal Type Format. This will save the file as a ".dat" for further use in MATLAB.
- In the BW tab under Hardware Bandwidth, select amplifier bandwidth of 0.13-500 Hz.
- Under Filter Display Selector, select WIDE to cover the widest range of signals.
- To select the desired file path to save, click on the Select Filename icon.
- Perform impact using an electromagnetic impactor.
NOTE: Impactor settings are 4 m/s impact speed and 5 mm deflection.- Turn the impactor on and ensure that the tip retracts and extends appropriately by flicking the 3-way toggle to retract the tip and extend the tip. The tip should be extended before placing the animal underneath the tip.
- Moving quickly, place the animal on a foam bed and slide the bed beneath the tip.
- Align the impactor tip at midline and 1 mm rostral of bregma.
- Lower the impactor tip until it is firmly touching the top of the animal's head.
NOTE: Do not push the tip down into the head. - Push the lever to retract the impactor tip and then manually lower it 5 mm before activating the impact lever.
- Immediately remove the animal from the foam bed and place the animal beneath the camera for imaging within 5 s, as the SD is initiated from the impact.
- Start iOS imaging by clicking on Acquire! in the Multi-Dimensional Acquisition window. Image for 4 min to capture the entire SD.
- Start the electrophysiology recording by clicking on the Record icon on the Intan software.
- Remove the animal from the foam pad and allow the animal to recover in an open arena or home cage.
NOTE: It is recommended that electrophysiology be recorded for at least 1 h to allow the cortical network function to recover. - Upon the completion of the recording, unplug the animal from the headstage and connector and place the animal back into its cage.
NOTE: It is recommended to allow 24 h between each impact and recording session. The animal can remain in its home cage for the entire time outside of recording sessions.
3. iOS image visualization and quantification
- In ImageJ19,20,21,22, open the image stack by clicking on the File > Import > Image sequence.
- Manually scroll through images to ensure the lack of movement or any other artifacts.
- To generate the plot of the SD, create an ROI by using the cursor to draw a square.
NOTE: It is recommended to use a 30 x 30-pixel square. One may use any shape or size, but ensure ROI size does not change across animals. - Create a plot by clicking on Image > Stacks > Plot Z-axis Profile. On the newly created graph, click on the live button. Go back to the image stack of the SD and move the ROI around until the graph shows the SD curve. The plot should look like a Sin wave with an initial rise followed by a drop below the baseline and a slow return to baseline within a few minutes.
NOTE: The plots can be expressed as arbitrary units (AU) or as percent baseline using the first few images as the baseline average. - On the generated plot, click on List to generate a list of values that can be plotted in other graph-generating software or Matlab.
- For visualization, turn the grayscale image into a lookup table in ImageJ by Image > Lookup Tables > Fire.
4. Electrophysiology analysis
NOTE: It is recommended that the expertise of the Buzsaki lab and their MATLAB-generated code be heavily utilized for visualizing and analyzing electrophysiology data. The analysis will require downloading MATLAB with an institutional activation code and the Buzsaki lab "Buzcode" zip file via GitHub (https://github.com/buzsakilab/buzcode).
- In the Intan electrophysiology software, ensure that the exported raw data is saved in ".dat" format.
NOTE: Matlab can be installed from the MathWorks website. An institutional or individual activation code is necessary for the purchase and use of Matlab. Some prior experience with running code in Matlab is necessary for performing this analysis. - Use the Buzaki code bz_LoadBinary function to load the data into Matlab.
NOTE: Example "'data' = bz_LoadBinary ('full data file path','frequency', sampling frequency, nChannels, number of channels); - Filter the AC noise using a notch filter centered at 60 Hz.
NOTE: Example notch_freq = 60; [b,a] = iirnotch(notch_freq/(sampling_frequency/2), notch_bandwidth/(sampling_frequency/2)); filtered_data = filtfilt(b, a, data); - Isolate the high-frequency activity using a bandpass filter with a range of 0.5-100 Hz, in accordance with the recommendations of the COSBID group for analyzing and visualizing spreading depolarizations (SDs).
- Calculate the power spectrogram using the pspectrum command in MatLab.
- To visualize, plot the high-frequency and the power spectrogram on the same time scale and in one column.
NOTE: From the plots, there will be an extension of the high-frequency depression and loss of power.
Representative Results
Figure 1 illustrates a graphical representation of the experimental setup. The recording skull screws are positioned in the primary visual cortex, with one skull screw electrode placed in the cerebellar vermis as a ground electrode. The skull screws and gold pins are secured to the skull using cyanoacrylate glue and dental cement. It is crucial to limit the dental cement to the caudal part of the skull to leave space for impact and imaging. The impact location, centered at midline and approximately 1 mm rostral to bregma, is indicated in Figure 1.
Representative intrinsic optical signals (iOS) images are shown in Figure 2A. Grayscale images are converted using a Fire Lookup Table to enhance the visualization of spreading depolarizations (SDs). The iOS encompasses various signals, including NAD(P)H, FADH, and tissue reflectance, making interpretation and comparison of these signals challenging. Large vessels should be clearly visible through the super glue window (denoted with arrows), while the dashed line in Figure 2A marks the propagating wave of the SD. Although SDs are difficult to observe in real-time, they become more apparent when the images are sped up (see Supplementary Video 1). Some small branches may appear to "disappear" due to vasoconstriction (Figure 2A). A small region of interest (ROI) extracted from the image stack should yield a Z-axis plot similar to Figure 2B. From the electrophysiology recordings, the extracted high-frequency signal should resemble the top panels of Figure 2C,D. These recordings were initiated immediately after the mild traumatic brain injury (mTBI), with animals remaining under isoflurane anesthesia during the 4 min of iOS imaging. Upon removal of isoflurane, high-frequency activity should return quickly within a minute in sham animals, as shown in Figure 2C (red box). Conversely, if an SD occurred, high-frequency activity would remain depressed for several minutes before recovery (Figure 2D, red box). The lower panels of Figure 2C,D display the calculated power (V²) for each high-frequency plot. These power plots help identify the suppression and recovery of high-frequency activity by the rapid rise in power (indicated by the right edge of the red boxes).
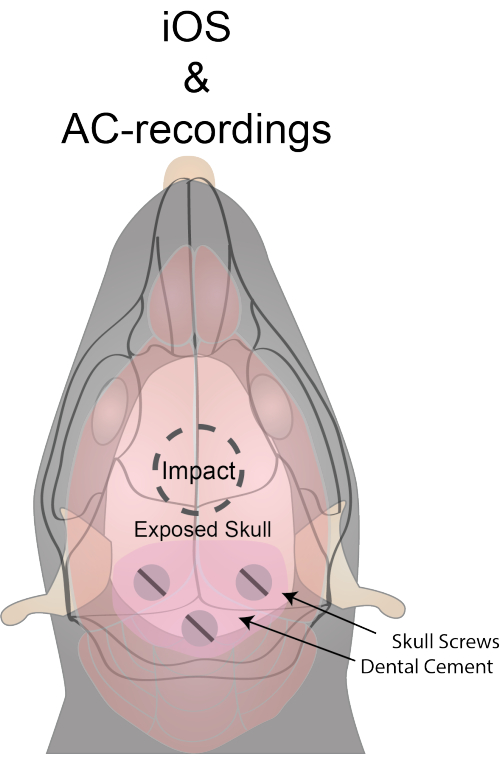
Figure 1: Experimental setup and location of impact and skull screws. The dorsal surface of the skull is exposed through a skin incision. The skull and skin are sealed with cyanoacrylate glue to enhance transparency. A burr hole for the ground is drilled at y = -6.00 and x = 0 from bregma. Burr holes for recording electrodes are placed at y = -2.79 and x = ±2.5 from bregma. Stainless steel screws are threaded through the skull and soldered to gold pins with uncoated stainless steel wire. Care is taken to avoid excessive coverage of the skull surface with dental cement to ensure adequate space for impact and imaging. Please click here to view a larger version of this figure.
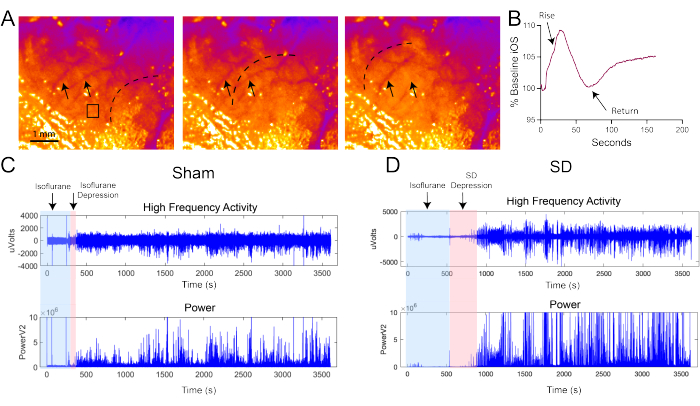
Figure 2: Representative iOS images and AC recordings. (A) Representative iOS images with arrows indicating blood vessels. Grayscale images are converted to the Fire lookup table in ImageJ for improved visualization. The dotted black line denotes the propagating SD, visualized as a wave of increased intensity in the iOS. The SD is clearly visible when quickly scrolling through the entire image stack. The iOS signal reflects various physiological and metabolic changes associated with the SD, including mitochondrial production of NAD(P)H and FADH and neuronal edema. The black box highlights the region of interest (ROI) used to generate the trace in (B). Scale bar: 1 mm. (B) Representative trace of the iOS SD expressed as a percentage of baseline. The iOS signal rises and returns near baseline within approximately 60 s. Although changes in iOS signals are small (about 10%), they are sufficient to identify the SD. In the AC recordings for both sham (C) and SD (D) animals, the top traces show high-frequency activity, and the bottom traces represent power (V²). Blue boxes indicate periods of isoflurane anesthesia, while the red box denotes the recovery period of high-frequency activity from isoflurane and SD. The prolonged depression induced by SD is contrasted with the rapid recovery of activity following isoflurane alone (sham). Please click here to view a larger version of this figure.
Supplementary Video 1: Propagating wave visualization. The video file is sped up to approximately 8x speed to facilitate visual identification of the propagating wave. The grayscale image was converted to the Fire lookup table and saved as an .AVI file in ImageJ. Please click here to download this File.
Supplementary File 1: List of abbreviations. Please click here to download this File.
Discussion
Spreading depolarizations are crucial in various disease models, including traumatic brain injury (TBI), stroke, and migraine1. Accurate detection of SDs is essential for understanding their role in acute behavioral deficits and/or the activation of the neuroimmune system following mild traumatic brain injuries (mTBIs)23,24. To mitigate the risk of missing SDs when using a single method, a multi-method approach is necessary for effective visualization and recording of SDs.
While combining techniques enables confirmation of spreading depolarizations (SDs), each technique may have potential pitfalls. The imaging techniques described in this protocol are accessible to most laboratories and are detailed by Zepeda and colleagues25. Preparation of the cranial window is crucial, as opaque windows can lead to inconclusive images26. This issue can be mitigated by minimizing the exposure of the skull to air before applying cyanoacrylate glue. Additionally, the transparency of the window can be improved with a drop of mineral oil prior to imaging. Movement artifacts can also complicate SD confirmation, so minimizing these artifacts is essential for obtaining clear video. To address this, it is important to keep the animal under isoflurane throughout the imaging process27. Using a nose cone facilitates efficient transfer of the animal between the impactor and recording setup. It is critical to quickly place the animal back under the nose cone after impact to capture as much of the SD propagation as possible. In this mild traumatic brain injury (mTBI) model, recording for approximately 4 min is usually sufficient to capture the entire SD. However, in more severe TBIs, spontaneous SDs may be generated by tissue damage28. Continuous imaging can detect these spontaneous SDs, but extended isoflurane anesthesia can make it challenging to observe high-frequency depression. Spontaneous SDs may be detected with electrophysiology by additional depressions in high-frequency activity, but confirming the relationship between depression and SD is difficult without imaging.
With the development of Open-Ephys and Intan amplifiers, in vivo electrophysiology is now accessible to many laboratories. All electrophysiological recordings require the implantation of skull screws and/or electrodes. In this protocol, there is a two-day latency period between the implantation of skull screws and recording to support recovery. While this period may not be sufficient for the brain to fully recover from the implantation, there should be minimal damage as the skull screws do not penetrate brain tissue. Additionally, the transparency of the cyanoacrylate-sealed skull decreases over time, so imaging should be performed as soon as possible to achieve optimal transparency. For electrophysiology, it is crucial to minimize 60 Hz noise. Ensure that the experimental area is isolated from AC power and properly grounded to the Intan amplifier. Although movement artifacts cannot be completely eliminated, accelerometers on the head stage can help identify and remove periods of movement during post-processing. Overall, these techniques can provide valuable insights into SD recovery and cortical network function following mild traumatic brain injuries (mTBIs).
One limitation of these techniques is that the animals are under anesthesia during imaging. However, most TBI models require anesthesia, and the extended anesthesia is unlikely to significantly impact the experimental design. Isoflurane significantly reduces high-frequency activity27. If isoflurane exposure extends beyond the imaging period, it may complicate the detection of high-frequency activity recovery following the SD. Therefore, it is critical to include a sham or isoflurane-alone group in the experimental design to compare the recovery of high-frequency activity, as shown in Figure 2. Another limitation is that electrophysiology recordings do not detect the DC shift. The amplitude and duration of the DC shift are indicators of tissue health and recovery from the SD28. Nonetheless, confirming SDs with these techniques will still provide valuable insights into TBI research.
All TBI researchers would benefit from the techniques described here, as it is highly likely that all TBI models produce spreading depolarizations (SDs). The TBI field would gain significantly from understanding the role of SDs in injury, injury progression, neuroimmune activation, and recovery. Recent research suggests that SDs contribute to behavioral deficits and may be an underlying mechanism of neurological impairment5,7. The techniques outlined in this protocol will aid in elucidating the connection between TBI and SD. Future applications of these techniques could extend to other research fields, including stroke, epilepsy, and migraine.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIGMS P20GM109089 and Department of Defense PR200891 grants.
Materials
| #00-90 x .118 Machine Screw | US Micro Screw | 00.90-118-M-SS-P, Amazon | |
| 0.8 mm diameter drill bit | PCB Drill Bit | WYTP21, Amazon | |
| 16-A Headstage | Intan | C3335 | |
| 2.1 mm Burrs micro drill bit | Fine Science Tools | 19007-21 | |
| AC amplifier | Intan | RHD recording system | |
| C57BL/6J mice | Jackson Laboratory | Jax#000664 | |
| CCD camera | Mightex | TCE-1304-U | |
| Extension lens | Zeikos | ZE-ETN | |
| Flexible cyanoacrylate glue | Stick Fast | CA Flexible | |
| Gold connector pin (female) | A-M Systems | 520100 | |
| Gold connector pin (male) | A-M Systems | 520200 | |
| ImageJ2/Fiji | Image J | NA | |
| Impactor One | Leica | 39463930 | |
| LED panel board | Supon | L122t | |
| MATLAB | MathWorks | Institutional or individual activation code | |
| Micro-Manager Camera Software | Image J | NA | |
| Nail drill/polisher | Makartt Nail Drill Electric Nail File | JD700 | |
| Omnetic connectors | Omnetics | A79014-001 | |
| RHX Data Acquisition Software | Intan | NA | |
| Stainless Steel uncoated wire (0.005") | A-M Systems | 792800 | |
| Stereotaxic Frame | Kopf Intruments | 1923-B | |
| Teets 'cold cure' dental cement | A-M systems | 525000, 526000 | |
| XLR camera lens | Nikon | NIKKOR 50 mm f/1.8-16 |
References
- Pietrobon, D., Moskowitz, M. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci. 15 (6), 379-393 (2014).
- Hartings, J. A., et al. Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurological Res. 30 (8), 876-882 (2008).
- Andrew, R. D., et al. Questioning glutamate excitotoxicity in acute brain damage: The importance of spreading depolarization. Neurocrit Care. 37, 11-30 (2022).
- Andrew, R. D., et al. Thecritical role of spreading depolarizations in early brain injury: Consensus and contention. Neurocrit Care. 37, 83-101 (2022).
- Pacheco, J., et al. Spreading depolarizations are associated with concussion-like behavior. eNeuro. 6 (6), (2019).
- Bouley, J., Chung, D. Y., Ayata, C. Y., Brown, R. H., Henninger, N. Cortical spreading depression denotes concussion injury. J Neurotrauma. 35, 1-10 (2018).
- Pinkowski, N. J., et al. Spreading depolarizations contribute to the acute behavior deficits associated with a mild traumatic brain injury in mice. J Neurotrauma. 41 (1-2), 271-291 (2023).
- Dreier, J. P., et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: Review and recommendations of the COSBID research group. J Cereb Blood Flow and Metab. 37 (5), 1595-1625 (2017).
- Herreras, O., Somjen, G. G. Analysis of potential shifts associated with recurrent spreading depression and prolonged unstable spreading depression induced by microdialysis of elevated K+ in hippocampus of anesthetized rats. Brain Res. 610 (2), 283-294 (1993).
- Boas, D. A., Dunn, A. K. Laser speckle contrast imaging in biomedical optics. J Biomed Opt. 15 (1), 011109 (2014).
- Hadjikhani, N., et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. PNAS. 98 (8), 4687-4692 (2001).
- Dietz, R. M., Weis, J. H., Shuttleworth, C. W. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J Neurochem. 109 (Suppl 1), 145-152 (2009).
- Reinhart, K. M., Shuttleworth, C. W. Ketamine reduces deleterious consequences of spreading depolarizations. Exp Neurol. 305, 121-128 (2018).
- Mané, M., Müller, M. Temporo-spectral imaging of intrinsic optical signals during hypoxia-induced spreading depression-like depolarization. PLoS One. 7 (8), (2012).
- Brennan, A. M., Connor, J. A., Shuttleworth, C. W. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow and Metab. 26 (11), 1389-1406 (2006).
- Shuttleworth, C. W., Brennan, A. M., Connor, J. A NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 23 (8), 3196-3208 (2003).
- Sword, J., Croom, D., Wang, P. L., Thompson, R. J., Kirov, S. A. Neuronal pannexin-1 channels are not molecular routes of water influx during spreading depolarization-induced dendritic beading. J Cereb Blood Flow and Metab. 37 (5), 1626-1633 (2017).
- Chung, D. Y., et al. Real-time non-invasive in vivo visible light detection of cortical spreading depolarizations in mice. J Neurosci Methods. 309, 143-146 (2018).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9 (7), 671-675 (2012).
- Rueden, C. T., et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 18 (1), 1-26 (2017).
- Edelstein, A., Amodaj, N., Hoover, K., Vale, R., Stuurman, N. Computer control of microscopes using µManager. Curr Protoc Mol Biol. 14, (2010).
- Edelstein, A. D., et al. Advanced methods of microscope control using µManager software. J of Biol Methods. 1 (2), e10 (2014).
- Soldozy, S., et al. Cortical spreading depression in the setting of traumatic brain injury. World Neurosurg. 134, 50-57 (2020).
- Zepeda, A., Arias, C., Sengpiel, F. Optical imaging of intrinsic signals: Recent developments in the methodology and its applications. J Neurosci Methods. 136 (1), 1-21 (2004).
- Steinzeig, A., Molotkov, D., Castrén, E. Chronic imaging through "transparent skull" in mice. PLoS One. 12 (8), (2017).
- Sword, J., Masuda, T., Croom, D., Kirov, S. A. Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. Brain. 136 (5), 1446-1461 (2013).
- Hudetz, A. G., Vizuete, J. A., Pillay, S. Differential effects of isoflurane on high-frequency and low-frequency oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology. 114 (3), 1588-1595 (2011).
- Reinhart, K. M., Morton, R. A., Brennan, K. C., Carlson, A. P., Shuttleworth, C. W. Ketamine improves neuronal recovery following spreading depolarization in peri-infarct tissues. J Neurochem. 168 (5), 855-867 (2023).

.
