Using Confocal Analysis of Xenopus laevis to Investigate Modulators of Wnt and Shh Morphogen Gradients
Summary
The manuscript here provides a simple set of methods for analysing the secretion and diffusion of fluorescently tagged ligands in Xenopus. This provides a context for testing the ability of other proteins to modify ligand distribution and allowing experiments that may give insight into mechanisms regulating morphogen gradients.
Abstract
This protocol describes a method to visualise ligands distributed across a field of cells. The ease of expressing exogenous proteins, together with the large size of their cells in early embryos, make Xenopus laevis a useful model for visualising GFP-tagged ligands. Synthetic mRNAs are efficiently translated after injection into early stage Xenopus embryos, and injections can be targeted to a single cell. When combined with a lineage tracer such as membrane tethered RFP, the injected cell (and its descendants) that are producing the overexpressed protein can easily be followed. This protocol describes a method for the production of fluorescently tagged Wnt and Shh ligands from injected mRNA. The methods involve the micro dissection of ectodermal explants (animal caps) and the analysis of ligand diffusion in multiple samples. By using confocal imaging, information about ligand secretion and diffusion over a field of cells can be obtained. Statistical analyses of confocal images provide quantitative data on the shape of ligand gradients. These methods may be useful to researchers who want to test the effects of factors that may regulate the shape of morphogen gradients.
Introduction
During early embryonic development, cells are progressively committed to follow specific lineages of differentiation: this means a group of totipotent (or pluripotent) cells become gradually restricted to establishing populations of progenitor cells determined to give rise to one cell type. Cell-cell signalling is central to the regulation of lineage specification during embryonic development. Manipulation of these signals will be required to direct stem cells toward particular fates to support novel medical treatments.
A relatively small number of signalling pathways are reiterated during development, including pathways responding to the TGF superfamily (nodals and BMPs)1-2, FGFs3, Wnts4, and Hedgehogs5. These secreted proteins bind receptors present on the cell membrane to activate signal transduction thereby altering gene expression and/or cell behaviour. The tight regulation of cell signalling is essential for cell lineage specification and normal development. While the cross-talk among these pathways is important in determining cell fate, a single ligand can itself elicit distinct responses at different concentrations. Morphogen gradients were described over 100 years ago as a theory to explain how different cell types can derive from a field of cells6. Signalling molecules produced by one group of cells may diffuse over a certain range, decreasing in concentration with a greater distance from the source. Cells exposed to the signal will respond to the local concentration at their position in the field of cells, with cells at distinct positions responding differently to different levels of the signal. Evidence for the existence of morphogens comes from studies of the early Drosophila embryo7 and the wing disc8, as well as the vertebrate limb9 and neural tube10.
Methods are needed to investigate how morphogen gradients are established and to identify other molecules important in regulating these gradients. Elegant experiments using immunohistochemistry to visualise endogenous proteins in vivo in the context of different genetic backgrounds have been used to investigate morphogen gradients11-12. However, good antibodies and specific mutants are not always available, so we describe here a protocol using overexpression of fluorescent ligands in Xenopus, to provide an alternative, simple method to dissect how exogenous gene products can influence distribution of ligands across a field of cells. Xenopus laevis provides an excellent system to undertake these types of experiments as their embryos develop externally so they are accessible at the earliest stages. Their large size (1-1.5mm in diameter) simplifies microinjection and surgical manipulation and by blastula stages the cells are easy to image as they are still relatively big (about 20µm across). Overexpression studies in Xenopus are simple to do: mRNA injected into the early embryo can be targeted to particular cells and is efficiently translated.
The fluorescently tagged Wnt8a/Wnt11b-HA-eGFP constructs were generated using pCS2 Wnt8a-HA13, pCS2 Wnt11b-HA14 and eGFP. The HA peptide is important to include, not only to provide an additional molecular tag, but also because it is thought to act as a spacer separating the Wnt and eGFP proteins allowing both gene products to function. The construct used for the visualisation of Shh was previously used to generate a transgenic mouse expressing a Shh-eGFP fusion protein15; this was kindly provided by Andy McMahon. Importantly, the GFP tag for all the constructs is cloned 3’ to the signal sequence such that it is retained after processing. It is also essential to ensure that the final protein includes sequences required for modifications, such the addition of lipids as is the case for Shh and Wnt ligands.The cDNAs were subcloned into the pCS2+ expression vector which is optimised for the prodution of synthetic mRNA; it includes an SP6 promoter and polyadenylation signal (http://sitemaker.umich.edu/dlturner.vectors).
The work described here provides a simple protocol for comparing the secretion and diffusion of fluorescently tagged Wnt and Shh tagged ligands. By injecting defined amounts of synthetic mRNA, the protocol circumnavigates any problems associated with variable expression from different vectors using different promoters. These methods have recently been applied to investigate the effects of the heparan sulfate endosulfatase Sulf1 on Shh-eGFP and Wnt8a/Wnt11b-HA-eGFP secretion and diffusion in Xenopus16-17.
Protocol
Ethics statement: Animal experiments were done under a UK Home office licence to MEP and the experiments carried out was approved by the University of York ethics committee in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. (https://www.nc3rs.org.uk/arrive-guidelines).
1. Strategy to Generate Fluorescently Tagged Ligands
Below is an example protocol for subcloning Wnt8a-HA and eGFP into pCS2, in order to produce the in frame fusion construct Wnta-HA-eGFP:
- Set up and run PCR reactions for Wnt8a-HA and eGFP. Use the primer information displayed in Table1 and the Example PCR reaction and Example PCR conditions displayed in Table 2. Note: Template DNA will vary depending on the construct being produced, in this example, Wnt8a-HA is used as template DNA.
- Clean up PCR reactions using a gel extraction kit, perform final elution in 30µl of molecular grade water.
- Check 2 µl of PCR product on a 1% agarose gel prepared in TAE.
- Digest Wnt8a-HA and eGFP PCR products and pCS2 using the appropriate restriction enzymes (see Table 1), set up reactions as described in Table 2 (Example PCR product digest).
- Incubate reactions for 3 hr at 37°C and then store Wnt8a-HA and GFP PCR products on ice.
- Add 1µl of calf alkaline intestinal phosphatase (CAIP) to pCS2 digest and incubate for 6 min at 37°C.
- Heat inactivate CAIP by incubating for 15 min at 65°C.
- Run pCS2 and PCR digests on an 1% ultrapure agarose gel prepared in TAE.
- Gel extract and clean up pCS2 and PCR products using a gel extraction kit, perform final elution in 30µl of molecular grade water.
- Set up ligation as described in Table 2 (Example T4 ligation) and incubate at 12°C for 16 hr.
2. mRNA Synthesis: Generating the Template
- Produce templates using restriction enzyme digestion of the following plasmids (all in the expression vector pCS2): membrane-Cerulean (memCerulean), membrane-RFP (memRFP), Shh-GFP, Wnt8a/Wnt11b-HA-eGFP.
- Set up restriction digests as described in Table 2 (Example template digest).
- Mix gently and incubate the reactions for 2 hr at 37°C.
- Run 2.5 µl of digested plasmid on a 1% agarose gel (TAE) to check that the reaction has cut to completion.
- Clean up the digests using a gel extraction kit, perform final elution in 50 µl of molecular grade water.
3. mRNA Synthesis: In Vitro Transcription
- Synthesize functional mRNA using the SP6 mRNA transcription kit. Assemble the reactions in 1.5 ml DNAse/RNAse free microcentrifuge tubes.
- Set up mRNA transcription reactions as described in Table 2 (Example mRNA synthesis).
- Mix the reactions gently with a pipette and then incubate at 37°C for 4 hr.
- Check 1 µl of the reaction on a 2% agarose gel (TAE).
- Add 1 µl of DNAse to the reaction, mix gently with a P20 and incubate the reaction for a further 15 min at 37°C.
- Add 340 µl of molecular grade water, 40 µl of ammonium acetate and 400 µl of phenol-chloroform to the reaction. Vortex the reactions for 30 sec.
- Spin the mRNA for 5 min, 14,000 x g, 4°C.
- Pipette the top (aqueous) layer from each reaction moving it to a fresh 1.5 ml screw cap microcentrifuge tube.
- Add an equal volume of chloroform to each of the decanted reactions. Vortex the samples for 30 sec.
- Spin the mRNA for 5 min, 14,000 x g, 4°C
- Pipette the top aqueous layer from each reaction moving it to a fresh 1.5 ml screw cap microcentrifuge tube.
- Add an equal volume of isopropanol to each of the reactions and precipitate the mRNA at -80°C for 30 min.
- Spin each of the reactions for 15 min at 14,000 x g, 4°C to pellet the mRNA
- Remove the supernatant taking care not to disturb the pelleted mRNA
- Add 200 µl of 70% ethanol to each of the reactions, mix the reactions and then spin for 10 min at 14,000 x g, 4°C.
- Remove the ethanol taking care not to disturb the mRNA, dry the pellet at room temperature using a vacuum desiccator or speed-vac.
- Re-suspend the mRNA in 10-20 µl of molecular grade water. Spin briefly and keep the samples on ice. Note: Heating the sample to 80 °C for 1 min can help it dissolve.
- Run 1µl of mRNA on a 2% agarose gel (TAE) and analyse 1.2 µl on a spectrophotometer to get an accurate readout of concentration.
CRITICAL STEP: A concentration of 400 ng/µl of synthetic mRNA is required to carry out the following experiments.
CRITICAL STEP: If the 260/280 ratio is outside of the range of 2.00-2.20, or the total amount of mRNA exceeds 12 µg, carry out a lithium chloride precipitation. - Store mRNA in 1 µl aliquots at -80°C. Use aliquots and then throw away; do not re-freeze mRNA.
4. Generating Xenopus laevis Embryos
- Prime Xenopus laevis females by subcutaneous injection with 50 units of human chorionic gonadotropin hormone (HCG; Chorulon) a week before the experiment.
- Induce Xenopus laevis females the night before the experiment by injecting 250 units of HCG and incubating them in the dark, O/N at 19 °C.
- Dissect out testes from euthanised male frog and store in 1xNAM at 4°C. Note: Male frog is euthanised by terminal anaesthesia in accordance with Schedule 1 of Animals (Scientific Procedures) Act 1986 (https://www.nc3rs.org.uk/euthanasia).
- Massage female Xenopus laevis to induce ovulation, collect the laid eggs in a 55 mm diameter round petri dish.
- Produce a sperm suspension by crushing ¼ of a Xenopus laevis testis in 1 ml of deionised water.
- Brush Xenopus oocytes with the crushed sperm suspension using a Pasteur pipette and pipette bulb. CRITICAL STEP: Perform fertilisation reactions at approximately 4:30pm on the day of the experiment.
- Culture embryos at 21°C in NAM/10 (see Table 3 for solutions) in 55 mm petri dishes coated with 1% agarose diluted in water (water).
- De-jelly embryos after 45 min using cysteine/HCL (Table 3). Note: At 21°C, embryos will reach the 2 cell stage after 90 min and cleave every 20 min after that.
5. Microinjecting Xenopus Embryos
- Using 1 X 90mm glass capillaries and a Narishige PC-10 dual-stage glass micropipette puller, pull micropipettes for injections. Adjust settings empirically to achieve a fine tip (less than one micron diameter).
- Attach a micropipette (needle) to a gas microinjector (for instance, the Harvard Appartaus PLI-100 PICO-INJECTOR) to repeatedly deliver precise volumes of liquid by applying a regulated pressure for a digitally set period of time. Adjust the pneumatic pressure to deliver a volume of 1.25 to 10 nanolitres as determined using a reticle or calibration slide.
- Coat a 55 mm petri dish with 5 ml of 1% agarose (water). Cut a 35-35 mm square of agarose out of the dish, this will provide a stable surface to microinject embryos.
- Transfer embryos into NAM/3 + Ficoll (Table 3) and move to injection dish.
- Inject embryos in the animal hemisphere once they reach the required stage for the experiment.
- To analyse the distribution of GFP tagged ligands on the cell surface, inject the embryos bi-laterally at the two cell stage, 10nl per cell. Example mRNA dilutions shown below for Wnt8a-HA-eGFP. CRITICAL STEP: Ensure that all mRNA concentrations start at 400 ng/µl.
Note: The amount of mRNA to be injected needs to be determined empirically by testing concentrations and finding the minimum amount needed to visualise the fluorescent ligand. Western blotting using an anti-HA antibody is an essential step to determine that the mRNAs are translated to similar levels in order to allow the comparison of two different fluorescently tagged ligands; we have done this for Wnt8a-HA-eGFP and Wnt11b-HA-eGFP in Fellgett et al., 2015.
Note: When assessing the effect of an injected mRNA (such as one coding for a candidate regulator), a typical control is to inject a non-active RNA, such as LacZ, into sibling embryos in order to control for any effects of the additional transcripts in the embryo.- Dilute memRFP mRNA 1 in 4 (1µl + 3µl dH2O) with molecular grade water to reduce the concentration to 100 ng/µl.
- Dilute Wnt8a-HA-eGFP mRNA 1 in 4 (1µl + 3µl dH2O) with molecular grade water to reduce the concentration to 100 ng/µl.
- Mix memRFP mRNA in a 1:1 ratio with Wnt8a-HA-eGFP mRNA.
- Mix mRNA from 5.5.1.3 in a 1:1 ratio with either the control mRNA LacZ or a modulator such as Sulf1.
- Inject embryos bilaterally at the 2 cell stage with 10nl of mRNA per cell (total of 20nl). Note: This results in 250 pg of memRFP, 250 pg of Wnt8a-HA-eGFP, 500 pg of Wnt11b-HA-eGFP and 2 ng of LacZ or Sulf1 mRNA being injected into each cell.
- To analyse the diffusion of GFP tagged ligands, inject mRNA coding for ligand-GFP together with a lineage marker such as memRFP at the 16-32 cell stage, 1.25 nl per cell.
- To analyse the effects of any potential modulator of ligand diffusion, co-inject mRNA coding for the modulator together with the ligand and lineage tracer. Alternatively, inject neighbouring cells: one with mRNAs coding for the GFP-tagged ligand and a lineage tracer (memRFP) and the other with mRNAs coding for the modulator and a different lineage tracer (memCerulean).
- To analyse the distribution of GFP tagged ligands on the cell surface, inject the embryos bi-laterally at the two cell stage, 10nl per cell. Example mRNA dilutions shown below for Wnt8a-HA-eGFP. CRITICAL STEP: Ensure that all mRNA concentrations start at 400 ng/µl.
- Transfer embryos to a 12.5°C incubator and culture until the following day, Nieuwkoop and Faber (NF) stage 8.
6. Excising Animal Cap Explants
- Transfer embryos to a 55 mm petri dishes coated with 5 ml of 1% agarose (water). Fill the dish with NAM/2 (Table 3).
- Take circular animal caps using Tungsten needles. CRITICAL STEP: Take large caps at this stage as these will heal better and be easier to image, keep control caps to ensure no convergent extension occurs.
- Transfer animal explants to 55 mm petri dishes coated with 1% agarose (water) and culture embryos at 21°C for 4 hr. CRITICAL STEP:The 4 hr incubation is required to allow fluorescent proteins to mature.
- Generate relief slides by sticking down two layers of PVC insulation tape onto microscope slides. Cut a 14 mm by 10 mm rectangle out of the tape to leave a chamber for mounting animal caps. CRITICAL STEP: Flatten both layers of tape thoroughly onto the slide before cutting the rectangle.
- Pipette 2 separate droplets of NAM/2 (Table 3) into the relief slide, approximately 30 µl per drop.
- Transfer animal explants to the relief slides using a cut off P20 tip and orientate so that the apical side faces upwards. Note: Each slide can hold 10-15 animal caps. CRITICAL STEP: At this stage the animal caps should have partially healed, this means they are less delicate and can be orientated using forceps.
- Gently lower a glass cover slip onto the relief slide and allow the cover slip to dry for 20 min. CRITICAL STEP: The size of the animal cap is crucial; ensure the cap is big enough that when the glass coverslip is lowered onto the relief slide it compresses the apical layer of animal cap cells enough to produce a flat surface to image. If the animal caps are too small still then reduce the PVC tape to one layer.
- Seal the slide using nail varnish. CRITICAL STEP: Do not use too much nail varnish to seal the relief slides because if the PVC tape becomes too damp it will raise up and ruin the slide.
- Dry relief slides for 20 min in the dark and they are ready to image, typically animal caps will remain healthy for 4-6 hr once sealed in the slide.
7. Imaging
Note: Imaging was carried out using an inverted confocal microscope. Lambda mode was chosen for imaging as this allowed multiple fluorophores with overlapping signatures to be used, and removed the problem of potential sample movement between scans.
- Find explants using a low magnification objective then switch to 63x/1.4 oil objective for higher resolution imaging.
- Switch on requisite lasers; for this example the 405 laser was used to excite memCerulean, the 488 laser was used to excite GFP and the 561 laser to excite memRFP using 405 and 488/561 dichroic mirrors.
- Use lambda mode (In Zen 2011, select lambda mode – 32 bins).
- Select the 405nm, 488nm and 561nm lasers.To permit a wider field of view, zoom out the image (to 0.6x).
- Set the frame size to the desired image size (1024 x 1024 with pixel sizes of 220nm was used for this data set).
- Set averaging to typically 4 to 8 to increase the signal to noise ratio.
- Optimise the pinhole (1 airy unit according to the 561nm laser was used for this data set).
- Optimise the laser powers. CRITICAL STEP: Balance the 405nm, 488nm and 561nm lasers such that all fluorophores can be detected using the 32 detector array whilst minimising the amount of voltage and gain required.
- Collect the light being emitted from memCerulean, GFP and memRFP. For this work light was collected every ~10nm from 415-720nm, although larger collection intervals are also possible (e.g., every ~20 nm).
8. Image Analysis
- Investigating the effects of modulators on the cell surface expression of GFP-tagged ligands
NOTE: The following steps are a detailed guide of how the analysis was performed in our laboratory. The end user may want to streamline the process by writing an ImageJ or FIJI script to remove some of the manual steps.
CRITICAL STEP: It is important the end user samples the spectra of any fluorophores used in the same conditions that the final experiment is performed in order to perform effective spectral unmixing of the multiple fluorophores used in the final experiment.
Two main types of analysis are performed in this section:- Calculating the total number of Wnt-HA-eGFP pixels co-localising with the cell membrane.
- Unmix the green, red and cerulean channels using spectra sampled from single injections of these fluorophores in ZEN software. Save these images as separate LSM documents to use in all of the following steps.
- Open LSM files directly in image editing software. Note: The following instructions are for FIJI Image J.
- Save the LSM images as image sequence documents, this will automatically split the images into the separate channels.
- Save the image sequence into the same folder as the scripts for the script analysis software that will be used to analyse the data(see supplemental code files for actual script). Note: The following instructions are for Matlab.
- Open up the script analysis software and open the directory in which the scripts are written.
- Enter the file name under Location analysis, the software will take the file name and perform the analysis.
Note: The file names will read ImageA0000 and ImageA0001 for each image. The script analysis software will use the image marked 0001 as the mask and analyse the percentage of pixels in image 0000 that co-localise with this mask. - Take the percentage co-localisation number (annotated as Cell membrane populated) and copy this to a spreadsheet. Note: The following instructions are for Excel.
- Plot the percentage co-localisation number on a bar chart either as an absolute or relative value.
- Analysing the number, size and shape of Wnt-HA-eGFP puncta.
- Open unmixed LSM files directly in image editing software. Note: the following instructions are for FIJI Image J.
- Split each image into its separate channels (Image > Colour > Split channels).
- Threshold both the Wnt-HA-eGFP and the membrane marker channels (Image > Adjust > Auto-threshold). CRITICAL STEP: Try a number of thresholds to see which one produces a representative image, without overexposing the channel.
- Convert the membrane marker channel to a mask (Process > Binary > Make mask).
- Invert this mask (Edit > Invert).
- Convert the Wnt-HA-eGFP puncta to a mask as above.
- Subtract the membrane marker mask from the ligand-GFP mask (Process > Image calculator > Wnt mask in top box, membrane mask in bottom box).
- Use the analyse particle function. From here, select the required parameters and analyse the data (Analyse > Analyse particles).
- Copy the number of particles, average particle size and circularity data into a spreadsheet and then plot graphs from these data. Note: For this analysis, only particles between 0.1-10µm2 size were analysed, no restrictions were placed on particle circularity. This instruction is for Excel.
- Calculating the total number of Wnt-HA-eGFP pixels co-localising with the cell membrane.
- Analysing the range of ligand diffusion
- Open up all unmixed images in confocal imaging software and then export the files in a TIF format, as raw data and as an RGB image. Note: This instruction is for Zen lite 2011.CRITICAL STEP: Include a scale bar on at least one of the images so that distance can be calculated during the analysis.
- Open the images to be analysed in image analysis software. Note: The following instructions are for Photoshop.8.2.3) Right click on the background of the image and select ‘layer from background’, to create a new layer.
- Set the membrane marker channels to 0, so that only the ligand-GFP channel is visible (Layer > New adjustment layer > Channel mixer). CRITICAL STEP: Clip the new adjustment layer to the image being analysed, the option to clip the new layer to this image is available as a tick box after clicking on the channel mixer option.
- Open a new workspace. Set the resolution to 300 pixels per inch and the colour mode to RGB colour (File > New > Clipboard).
- Copy all of the images being analysed into the single workspace (Click on the image and it’s clipped channel mixer by holding control then hold control and Alt and drag the image into the workspace).
- Orientate all of the images so that the maximum length of diffusion for each image can be measured along the horizontal axis, with the cells expressing Wnt-HA-eGFP orientated to the left.
- Save the work space as a TIF and then open this in the image analysis software. Note: The following instructions are for FIJI Image J.
- Open the ROI manager window (Analyse > Tools > ROI manager).
- Draw a rectangle on the first image, the exact size of the rectangle can be specified (Select rectangle tool and draw any rectangle > Edit > Selection > Specify). Note: A size of 650 x 100 pixels length x width was used in this study.
- Position the rectangle on the very edge of the domain expressing Wnt-HA-eGFP. Place the rectangle over the region with the maximum distance of Wnt-HA-eGFP diffusion. CRITICAL STEP: Even without the membrane marker the regions expressing Wnt-HA- eGFP should be visible due to background fluorescence; if not then consult raw images.
- Shift the box 10 µM to the right. Determine the number of micrometres by measuring the length of the scale bar included in one of the images (Draw a line tracing the scale bar > Analyse > set scale). Note: This provides a value for 10 uM in pixels.
- Add the box to the ROI manager by pressing the add button in ROI manager window.
- Move rectangle to the next image to be measured by clicking back on the rectangle tool to move the rectangle. Repeat steps 8.2.10-11.
- Once all of the panels have been measured, select multiplot from the ROI manager menu (ROI manager > More > Multiplot > List).
Note: the data represents Wnt pixel intensity (Y) with increasing distance along the horizontal axis (X, measured in Pixels). The value for Y is the average signal intensity of ligand-GFP at the point X and the average is calculated from all of the pixels in the Y axis for each X value. Consequently the taller the box, the more pixels will be analysed to produce this average. A small box (in the Y axis) will be noisier; a tall box will have very low average pixel intensity. - Copy the data from the multiplot box into a spreadsheet and re-label accordingly. Note: The following instructions are for Excel.
- Discard the first 10-20µm of data from each analysis as this represents background fluorescence from ligand-GFP expressing cells and distorts the graphs.
- Open up scientific analysis and graphing software and create a new notebook. Note: The following instructions are for Sigmaplot 13.
- Label the required number of columns for the data by double clicking on the horizontal numbers on the worksheet and labelling them distance in µm, Wnt8a-HA-eGFP and Wnt11b-HA-eGFP.
- Copy data from the spreadsheet into the relevant columns in the scientific analysis and graphing software.
- Create a scatter graph (Create graph > Scatter > Multiple scatter > Select X many Y > Select the distance column for X > Select Wnt8a-HA-eGFP and Wnt11b-HA-eGFP for the Y values > Finish).
- Perform regression analysis on Wnt8a-HA-eGFP curve by left clicking on a Wnt8a-HA-eGFP point on the scatter graph. All of the points should highlight. Click on Analysis > Regression wizard.
- Select the curve equation category (Exponential decay) and the equation name (Single, 3 parameter) then press next.
- Select the X and Y values to which the curve should be fitted (if the data was highlighted previously, this should be done automatically) then press next.
- Continue through the wizard until the Numeric Output options page, ensure ‘create report’ is ticked then press next. Note: The parameters for the fitted curve will be shown in Report 1*.
- On the Graph options page ensure ‘add equation to graph title’ is ticked then press finish. Note: The wizard will have created Report 1* and Graph 1* tabs at the top of the note book. In addition the curve will have been added to the graph produced in (8.2.20).
- Repeat steps 8.2.21-8.2.25 to fit a curve for Wnt11b-HA-eGFP. CRITICAL STEP: try fitting multiple equation categories and equation names to the data. Note: The curve with the best fit should produce the highest R2 value with the lowest residual sum of squares.
Representative Results
Confocal analysis of animal cap explants expressing fluorescently tagged proteins provides an effective system for visualising ligand distribution under different experimental conditions. In one example, the distribution of GFP tagged Shh is shown (Figure 1). At the 2-cell stage, Xenopus embryos are injected into both cells with either with a control mRNA or with mRNA coding for Sulf1, an enzyme that modifies cell surface heparan sulfate and influences the Shh morphogen gradient16. These embryos are cultured until the 32-cell stage and a single cell is co-injected with mRNAs coding for Shh-GFP and memRFP (as a lineage tracer). This creates a clone of cells expressing Shh-GFP that is marked with the cell membrane tethered RFP. At the blastula stage, animal cap explants are taken for analysis by confocal microscopy. Figure 1 shows that under control conditions, Shh-GFP is secreted and diffuses outside of the region expressing the injected mRNAs. However, in the presence of Sulf1, Shh-GFP is more restricted in its distribution and, in this sample, is not detected outside the clone of cells producing it. The effects of Sulf1 on Shh-GFP distribution has been analysed more fully16.
When expressed in Xenopus embryos, fluorescently tagged Wnt ligands are secreted, accumulate on the cell membrane, and diffuse across the field of cells. In Figure 2, we characterise the quantitaive and qualitative properties of the two different fluorecently tagged Wnt ligands in cells injected and expressing the proteins. Figure 2A-H shows examples of how Wnt8a and Wnt11b-HA-eGFP accumulate on the membrane of animal cap cells. By comparing panels 2B and 2F it is clear that Wnt8a-HA-eGFP accumulates more efficiently on the cell membrane than Wnt11b-HA-eGFP. Quantitative information has been extracted from the confocal images using a combination of image and script analysis software (supplemental code file). Figure 2I shows the relative accumulation of Wnt8a-HA-eGFP on the cell membrane compared to Wnt11b-HA-eGFP. The data was obtained using a computer script that determines the total number of Wnt-HA-eGFP pixels co-localising with cell membrane pixels in each image. In another approach, the total number of Wnt-HA-eGFP puncta that co-localise with the cell membrane are counted using image analysis software (Figure 2J). Qualitative information about the size and shape of individual puncta can also be extracted from the data using image analysis software. In addition to fewer Wnt11b-HA-eGFP puncta co-localising with the cell membrane (Figure 2J) these puncta also have a smaller average size than Wnt8a-HA-eGFP puncta (Figure 2K). Moreover, Wnt11b-HA-eGFP puncta have a reduced circularity compared to Wnt8a-HA-eGFP puncta (Figure 2L). Circularity is a measure of how closely an object resembles a perfect circle, with 1 representing a perfect circle and 0.1 an elongated non-circular shape. This approach can be used to test any candidate regulator of Wnt ligand diffusion. To do this, control cells should be injected with a control mRNA coding for an inactive form of the candidate regulator or an irrelevant protein such as beta-galactosidase (lacZ); this provides a more valid control than simply not injecting the regulator. Data in these examples was analysed using the non-parametric test Mann-Whitney U18-19. A statistical analysis programme was used to perform the test.
In Figure 3, we investigate Wnt ligand diffusion. A single blastomere was injected at the 4-cell stage such that the animal cap explants represent a field of cells in which only some cells express either Wnt8a-HA-eGFP or Wnt11b-HA-eGFP. By including a cell lineage tracer, we can identify expressing versus non-expressing cells and this allows the range of Wnt8a-HA-eGFP and Wnt11b-HA-eGFP ligand diffusion away from the source cells to be analysed (Figure 3B and 3C). Due to the curvature of the animal cap explants, the maximum distance that could be reliably measured using this assay was 160µm, which was not enough to measure the absolute distance of ligand diffusion. However, by using a combination of confocal analysis, image analysis and scientific analysis and graphing software the overall shape of the Wnt-HA-eGFP morphogen gradient could be analysed (Figure 3D). This data can be used to investigate whether overexpressing another protein together with the tagged ligands in the same cells can affect Wnt secretion or diffusion. In another type of experiment (Figure 3E-3J) the effects of a potential regulator in receiving cells can be measured by overexpressing the candidate protein in clones of cells adjacent to cells expressing Wnt8a or Wnt11b-HA-eGFP. This allows any non-cell-autonomous effects of the regulator on Wnt-HA-eGFP ligand diffusion to be examined. Consistent with Figure 2, more Wnt8a-HA-eGFP can be detected diffusing away from cells than Wnt11b-HA-eGFP in both diffusion experiments.
The types of experiments described in this paper have been used to analyse the effect of Sulf1 on Shh and Wnt signalling16,17.
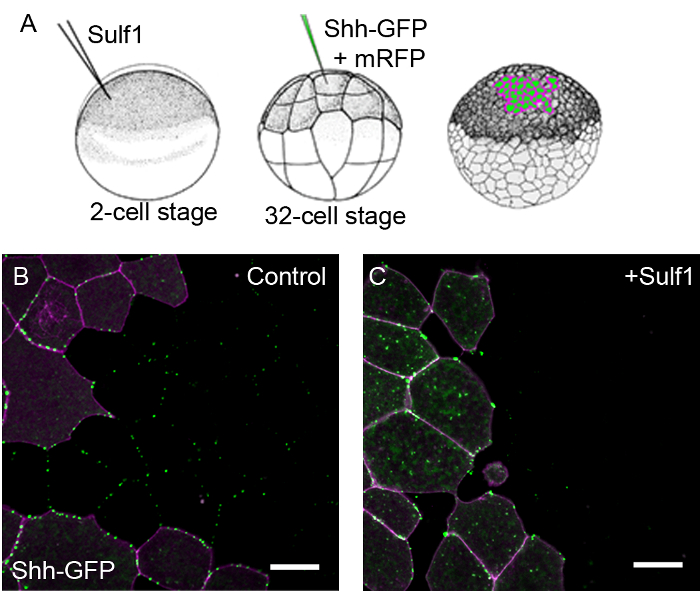
Figure 1. Modulation of Shh-GFP distribution can be detected by confocal analysis of Xenopus animal caps. (A) A cartoon depicting experimental assay where embryos are first injected with mRNA coding for Sulf1 or a control mRNA, the same embryos are later injected at the 32-cell stage with mRNA coding for Shh-GFP into a single cell. (B-C) Animal cap explants were taken at stage 8 and imaged after 4 hr. Images are shown at the edge of a Shh-GFP + memRFP expressing clone of cells. In control embryos (B), Shh-GFP is distributed away from the memRFP marked clone of signal producing cells. In embryos expressing Sulf1 (C), Shh-GFP is more restricted in its distribution, see16. memRFP is shown in magenta and Shh-GFP in green. Scale bars represent 20 µm. Please click here to view a larger version of this figure.
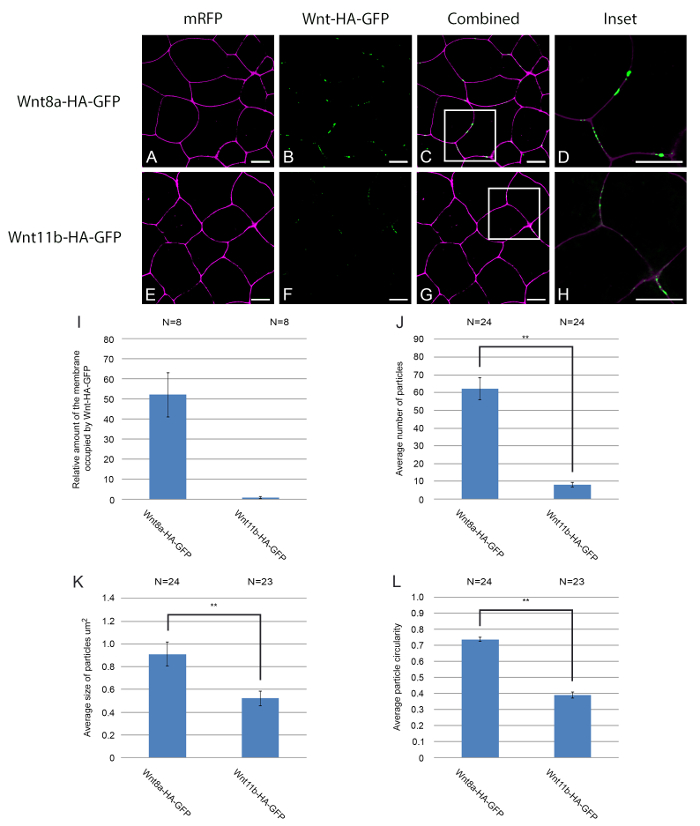
Figure 2. Quantitative and qualitative analysis of Wnt8a and Wnt11b-HA-eGFP puncta on the cell membrane. (A-H) Embryos were microinjected bi-laterally with mRNA encoding memRFP (500 pg) into the animal hemisphere at the two cell stage. In addition embryos were injected with mRNA encoding (A-D) Wnt8a-HA-eGFP (500 pg) or [E-H] Wnt11b-HA-eGFP (1ng). The embryos were also injected with mRNA coding for LacZ to provide a control for additional mRNA/protein when analysing the effects of any potential regulator. The white boxes in (C) and (G) mark the areas enlarged in panels (D) and (H) respectively. The total amount Wnt-HA-eGFP fluorescence co-localising with the cell membrane was calculated using image and script analysis software and then normalised (I). Qualitative information was extracted using image analysis software. Wnt8a and Wnt11b-HA-eGFP punctae were analysed for particle number (J), particle size (K) and particle circularity (L). Mann-Whitney U (**P<0.01), N=number of embryos. memRFP is shown in magenta, and Wnt8a/11b-HA-GFP is shown in green, scale bars represent 20µm. Please click here to view a larger version of this figure.
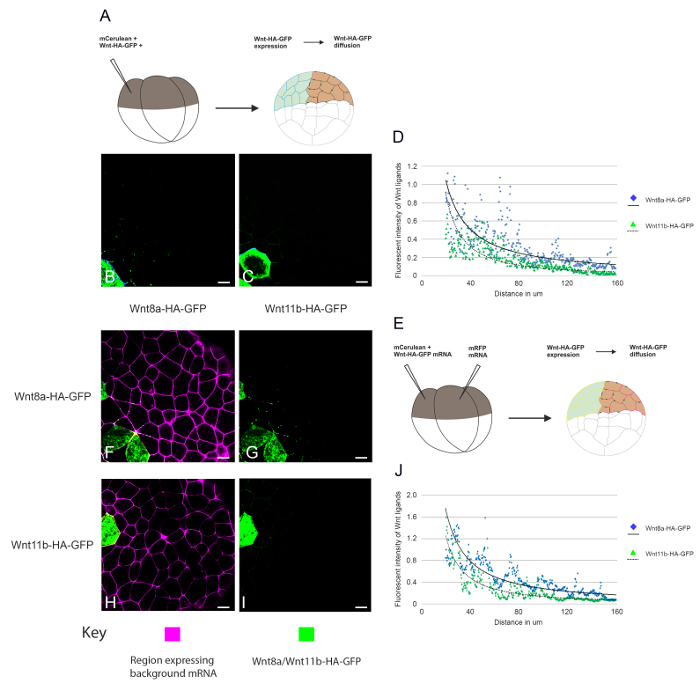
Figure 3. Measuring the range of diffusion of fluorescently tagged Wnt ligands. (A) Diagram depicting the assay used to measure Wnt8a and Wnt11b-HA-eGFP secretion and diffusion away from expressing cells. (B-C) mRNA encoding (B) memCerulean (600 pg), LacZ (4ng) and Wnt8a-HA-eGFP (2ng) or (C) memCerulean (600 pg), LacZ (4ng) and Wnt11b-HA-eGFP (2 ng) was injected into the animal hemisphere of one blastomere at the four cell stage. (D) The range of diffusion of Wnt8a-HA-eGFP and Wnt11b-HA-eGFP was measured through a control background. (E) Diagram depicting the assay used to measure Wnt8a and Wnt11b-HA-eGFP diffusion through a background expressing LacZ, see method for details. (F-G) mRNA encoding memCerulean (600 pg) and (F and H) Wnt8a-HA-eGFP (2 ng) or (G and I) Wnt11b-HA-eGFP was injected into the animal hemisphere of one blastomere at the four cell stage. An adjacent blastomere was injected with mRNA encoding memRFP (600 pg) and LacZ (4 ng) see Key for details. (J) The range of Wnt8a-HA-eGFP and Wnt11b-HA-eGFP diffusion was measured through a background expressing LacZ. The data was quantified and plotted using confocal analysis, image analysis and scientific analysis and graphing software. memCerulean (blue (C-D) and Yellow (F and H)), Wnt-HA-eGFP (green), memRFP (magenta), scale bars represent 20 µm. Please click here to download a larger version of this file.

Table 1. Primers used to subclone Wnt8a/Wnt11b-HA-eGFP and Shh-GFP into pCS2.
| Reaction | Components | |
| Example CS2+ digest | 1.5 μg of pCS2+ | |
| 2 μl of Restriction enzyme 1 | ||
| 2 μl of Restriction enzyme 2 | ||
| 5 μl of Restriction enzyme buffer (10X) | ||
| Made up to 50 μl with molecular grade water | ||
| Example mRNA synthesis | 2 μl linearised template | |
| 2 μl 10x Megascript trx mix | ||
| 2 μl 50mM ATP | ||
| 2 μl 50mM CTP | ||
| 2 μl 50mM UTP | ||
| 2 μl 5mM GTP | ||
| 2.5 μl 40mM Cap Analog (m7G(5') | ||
| 2 μl SP6 enzyme mix | ||
| 3.5 μl Molecular grade water | ||
| Example PCR reaction | 0.5 μl High fidelity DNA polymerase (2,000 units/ml) | |
| 2 ng Template DNA | ||
| 2.5 μl of forward and reverse primers (10μM) | ||
| 0.5 μl of dNTPs | ||
| 5 μl of DNA ploymerase buffer (10X) | ||
| Made up to 50 μl with molecular grade water | ||
| Example PCR conditions | Intial denaturation 2 min at 98 °C | |
| 15 sec 98 °C | ||
| 15 sec 65 °C | 30 cycles | |
| 40 sec 72 °C | ||
| Final extension 10 min at 72 °C | ||
| Example PCR product digest | 28 μl of PCR product | |
| 2 μl of Restriction enzyme 1 | ||
| 2 μl of Restriction enzyme 2 | ||
| 5 μl of Restriction enzyme buffer (10X) | ||
| 13 μl Molecular grade water | ||
| Example T4 ligation | 1 μl Cut CS2+ | |
| 3 μl Cut PCR product 1 | ||
| 3 μl Cut PCR product 2 | ||
| 1 μl of T4 DNA ligase | ||
| 1 μl T4 DNA ligase buffer (10X) | ||
| 1 μl Molecular grade water | ||
| Example template digest | 5 ug Plasmid DNA | |
| 10 μl Restriction enzyme buffer (10X) | ||
| 3 μl Not1 (except Shh-GFP, Kpn1 is used) | ||
| Made up to 100 μl with molecular grade water |
Table 2. Example reaction conditions used for subcloning and producing synthetic mRNA for Wnt8a-HA-eGFP.
| Solution | Components |
| Cysteine-HCL | 0.1X NAM |
| 2.5% L-cysteine hydrochloride monohydrate (pH7.8) | |
| NAM salts | 110 mM NaCL |
| 2 mM KCl | |
| 1 mM CA(NO3)2 | |
| 0.1 mM EDTA | |
| NAM/2 | 0.5X NAM salts |
| 5 mM HEPES pH7.4 | |
| 0.25 mM Bicarbonate | |
| 25 ug/ml Gentamycin | |
| NAM/3 + Ficoll | 0.33X NAM salts |
| 5 mM HEPES pH7.4 | |
| 0.25 mM Bicarbonate | |
| 2 5ug/ml Gentamycin | |
| 5 % Ficoll | |
| NAM/10 | 0.1X NAM salts |
| 5 mM HEPES pH7.4 | |
| 25 ug/ml Gentamycin |
Table 3. Solutions used during the production and microinjection of Xenopus laevis embryos.
Discussion
An essential part of this protocol is generating biologically active ligands that are normally processed, secreted, and able to elicit a response in the receiving cell, despite having a fluorescent moiety attached. It is critical to establish that the fluorescently-tagged gene product is biologically active using an appropriate assay. For Shh-GFP, the ability to activate the expression of ptc1 was confirmed16. Wnt8a has a remarkably potent ability to induce a secondary axis when expressed in a single ventral blastomere20-21, and Wnt8a-HA-eGFP was shown retain this biological activity. Wnt11b inhibits activin treated ectodermal explants from undergoing convergent extension21-22, and Wnt11b-HA-eGFP also retains its biological activity17. The ability of the tagged ligands to elicit responses equivalent to the untagged proteins suggests that the conclusions based on experiments using the fluorescent ligands will be relevant to the normal protein. The addition of the HA epitope between the ligand and the fluorescent protein provided a spacer region that allows the Wnt constructs to be both active and fluorescent.
The major limitation of this type of analysis is that it does not directly inform on endogenous morphogen gradients. For instance, the diffusion of Shh across the field of cells in animal cap may not reflect the way endogenous Shh moves through the columnar neural epithelium in the embryo. However, the simplicity of this protocol allows experiments where the effect of exogenous regulators on the distribution of ligands can be tested. The results from these approaches can form the basis of hypotheses to be tested using more demanding in vivo analyses. For instance, the ability of over-expressed Sulf1 to restrict the distribution of Shh-GFP using the methods described in this paper pointed to the potential for Sulf1 in modulating the Shh morphogen gradient. This was directly tested and validated using antisense morpholino knock-down of Sulf1 and immunocytochemistry to visualise endogenous Shh in the neural tube16. A similar method to ours has been used to investigate specific mechanisms that could regulate ligand diffusion. Smith and colleagues demonstrated that a GFP-tagged nodal (Xnr2) used simple diffusion, and not cell extensions (cytonemes) or transcytosis (using vesicles) to form a gradient23.
Further elaborations of these methods are possible where other proteins that block particular pathways can be expressed in targeted cells to investigate whether a response to the signalling molecule is needed as an intermediary to relay signals from one cell to another. For instance, expressing a dominant negative activin receptor between the source of activin and the responding cells showed that simple ligand diffusion could elicit a response several cell diameters away from the source even with non-responsive cells in between24. This conclusion was supported by work in zebrafish where wild type cells can respond to a source of nodal, despite being surrounded by non-responsive mutant cells lacking an essential co-receptor for nodal25. Other studies have used zebrafish to investigate diffusion of fluorescently tagged ligands28,29. Some studies has observed that epitope tagging the C-terminus of Wnt proteins can impact on signalling activity, possibly because of the highly conserved cysteines that contribute to protein conformation. Our previous work has shown that including a spacer (such as the HA tag) results in tagged Wnt ligands that are biologically active17. This is likely because the spacer provides flexibility needed for ligand –receptor interaction, such as in the thumb-forefinger model of Wnt-Frz binding30.
The next step for advancing our studies is to include a visual readout for the activation of the signalling pathway. For instance, expressing GFP-tagged Dvl26 in cells distant from the source of ligand will allow the range across which Wnt11b has ability to signal to be measured. The range of Wnt8a signalling activity could be shown using antibody staining to measure nuclear localisation of b-catenin in cells27 at a distance from the source. These types of experiments are possible using the techniques described in this paper. By employing a variety of different fluorescent proteins or fluorophores, an additional level of information would be provided where one not only measures the distribution of a ligand but the also the output of the signalling pathways, and in this way determining the threshold range of a morphogen.
Xenopus laevis is a useful model for visualising GFP-tagged ligands and further details on the methods for procuring embryos, mRNA synthesis, microinjection and dissection are available31.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by a BBSRC grant to MEP (BB/H010297/1), a BBSRC quota studentship to SAR, and an MRC studentship to SWF.
Materials
| Agarose | Melford | MB 1200 | |
| Ammonium acetate | Ambion | From Megascript SP6 Kit AM 1330 | |
| Bicarbonate | VWR International | RC-091 | |
| Calcium nitrate | Sigma | C13961 | |
| Cap analog (m7G(5')) | Applied Biosystems | AM 8050 | |
| Chloroform | Sigma | C 2432 | |
| L-Cysteine hydrochloride monohydrate | Sigma | C7880 | |
| dNTPs | Invitrogen | 18427-013 | |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma | O3690 | |
| Ethanol | VWR International | 20821.33 | |
| Ficoll 400 | Sigma | F 4375 | |
| Fiji image J software | N/A | N/A | Free download http://fiji.sc/Fiji |
| Gentamycin | Melford | G 0124 | |
| Glacial acetic acid | Fisher Scientific | A/0400/PB17 | |
| Glass cover slips, No.1.5 | Scientific Laboratory Supplies | 22X22-SGJ3015. 22X50-SGJ3030 | |
| Glass needle puller | Narishige | Narishige PC -10 | |
| Glass pull needles | Drummond Scientific | 3-000-203-G/X | |
| Human chronic gonadotropin (HCG) | Intervet | ||
| Isopropanol | Fisher Scientific | P/7500/PB17 | |
| Lithium chloride (LiCl) | Sigma | L-7026 | |
| LSM710 and Zen software (2008-2010) | Carl Zeiss | ||
| Matlab software | Mathworks | http://uk.mathworks.com/ | |
| Molecular grade water | Fisher Scientific | BP 2819-10 | |
| Nail varnish | Boots | Bar code 3600530 373048 | |
| Spectrophotometer | Lab.tech International | ND-1000 / ND8000 | |
| Petri dish (55mm) | VWR International | 391-0865 | |
| Phenol-chloroform | Sigma | P3803 | |
| Photoshop software | Adobe | N/A | http://www.photoshop.com/products |
| High fidelity DNA polymerase and buffers | Biolabs | M0530S Buffer – M0531S | |
| Potassium chloride (KCl) | Fisher Scientific | P/4280/53 | |
| PVC insulation tape | Onecall | SH5006MPK | |
| Gel extraction kit | Qiagen | S28704 | |
| Restriction enzymes buffers | Roche | SuRE/CUT Buffer Set 11082 035 001 | |
| RNAse-free DNAse | Promega | ME10A | |
| Steel back single edge blades | Personna | 66-0403-0000 | |
| Sodium chloride (NaCl) | Fisher Scientific | 27810.364 | |
| SP6 transcription kit | Ambion | AM1330 | |
| Glass slides | Thermo Fisher | SHE 2505 | |
| Tris base | Invitrogen | 15504-020 | |
| Tungsten needles | N/A | N/A | homemade |
| Zen lite software | Carl Zeiss | N/A | Free download http://www.zeiss.co.uk/microscopy/en_gb/downloads/zen.html |
References
- Shen, M. M. Nodal signaling: developmental roles and regulation. Development. 134 (6), 1023-1034 (2007).
- Shimmi, O., Newfeld, S. J. New insights into extracellular and post-translational regulation of TGF-beta family signalling pathways. J Biochem. 154 (1), 11-19 (2013).
- Pownall, M. E., Isaacs, H. V. . FGF Signalling in Vertebrate Development. 1, 1-75 (2010).
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell. 127 (3), 469-480 (2006).
- Ingham, P. W., McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes and Development. 15 (23), 3059-3087 (2001).
- Wolpert, L. One hundred years of positional information. Trends in Genetics. 12 (9), 359-3564 (1996).
- Rushlow, C. A., Shvartsman, S. Y. Temporal dynamics, spatial range, and transcriptional interpretation of the Dorsal morphogen gradient. Current Opinion in Genetics and Development. 22 (6), 542-546 (2012).
- Erickson, J. L. Formation and maintenance of morphogen gradients: an essential role for the endomembrane system in Drosophila melanogaster wing development. Fly (Austin). 5 (3), 266-271 (2011).
- Towers, M., Wolpert, L., Tickle, C. Gradients of signalling in the developing limb. Current Opinions in Cell Biology. 24 (2), 181-187 (2012).
- Dessaud, E., McMahon, A. P., Briscoe, J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 135 (15), 2489-2503 (2008).
- Briscoe, J., et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 398 (6728), 622-627 (1999).
- Ribes, V., et al. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes and Development. 24 (11), 1186-1200 (2010).
- Freeman, S. D., Moore, W. M., Guiral, E. C., Holme, A., Turnbull, J. E., Pownall, M. E. Extracellular regulation of developmental cell signaling by XtSulf1. Biologie du développement. 320 (2), 436-445 (2008).
- Tao, Q., et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 120 (6), 857-871 (2005).
- Chamberlain, C. E., Jeong, J., Guo, C., Allen, B. L., McMahon, A. P. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 135 (6), 1097-1106 (2008).
- Ramsbottom, S. A., Maguire, R. J., Fellgett, S. W., Pownall, M. E. Sulf1 influences the Shh morphogen gradient during the dorsal ventral patterning of the neural tube in Xenopus tropicalis. Biologie du développement. 391 (2), 207-218 (2014).
- Fellgett, S. W., Maguire, R. J., Pownall, M. E. Sulf1 has ligand dependent effects on canonical and non-canonical WNT signalling. Journal of Cell Science. 128 (7), 1408-1421 (2015).
- Dytham, C. . Choosing and using statistics : A biologist’s guide. , (2005).
- Fay, M. P., Proschan, M. A. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Statistical Surveys. 4, 1-39 (2010).
- Christian, J. L., McMahon, J. A., McMahon, A. P., Moon, R. T. Xwnt-8, a Xenopus Wnt-1 /int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 111 (4), 1045-1055 (1991).
- Du, S. J., Purcell, S. M., Christian, J. L., McGrew, L. L., Moon, R. T. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Molecular and Cellular Biology. 15 (5), 2625-2634 (1995).
- Tada, M., Smith, J. C. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 127 (10), 2227-2238 (2000).
- Williams, P. H., Hagemann, A., Gonzalez-Gaitan, M., Smith, J. C. Visualizing long-range movement of the morphogen Xnr2 in the Xenopus embryo. Current Biology. 14 (21), 1916-1923 (2004).
- McDowell, N., Zorn, A. M., Crease, D. J., Gurdon, J. B. Activin has direct long-range signalling activity and can form a concentration gradient by diffusion. Current Biology. 7 (9), 671-681 (1997).
- Chen, Y., Schier, A. F. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 411 (6837), 607-610 (2001).
- Miller, J. R., Rowning, B. A., Larabell, C. A., Yang-Snyder, J. A., Bates, R. L., Moon, R. T. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. Journal of Cell Biology. 146 (2), 427-437 (1999).
- Schohl, A., Fagotto, F. B. e. t. a. -. c. a. t. e. n. i. n. MAPK and Smad signaling during early Xenopus development. Development. 129 (1), 37-52 (2002).
- Muller, P., et al. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 336, 721-724 (2012).
- Yu, S. R., Burkhardt, M., Nowak, M., Ries, J., Petrasek, Z., Scholpp, S., Schwille, P., Brand, M. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 461, 533-536 (2009).
- Janda, C. Y., Waghray , D., Levin, A. M., Thomas, C., Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science. 337, 59-64 (2012).
- Sive, H. L., Grainger, R. M., Harland, R. M. . Early development of Xenopus laevis: a laboratory manual. , (2010).

