12.6:
Physical Properties Affecting Solubility
18,972 Views
•
•
Solutions of Gases in Liquids
As for any solution, the solubility of a gas in a liquid is affected by the attractive intermolecular forces between solute and solvent species. Unlike solid and liquid solutes, however, there is no solute-solute intermolecular attraction to overcome when a gaseous solute dissolves in a liquid solvent since the atoms or molecules comprising a gas are far separated and experience negligible interactions. Consequently, solute-solvent interactions are the sole energetic factor affecting solubility. For example, the water solubility of oxygen is approximately three times greater than that of helium (there are greater dispersion forces between water and the larger oxygen molecules) but 100 times less than the solubility of chloromethane, CHCl3 (polar chloromethane molecules experience dipole-dipole attraction to polar water molecules). Likewise, note the solubility of oxygen in hexane, C6H14, is approximately 20 times greater than it is in water because greater dispersion forces exist between oxygen and the larger hexane molecules.
Temperature is another factor affecting solubility, with gas solubility typically decreasing as temperature increases. This inverse relation between temperature and dissolved gas concentration is responsible for one of the major impacts of thermal pollution in natural waters.
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Gas solubility increases as the pressure of the gas increases.
For many gaseous solutes, the relation between solubility, Sgas, and partial pressure, Pgas, is a proportional one:
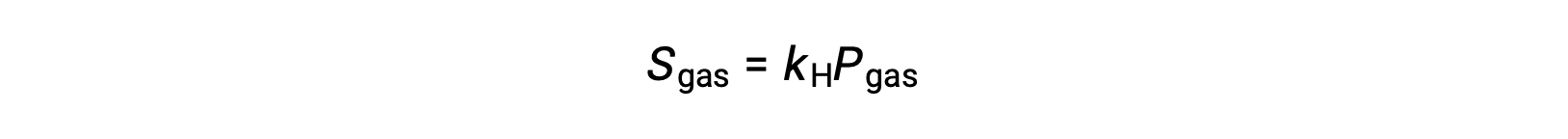
where kH is a proportionality constant that depends on the identities of the gaseous solute and solvent and on the solution temperature. This is a mathematical statement of Henry’s law: The quantity of an ideal gas that dissolves in a definite volume of liquid is directly proportional to the pressure of the gas.
This text is adapted from Openstax, Chemistry 2e, Section 11.3: Solubility.
