Robotic Left Hepatectomy using Indocyanine Green Fluorescence Imaging for an Intrahepatic Complex Biliary Cyst
Summary
Robotic liver surgery has gained more acceptance as a feasible, safe, and effective procedure for the treatment of both benign and malignant indications. However, robotic left hepatectomy is still technically demanding. We describe our surgical technique of a robotic left hepatectomy using indocyanine green fluorescence imaging for a large biliary cyst.
Abstract
Biliary cysts (BC) are rare congenital dilatations of intra- and extrahepatic parts of the biliary tract and bear a significant risk of carcinogenesis. Surgery is the cornerstone treatment for patients with BC. While total BC excision and Roux-Y hepaticojejunostomy is the treatment method of the choice in patients with extrahepatic BC (i.e., Todani I-IV), patients with intrahepatic BC (i.e., Todani V) benefit the most from a surgical liver resection. In recent years, minimally invasive liver surgery (MILS) including robotic MILS has gained more acceptance as a feasible, safe, and effective procedure for the treatment of both benign and malignant indications. Robotic major MILS is still considered technically demanding and a detailed description of the technical approach during robotic major MILS has only been limitedly discussed in the literature. The current article describes the main steps for a robotic left hepatectomy in a patient with a large BC Todani Type V. The patient is in French position with 5 trocars placed (4 robotic, 1 laparoscopic assistant). After mobilizing the left hemiliver, the left and right hepatic artery are dissected carefully followed by a cholecystectomy. Intraoperative ultrasound is performed to confirm localization and margins of the BC. The Left hepatic artery and left portal vein are isolated, clipped, and divided. Indocyanine green (ICG) fluorescence imaging is used regularly during the entire procedure to visualize and confirm biliary tract anatomy and the BC. Parenchymal transection is performed with robotic cautery hook for the superficial part and robotic cautery spatula, bipolar cautery, and vessel sealer for the deeper parenchyma. The postoperative course was uncomplicated. A robotic left hepatectomy is technically demanding, yet a feasible and safe procedure. ICG-fluorescence imaging aids in delineating the BC and bile duct anatomy. Further, comparative studies are needed to confirm clinical benefits of robotic MILS for benign and malignant indications.
Introduction
Biliary cysts (BC) are rare congenital dilatations of intra- and extrahepatic parts of the biliary tract1. Approximately 1% of all benign biliary diseases are BC with an incidence of 1:1000 in Asian countries and 1:100,000 to 1:150,000 in western countries1,2. While the majority of cases are diagnosed during infancy or childhood, 20% of the cases are diagnosed in adults2. BC are divided into groups as per the Todani classification3. The early diagnosis and treatment are crucial since BC are associated with a risk of carcinogenesis, not only occurring more often in these patients but also 10-15 years before the disease is manifested4,5,6. The overall risk of malignancy has been reported to be 10%-15%, and depends on the Todani classification and age1,6. While patients aged 31-50 years with BC have a risk of 19% of carcinogenesis, 51-70-year-old patients with BC were reported to have a risk of at least 50% of carcinogenesis7. Surgery is the cornerstone treatment of BC8. While total BC excision and Roux-Y hepaticojejunostomy is the treatment method of the choice in patients with extrahepatic BC (i.e., Todani I-IV), patients with intrahepatic BC (i.e., Todani V) benefit the most from a surgical liver resection or liver transplantation in case of bilobar Todani V8.
In recent years, minimally invasive liver surgery (MILS), including laparoscopic and robotic MILS has gained more acceptance as a feasible, safe, and effective procedure for the treatment of both benign and malignant indications9,10,11,12. According to the most recent international Southampton guidelines on laparoscopic liver surgery, laparoscopy is now seen as the gold standard for minor liver resections and laparoscopic major liver resections are considered feasible and safe in selected patients if performed by surgeons who have completed the learning curve for minor laparoscopic liver surgery. However, laparoscopic liver surgery has some persistent limitations, including restriction of movements, presence of physiologic tremors and reduced visualization13,14. Robotic MILS is, therefore, a valuable alternative to laparoscopic MILS. It is suggested that robotic MILS provides a better magnified three-dimensional view, tremor filtration, improved dexterity with several degrees of freedom, ease of suturing, and better motion scaling, as compared to laparoscopic liver surgery15,16,17. Furthermore, robotic MILS allows the surgeon to remain in a seated posture, reducing fatigue during surgery18. While some studies reported on the potential advantages of robotic MILS as compared to open liver surgery, several high-volume expert centers showed similar outcomes of both minor and major robotic and laparoscopic MILS14,18,19,20. However, major robotic MILS, defined as the resection of three or more Couinaud's segments21, is still considered technically demanding and a detailed description of the technical approach during robotic major MILS had only been discussed limitedly in the literature. Studies describing the technique and use of robotic MILS for the treatment of BC Todani Type V are lacking.
Here, we describe our robotic technique of a left hepatectomy using Indocyanine green (ICG) fluorescence imaging for a symptomatic complex BC. This case involves a 68-year-old woman who had elevated liver enzymes during a routine check-up without any clinical symptoms. An abdominal ultrasound of the liver revealed intrahepatic dilatation of the biliary ducts specifically in the left hemi liver without a clear lesion. Further diagnostic examinations, including an abdominal CT scan, MRI scan, (Figure 1) and MRCP, showed a large intrahepatic complex cystic lesion of 40 mm on the border of segment 4a and 4b in continuity with the biliary tree with intrahepatic dilatation of biliary ducts in the left lobe. The patient was diagnosed with a large BC Todani Type V of the left hepatic duct and was recommended for a robotic left hepatectomy. Since there were no signs of biliary obstruction, preoperative biliary drainage was not performed.
Protocol
Written informed consent has been obtained from the patient to use medical data and the operative video for education and scientific purposes. This research was performed in compliance with all institutional, national, and international guidelines for human welfare.
1. Positioning and robot docking
- Position the patient on a vacuum mattress in a supine French position. Lower the right arm alongside the body on an arm support and extend the left arm. Tilt the operating table 10-20° in anti-Trendelenburg and 5-10° to the right.
- After all safety procedures (hood, sterile glove, and sterile scrub) are ascertained, create a sterile exposition. Make a 2 mm incision in the left hypochondrium on the midclavicular line and create a pneumoperitoneum with CO2 to 15 mmHg by placing a Veress needle.
- Insert the robotic camera through a visiport 12 mm trocar in the right pararectal space just below the umbilicus and perform a diagnostic laparoscopy. Once diagnostic laparoscopy confirms no contraindication for surgery, place the remaining trocars as shown in Figure 2.
- Place four 8 mm trocars above the umbilicus and introduce a 12 mm laparoscopic assistant trocar for the bedside surgeon on the right side of the umbilicus.
- Ensure that the bedside surgeon can reach the transection area for suctioning, compression, clipping, and stapling without difficulty. The distance between the four ventral trocars is approximately 8 cm.
- Place the robot on the right side next to the patient and dock the arms to the four robotic trocars.
- Ensure that the first surgeon takes place at the robot console and the bedside surgeon between the patient's legs.
2. Mobilization
- Start with the mobilization of the left lobe. Divide the round and falciform ligaments using the robotic cautery hook and vessel sealer.
- Then, continue the mobilization by dividing the left coronary and triangular ligaments using the robotic cautery hook and/or vessel sealer.
NOTE: It is important not to injure the left hepatic vein and branches of the phrenic vein, often located nearby and draining into the left hepatic vein. - Open the triangular ligament using the robotic cautery hook and/or vessel sealer all the way toward the origin of the left hepatic vein. Dissection is completed until the origin of the left hepatic vein is reached.
- Visualize the lesser omentum by lifting the inferior aspect of the liver cranially. Dissect the lesser omentum using a vessel sealer.
NOTE: If an aberrant left hepatic artery is present, ligate using the robotic cautery hook and/or vessel sealer.
3. Hilar dissection
- Identify the proper and left hepatic artery in the hepatoduodenal ligament by lifting the liver cranially and moving the robotic camera to the hilum.
- Dissect and isolate the left hepatic artery using both the robotic cautery hook and bipolar forceps (optional: Maryland bipolar forceps).
- After visualizing the left hepatic artery, identify and dissect the origin of the right hepatic artery to make sure it is preserved.
- Then, dissect and isolate the left portal vein carefully. Switch the view to ICG-fluorescence imaging to identify the exact localization and trajectory of the left bile duct with respect to the left portal vein.
NOTE: ICG was administered preoperatively in parallel to induction of general anesthesia prior to the start of the surgery.
4. Cholecystectomy
- Identify the cystic duct and artery.
- First, dissect and isolate the cystic duct and artery using the robotic cautery hook to achieve the critical view of safety, also known as the Calot's Triangle.
- Clip both cystic duct and artery using polymer locking clips. Place two clips proximally and one distally on the cystic duct. Place one clip proximally and one clip distally for the cystic artery.
- Divide the cystic duct and artery between the clips with robotic scissors.
- Second, dissect the gallbladder circumferentially off the liver using a robotic cautery hook until the gallbladder is detached from the liver.
- Place the resected gallbladder in an extraction bag and position it outside of the working field.
5. Vascular transection
- Prepare a pringle loop by passing a vessel loop around the hepatoduodenal ligament. During this procedure, the pringle maneuver was not applied.
- Perform an intraoperative ultrasound (IOUS) of the liver to confirm the localization, borders, and depth of the biliary cyst.
- Switch the view to ICG-fluorescence imaging to confirm the trajectory of the right and left hepatic duct before heading to the arterial and venous hilar transection.
- First, clip the left hepatic artery carefully with polymer locking clips by placing two clips proximally and one distally.
- Divide the left hepatic artery between the clips with robotic scissors.
- Pass a vessel loop around the left portal vein using the Maryland bipolar forceps to ensure isolation of the left portal vein with preservation of the segment 1 branch.
- Then, clip the left portal vein with polymer locking clips by placing two clips proximally and one distally.
- Divide the left portal vein between the clips with robotic scissors.
NOTE: The left hepatic bile duct is not divided during this phase of the procedure to ensure no injury to the right hepatic duct.
6. Parenchymal transection
- Visualize the ischemia line on the liver surface. The ischemia line should overlap the Cantlie's line since the aim is to perform anatomical left hepatectomy. Mark the transection line following the ischemia line using a cautery hook.
- Perform the superficial part of the transection using a cautery hook until a depth of 1 cm parenchyma is reached. For the deeper parenchyma, use the vessel sealer, the cautery spatula, and Maryland bipolar forceps.
- Control intrahepatic vascular and biliary structures with the vessel sealer as well. Control any intrahepatic small bleeding using the cautery spatula or bipolar forceps. Now carefully identify the branch of the middle hepatic vein for preservation.
- Transect the parenchyma until the left hepatic vein is reached. Before completion of the parenchymal transection, move back to the hilum to focus on the left hepatic duct.
- Switch the view to ICG-fluorescence imaging to confirm the exact trajectory, size, and localization of the left hepatic duct.
- Dissect the left hepatic duct carefully using the Maryland bipolar forceps.
- At last, clip the left hepatic duct with polymer locking clips by placing one clip proximally and one clip distally. Divide the left hepatic duct between the clips with robotic scissors. The procedure ends with the division of the left hepatic vein.
- Pass a vessel loop around the remaining liver parenchyma and left hepatic vein for the hanging maneuver.
NOTE: This allows retraction of the right lobe of the liver toward the right side and puts tension on the remaining liver parenchyma and left hepatic vein to be able to get a better vision and grip on the left hepatic vein. - Then, divide the left hepatic vein using a laparoscopic stapler.
- After completion of the left hepatectomy, place the resected specimen in an extraction bag and take both the specimen and gallbladder out through a Pfannenstiel incision. No intra-abdominal drain was placed.
Representative Results
Representative results are shown in Table 1. Following the surgical technique in the protocol, the operative time was 189 min with an intraoperative blood loss of 10 mL. No conversion to laparotomy was needed and no intraoperative incidents occurred. The postoperative course was uncomplicated without any postoperative complications. The patient was discharged on postoperative day 4.
The final histopathological examination revealed a large complex cyst of 3.1 cm in continuity with a biliary branch of the left hepatic duct without any suspicion for malignancy.
Comparable result from literature
Several studies investigated outcomes of major robotic liver surgery, including robotic left hepatectomy22,23,24. An operative time of 383 min (IQR 240-580 min)23 with an estimated intraoperative blood loss of 300 mL (IQR 100-1,000)23 has been described previously. With regards to postoperative outcomes, a length of hospital stay of 3 days (IQR 3-5 days)22,24, a favorable Clavien-Dindo ≥ grade III complication rate of 7.0%24 and a remarkable low mortality rate (0%)22,23,24 were reported.
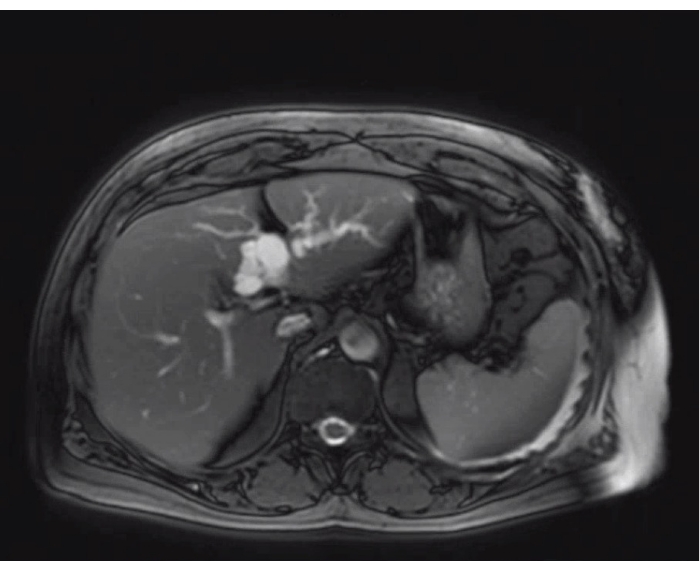
Figure 1: The appearance of the biliary cyst and the relationship with the left biliary tree on MRI-scan Please click here to view a larger version of this figure.
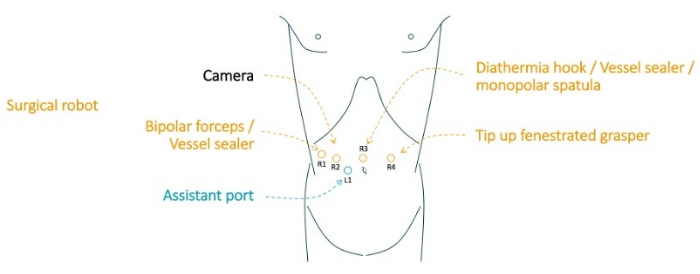
Figure 2: Trocar placement. R1: robotic trocar at right anterior axillary line; R2: robotic trocar at right mid-clavicular line; R3: robotic trocar on midline; R4: robotic trocar at left mid-clavicular line. L1: laparoscopic assistant trocar on the right side of the umbilicus. This figure is adapted from Kaçmaz, E. et al. 202025. Please click here to view a larger version of this figure.
| Variable | Outcome |
| Intraoperative | |
| Operative time (min) | 189 |
| Conversion to laparotomy | No |
| Estimated intraoperative blood loss (mL) | 10 |
| Intraoperative incidents | No |
| Postoperative | |
| Clavien-Dindo complication | No |
| Clavien-Dindo complication ≥ grade III | No |
| 90-day Reoperation | No |
| Length of hospital stay, days | 4 |
| 90-day readmission | No |
| 90-day/in-hospital mortality | No |
| Pathological diagnosis | Large complex biliary cyst without malignancy |
Table 1: Outcome of the surgery
Discussion
The use of robotic major MILS has increased gradually over the years for both benign and malignant indications. However, robotic major left hepatectomy is still a technically demanding procedure and it is, therefore, suggested to follow a structured approach, including six main steps: positioning and docking of the robotic system, mobilization of the left lobe, hilar dissection, cholecystectomy, vascular transection, and parenchymal transection.
ICG-fluorescence imaging is emerging as a promising and useful tool during robotic liver surgery as applied in the current procedure. While IOUS is routinely performed during robotic MILS and provides the most actual information on number and size of lesions, and its relation to anatomical structures26, it may be technically challenging due to limitations in free-range of motion and lack of information on precise biliary tract anatomy27. ICG-fluorescence imaging can, therefore, aid the surgeon in both visualizing liver lesions and the exact trajectory of intra- and extrahepatic biliary ducts to perform an uncomplicated robotic liver resection. Previously published retrospective studies on ICG-fluorescence imaging during liver surgery primarily focused on the sensitivity of ICG-fluorescence imaging and detection of additional liver lesions as compared to IOUS rather than focusing on the intra- and postoperative impact of enhanced intraoperative visualization of biliary tract anatomy28,29,30. These studies showed that significantly more additional lesions were identified in patients where ICG-imaging was performed compared to IOUS with comparable intra- and postoperative outcomes between both groups. Of note, these studies didn't include robotic MILS.
Parenchymal transection is one of the most critical steps during robotic MILS and accounts for the majority of blood loss, being a major determinant of morbidity and mortality. A careful and structured approach with the use of appropriate robotic instruments is therefore necessary. Transection techniques have evolved over time from the clamp-crush technique to the use of a variety of energy devices31,32. Ultrasonic dissection devices such as the Cavitron Ultrasonic Aspirator (CUSA) offer superior visualization of intrahepatic structures and are often used during parenchymal transection32. However, the laparoscopic CUSA is the only available ultrasonic dissection device successfully integrated into laparoscopic MILS, not available for robotic MILS33,34. During the current robotic procedure, a cautery hook was used for the superficial part of the liver and both the vessel sealer and the cautery spatula for the deeper parenchyma. Of note, a recent survey study highlighted that 70% of the surgeons performing robotic MILS were dissatisfied with the available robotic instruments for liver parenchymal transection34. The development of new instruments for robotic parenchymal transection might help to further improve outcomes after liver surgery and increase the adoption of robotic MILS.
Blood loss, operative time, and length of hospital stay of the current procedure were favorable and comparable with recent series on major robotic MILS22,23. Furthermore, the robotic procedure has similar intra- and postoperative outcomes as compared to laparoscopic MILS35,36. However, it is important to emphasize that robotic MILS is costly and more challenging as compared to the laparoscopic and open approach. Specific training in robotic MILS in combination with extensive experience in both open and laparoscopic liver surgery is needed to perform robotic MILS safely37. We therefore believe that robotic major MILS such as a robotic left hepatectomy should be limited to high-volume MILS centers and a careful selection of patients should be applied.
In summary, this manuscript provides the detailed steps of a robotic left hepatectomy, as performed at Amsterdam UMC in the Netherlands. A robotic left hepatectomy is technically demanding, yet a feasible and safe procedure. ICG-fluorescence imaging may be helpful in delineating BC and bile duct anatomy. Further comparative studies are needed to confirm clinical benefits of robotic MILS for benign and malignant indications.
Divulgations
The authors have nothing to disclose.
Materials
| Systems | |||
| Arietta V70 Ultrasound | Hitachi | – | The ultrasound system. |
| da Vinci Surgeon Console | IS | SS999 | Used to control the surgical robot. |
| da Vinci Vision Cart | IS | VS999 | The vision cart houses advanced vision and energy technologies and provides communications across da Vinci system components. |
| da Vinci Xi | IS | K131861 | The surgical robot: ’patient side-cart’. |
| Robotic ultrasonography transducer | Hitachi | L43K | Used for intraoperative laparoscopic ultrasonography. |
| Instruments | |||
| da Vinci Xi Endoscope with Camera, 8 mm, 30˚ | IS | 470027 | The camera of the da Vinci robot. |
| EndoWrist Fenestrated Bipolar Forceps | IS | 470205 | Used for dissection and coagulation. |
| EndoWrist HOT SHEARS | IS | 470179 | Used for cutting and coagulation. |
| EndoWrist Maryland Bipolar Forceps | IS | 470172 | Used for dissection. |
| EndoWrist Permanent Cautery Hook | IS | 470183 | Used for coagulation. |
| EndoWrist Medium-Large Clip Applier | IS | 470327 | Used for clipping with Weck Hem-o-lok medium-large polymer clip |
| EndoWrist Stapler 45 Instrument | IS | 470298 | Used for stappling |
| Vessel sealer | IS | 480322 | Used for vessel sealing and dividing. |
References
- Jabłońska, B. Biliary cysts: Etiology, diagnosis and management. World Journal of Gastroenterology. 18 (35), 4801-4810 (2012).
- Singham, J., Yoshida, E. M., Scudamore, C. H. Choledochal cysts part 1 of 3: Classification and pathogenesis. Canadian Journal of Surgery. 52 (5), 434-440 (2009).
- Todani, T., Watanabe, Y., Narusue, M., Tabuchi, K., Okajima, K. Congenital bile duct cysts. Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. American Journal of Surgery. 134 (2), 263-269 (1977).
- Tsuchiya, R., Harada, N., Ito, T., Furukawa, M., Yoshihiro, I. Malignant tumors in choledochal cysts. Annals of Surgery. 186 (1), 22-28 (1977).
- Jan, Y. Y., Chen, H. M., Chen, M. F. Malignancy in choledochal cysts. Hepatogastroenterology. 47 (32), 337-340 (2000).
- Okada, A., Hasegawa, T., Oguchi, Y., Nakamura, T. Recent advances in pathophysiology and surgical treatment of congenital dilatation of the bile duct. Journal of Hepato-Biliary-Pancreatic Surgery. 9 (3), 342-351 (2002).
- Nicholl, M., et al. Choledochal cysts in western adults: Complexities compared to children. Journal of Gastrointestinal Surgery. 8 (3), 245-252 (2004).
- Singham, J., Yoshida, E. M., Scudamore, C. H. Choledochal cysts: Part 3 of 3: Management. Canadian Journal of Surgery. 53 (1), 51 (2010).
- vander Poel, M. J., et al. Implementation and outcome of minor and major minimally invasive liver surgery in the Netherlands. HPB. 21 (12), 1734-1743 (2019).
- Ciria, R., et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: Updated results from the European guidelines meeting on laparoscopic liver surgery, Southampton, UK, 2017. Annals of Surgical Oncology. 26 (1), 252-263 (2017).
- Nota, C. L., et al. Robot-assisted laparoscopic liver resection: a systematic review and pooled analysis of minor and major hepatectomies. HPB. 18 (2), 113-120 (2016).
- Nota, C., Molenaar, I. Q., Hagendoorn, J., Borel Rinkes, I. H. M., van Hillegersberg, R. Robot-assisted laparoscopic liver resection: First dutch experience. HPB. 18 (1), 265 (2016).
- Alkhalili, E., Berber, E. Laparoscopic liver resection for malignancy: a review of the literature. World Journal of Gastroenterology. 20 (37), 13599-13606 (2014).
- Cai, J. P. Comparison between robotic-assisted and laparoscopic left hemi-hepatectomy. Asian Journal of Surgery. 45 (1), 265-268 (2021).
- Troisi, R. I., et al. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints. Surgical Oncology. 33, 239-248 (2019).
- Sucandy, I., et al. Robotic hepatectomy for benign and malignant liver tumors. Journal of Robotic Surgery. 14 (1), 75-80 (2020).
- Beard, R. E., et al. Long-term and oncologic outcomes of robotic versus laparoscopic liver resection for metastatic colorectal cancer: A multicenter, propensity score matching analysis. World Journal of Surgery. 44 (3), 887-895 (2020).
- Wang, J. -. M., Li, J. -. F., Yuan, G. -. D., He, S. -. Q. Robot-assisted versus laparoscopic minor hepatectomy: A systematic review and meta-analysis. Medicine (Baltimore). 100 (17), 25648 (2021).
- Ciria, R., et al. The impact of robotics in liver surgery: A worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. Journal of Hepatobiliary Pancreatic Sciences. 29 (2), 181-197 (2020).
- Wong, D. J. Systematic review and meta-analysis of robotic versus open hepatectomy. ANZ Journal of Surgery. 89 (3), 165-170 (2019).
- Strasberg, S. M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. Journal of Hepato-Biliary-Pancreatic Surgery. 12 (5), 351-355 (2005).
- Sucandy, I., Gravetz, A., Ross, S., Rosemurgy, A. Technique of robotic left hepatectomy how we approach it. Journal of Robotic Surgery. 13 (2), 201-207 (2019).
- Magistri, P., Assirati, G., Ballarin, R., Di Sandro, S., Di Benedetto, F. Major robotic hepatectomies: technical considerations. Updates in Surgery. 73 (3), 989-997 (2021).
- Fruscione, M., et al. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. Hpb. 21 (7), 906-911 (2019).
- Kaçmaz, E., et al. Robotic enucleation of an intra-pancreatic insulinoma in the pancreatic head. Journal of Visualized Experiments:JoVE. (155), e60290 (2020).
- Shah, A. J., Callaway, M., Thomas, M. G., Finch-Jones, M. D. Contrast-enhanced intraoperative ultrasound improves detection of liver metastases during surgery for primary colorectal cancer. HPB. 12 (3), 181-187 (2010).
- Bijlstra, O. D., Achterberg, F. B., Grosheide, L., Vahrmeijer, A. L., Swijnenburg, R. -. J. Fluorescence-guided minimally-invasive surgery for colorectal liver metastases, a systematic review. Laparoscopic Surgery. 5, (2021).
- Handgraaf, H. J. M., et al. Long-term follow-up after near-infrared fluorescence-guided resection of colorectal liver metastases: A retrospective multicenter analysis. European Journal of Surgical Oncology. 43 (8), 1463-1471 (2017).
- Vahrmeijer, A. L., Hutteman, M., Van Der Vorst, J. R., Van De Velde, C. J. H., Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nature Reviews. Clinical Oncology. 10 (9), 507-518 (2013).
- Van Der Vorst, J. R., et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 119 (18), 3411-3418 (2013).
- Eeson, G., Karanicolas, P. J. Hemostasis and hepatic surgery. The Surgical Clinics of North America. 96 (2), 219-228 (2016).
- Otsuka, Y., et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. Journal of Hepato-Biliary-Pancreatic Sciences. 22 (5), 363-370 (2015).
- Hawksworth, J., et al. Improving safety of robotic major hepatectomy with extrahepatic inflow control and laparoscopic CUSA parenchymal transection: technical description and initial experience. Surgical Endoscopy. 36 (5), 3270-3276 (2021).
- Zwart, M. J. W., et al. Pan-European survey on the implementation of robotic and laparoscopic minimally invasive liver surgery. HPB. 24 (3), 322-331 (2021).
- Fruscione, M., et al. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB. 21 (7), 906-911 (2019).
- Cipriani, F., et al. Pure laparoscopic versus robotic liver resections: Multicentric propensity score-based analysis with stratification according to difficulty scores. Journal of Hepato-Biliary-Pancreatic Sciences. , (2021).
- Coletta, D., Sandri, G. B. L., Giuliani, G., Guerra, F. Robot-assisted versus conventional laparoscopic major hepatectomies: Systematic review with meta-analysis. The International Journal of Medical Robotics + Computer Assisted Surgery. 17 (3), 2218 (2021).

