Generation and Culture of Lingual Organoids Derived from Adult Mouse Taste Stem Cells
Summary
The protocol presents a method for culturing and processing lingual organoids derived from taste stem cells isolated from the posterior taste papilla of adult mice.
Abstract
The sense of taste is mediated by taste buds on the tongue, which are composed of rapidly renewing taste receptor cells (TRCs). This continual turnover is powered by local progenitor cells and renders taste function prone to disruption by a multitude of medical treatments, which in turn severely impacts the quality of life. Thus, studying this process in the context of drug treatment is vital to understanding if and how taste progenitor function and TRC production are affected. Given the ethical concerns and limited availability of human taste tissue, mouse models, which have a taste system similar to humans, are commonly used. Compared to in vivo methods, which are time-consuming, expensive, and not amenable to high throughput studies, murine lingual organoids can enable experiments to be run rapidly with many replicates and fewer mice. Here, previously published protocols have been adapted and a standardized method for generating taste organoids from taste progenitor cells isolated from the circumvallate papilla (CVP) of adult mice is presented. Taste progenitor cells in the CVP express LGR5 and can be isolated via EGFP fluorescence-activated cell sorting (FACS) from mice carrying an Lgr5EGFP-IRES-CreERT2 allele. Sorted cells are plated onto a matrix gel-based 3D culture system and cultured for 12 days. Organoids expand for the first 6 days of the culture period via proliferation and then enter a differentiation phase, during which they generate all three taste cell types along with non-taste epithelial cells. Organoids can be harvested upon maturation at day 12 or at any time during the growth process for RNA expression and immunohistochemical analysis. Standardizing culture methods for production of lingual organoids from adult stem cells will improve reproducibility and advance lingual organoids as a powerful drug screening tool in the fight to help patients experiencing taste dysfunction.
Introduction
In rodents, lingual taste buds are housed in fungiform papillae distributed anteriorly, bilateral foliate papillae posteriorly, as well as a single circumvallate papilla (CVP) at the posterodorsal midline of the tongue1. Each taste bud is composed of 50-100 short-lived, rapidly renewing taste receptor cells (TRCs), which include type I glial-like support cells, type II cells that detect sweet, bitter, and umami, and type III cells that detect sour2,3,4. In the mouse CVP, LGR5+ stem cells along the basal lamina produce all TRC types as well as non-taste epithelial cells5. When renewing the taste lineage, LGR5 daughter cells are first specified as post-mitotic taste precursor cells (type IV cells) that enter a taste bud and are capable of differentiating into any of the three TRC types6. The rapid turnover of TRCs renders the taste system susceptible to disruption by medical treatments, including radiation and certain drug therapies7,8,9,10,11,12,13. Thus, studying the taste system in the context of taste stem cell regulation and TRC differentiation is vital for understanding how to mitigate or prevent taste dysfunction.
Mice are a traditional model for in vivo studies in taste science since they have a taste system organized similarly to humans14,15,16. However, mice are not ideal for high throughput studies, as they are expensive to maintain and time-consuming to work with. To overcome this, in vitro organoid culture methods have been developed in recent years. Taste organoids can be generated from native CVP tissue, a process in which organoids bud off from isolated mouse CVP epithelium cultured ex vivo17. These organoids display a multilayered epithelium consistent with the in vivo taste system. A more efficient way to generate organoids that does not require ex vivo CVP culture was developed by Ren et al. in 201418. Adapting methods and culture media first developed to grow intestinal organoids, they isolated single Lgr5-GFP+ progenitor cells from mouse CVP and plated them in matrix gel19. These single cells generated lingual organoids that proliferate during the first 6 days of culture, begin to differentiate around day 8, and by the end of the culture period contain non-taste epithelial cells and all three TRC types18,20. To date, multiple studies utilizing the lingual organoid model system have been published17,18,20,21,22; however, methods and culture conditions used to generate these organoids vary across publications (Supplementary Table 1). Thus, these methods have been adjusted and optimized here to present a detailed standardized protocol for the culture of lingual organoids derived from LGR5+ progenitors of adult mouse CVP.
Lingual organoids provide a unique model for studying the cell biological processes driving taste cell development and renewal. As the applications of lingual organoids expand and more labs move toward utilizing in vitro organoid models, it is important that the field strives to develop and adopt standardized protocols to improve reproducibility. Establishing lingual organoids as a standard tool within taste science would enable high throughput studies that tease apart how single stem cells generate the differentiated cells of the adult taste system. Additionally, lingual organoids could be employed to rapidly screen drugs for potential impacts on taste homeostasis, which could then be investigated more thoroughly in animal models. This approach ultimately will enhance efforts to devise therapies that improve the quality of life for future drug recipients.
Protocol
All the animal procedures were performed in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals, Animal Welfare Act, and Public Health Service Policy, and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Colorado Anschutz Medical Campus. Lgr5EGFP-IRES-CreERT2 mice used in this protocol are from The Jackson Laboratory, Stock No. 008875.
NOTE: The following steps should be completed before beginning to ensure smooth and timely progression of the protocol: set water bath to 37 °C, set centrifuge to 4 °C, make injection and dissociation enzyme solutions from the 10 mg/mL Dispase, Collagenase, and Elastase stock solutions (see Table of Materials), remove matrix gel from -20 °C freezer (~750 µL needed for a 48-well plate) and thaw by submerging the vial in ice for at least 3-4 h, pre-coat microcentrifuge tubes in undiluted FBS by rocking gently at room temperature for at least 30 min (two 2 mL tubes for tissue collection, two 1.5 mL tubes for dissociated cells, and one 1.5 mL tube for collection of single cells from cell sorter; remove excess FBS before use).
1. Isolation of CVP epithelium
NOTE: To obtain enough LGR5+ cells for a full 48-well plate, collect three Lgr5-EGFP CVPs in the same tube and process simultaneously. Importantly, harvest and process the CVP of at least one wild type littermate in parallel in a separate tube and utilize it as a gating control to set FACS parameters (see Representative Results).
- Euthanize the mice with CO2 asphyxiation according to IACUC regulations, followed by an approved secondary method such as bilateral thoracotomy, cervical dislocation, decapitation, or exsanguination.
- Use large sterile dissection scissors to cut the cheeks and break the jaw. Lift the tongue and cut the lingual frenulum to separate the tongue from the floor of the oral cavity. Cut out the tongue and collect it in sterile ice-cold Dulbecco's phosphate-buffered saline (dPBS) with Ca2+ and Mg2+.
- Remove and discard the anterior tongue by cutting just anterior of the intermolar eminence with a razor blade (Figure 1A, dashed line). Use a delicate task wipe to remove any hair and excess liquid from the posterior tongue.
- Fill 1 mL syringe with 200-300 µL of injection enzyme solution (final concentration: 2 mg/mL type-I Collagenase and 5 mg/mL Dispase II in Ca2+/Mg2+-containing dPBS, diluted from 10 mg/mL stock solutions) and insert a 30 G x ½ needle just above the intermolar eminence (Figure 1B, black arrow) until just anterior to the CVP (Figure 1B, black box). Inject the enzyme solution underneath and at the lateral edges of the CVP between the epithelium and the underlying tissues (lamina propria, muscle). Withdraw the syringe slowly and continuously from the tongue as the injection is performed.
- Incubate the tongue in sterile Ca2+/Mg2+-free dPBS at room temperature for precisely 33 min.
- Make small cuts in the epithelium bilaterally and just anterior to the CVP using extra fine dissection scissors, and gently peel the epithelium by lifting it with fine forceps. Once the trench epithelium is free of the underlying connective tissue, place it in an empty 2 mL microcentrifuge tube pre-coated with FBS. Do the epithelial trimming before or after detaching the CVP epithelium (Figure 1C,D).
2. Dissociation of CVP epithelium
NOTE: Dissociation of the CVP epithelium and plating are represented graphically in Figure 2.
- Add the dissociation enzyme cocktail (final concentration: 2 mg/mL type-I Collagenase, 2 mg/mL Elastase, and 5 mg/mL Dispase II in Ca2+/Mg2+-containing dPBS, diluted from 10 mg/mL stock solutions) to tubes containing peeled CVP epithelia (200 µL per CVP). Incubate in a 37 °C water bath for 45 min. Vortex briefly every 15 min.
NOTE: Prewarm 0.25% Trypsin-EDTA in 37 °C water bath during the last 15 min of enzyme cocktail incubation. - Following incubation, vortex (three pulses) then triturate with a glass Pasteur pipette for 1 min. After tissue pieces settle, pipette the supernatant containing first collection of dissociated cells, into new FBS-coated 1.5 mL microcentrifuge tubes corresponding to the genotype. Process the remaining tissue pieces further as described in step 2.3. below.
- Spin the supernatant for 5 min at 370 x g and 4 °C to pellet cells.
- Remove the resulting supernatant and resuspend the cell pellet in Fluorescence-Activated Cell Sorting (FACS) Buffer (1 mM EDTA, 25 mM HEPES (pH 7.0) and 1% FBS in Ca2+/Mg2+-free PBS (50 µL per CVP)). Store on ice.
- While carrying out steps 2.2.1 and 2.2.2, dissociate the remaining tissue pieces from step 2.2 by adding pre-warmed 0.25% trypsin-EDTA (200 µL per CVP) to the original 2 mL microcentrifuge tubes and incubate in a 37 °C water bath for 30 min. Vortex briefly every 10 min.
- Following incubation, vortex the tube containing tissue pieces (three pulses) then triturate with a glass Pasteur pipette for 1 min. After tissue pieces settle, pipette the supernatant into the 1.5 mL microcentrifuge tubes containing cells from step 2.2.2. Discard the tubes containing the remaining tissue pieces.
- Spin the tubes with dissociated cells for 5 min at 370 x g and 4 °C to pellet cells.
- Remove the supernatant and resuspend cell pellets in FACS Buffer (100 µL per CVP). Store on ice.
- Pass the cells through a 30 µm nylon mesh filter and add DAPI (λemission = 450 nm) to cell mixtures prior to FACS. Isolate Lgr5-GFP+ cells via FACS using the green fluorescent protein channel (λexcitation = 488 nm; λemission = 530 nm). Sort the cells using a 100 µm nozzle into a fresh FBS-coated 1.5 mL microcentrifuge tube containing 300 µL of Ca2+/Mg2+ free dPBS. Place the cells on ice until plating.
3. Plating of Lgr5-EGFP cells
- Determine the volume of LGR5+ cell suspension received from the flow cytometer.
- Based on the number of cells obtained from the sorter, calculate the number of cells per µL. Then, determine the volume needed to obtain the desired number of cells for plating (we use 200 cells per well of a 48-well plate) and transfer that total volume of suspended cells into a new microcentrifuge tube.
- Spin the tube for 5 min at 370 x g and 4 °C to pellet cells (pellet may not be visible). Remove the supernatant and place the tube on ice.
- Gently resuspend the cell pellet in the appropriate amount of matrix gel (15 µL per well for 48-well plates); pipette up and down gently to thoroughly distribute cells in matrix gel. Place 15 µL of matrix gel/cell mixture in the center of each well. Keep the microcentrifuge tube on ice in a 50 mL conical tube during plating to prevent matrix gel from gelling. Continue to mix matrix gel/cell mixture throughout plating by pipetting up and down every three wells to ensure an even distribution of cells across wells.
- Place the plate in the incubator (37 °C, 5% CO2, ~95% humidity) for 10 min to allow matrix gel gelling. Then, add 300 µL of room temperature WENRAS + Y27632 media to each well and return the plate to the incubator.
4. Organoid maintenance
NOTE: Organoids are grown in conventional organoid media (WENR) comprising recombinant EGF and 50% conditioned media containing Wnt3a, Noggin, and R-spondin23. Drugs A8301 and SB202190 are added for the first 6 days of the culture period to optimize growth (WENRAS media) (Figure 5), then removed to promote differentiation (WENR media)20. Y27632 is added for the first 2 days of culture to promote survival. Media conditions relative to the culture timeline are presented in Figure 4.
- Two days after plating, remove WENRAS + Y27632 media from each well using a 1 mL pipette, ensuring no cross-contamination. Add 300 µL of WENRAS media down the side of the well to not disrupt the matrix gel. Return the plate to the incubator.
- Change the media every 2 days, using the appropriate media for the culture stage (Figure 4). Maintain organoids until day 12, when the organoids are ready to harvest.
5. RNA processing
- Harvesting organoids for RNA
- Place 48-well plate on ice for 30 min to depolymerize the matrix gel.
- Using a 1 mL pipette, pull up the organoid media; then, as the media is returned to the well, use the tip of the pipette to scratch and further break up the matrix gel. Transfer the contents to a 1.5 mL microcentrifuge tube, pooling contents of three wells in one tube. Centrifuge the tubes for 5 min at 300 x g at room temperature.
- Remove as much media supernatant as possible without removing any organoids; then, spin down tubes again for 5 min at 300 x g at room temperature.
- Remove the remaining media and resuspend the organoids in 350 µL lysis buffer + β-mercaptoethanol (βME) (10 µL βME per 1 mL lysis buffer). Place the samples on ice for immediate RNA extraction or store at -80 °C.
- Quantitative RT-PCR analysis
- Measure RNA quantity via spectrophotometer. Reverse-transcribe RNA using a cDNA Synthesis Kit.
- Mix cDNA equivalent to 5 ng RNA with 200 nM pre-validated forward and reverse primers (Table 1) and fluorescent PCR Master Mix. Run the qRT-PCR reaction for 40 cycles at: 95 °C for 15 s, then 60 °C for 60 s.
6. Immunohistochemistry
- Harvesting and fixing the organoids
- Place 48-well plate on ice for 30 min to loosen the matrix gel.
- Remove the organoid media and add 400 µL of ice-cold PBS to each well. Then, remove PBS and add 400 µL of ice-cold Cell Recovery Solution to each well. Rock gently at 4 °C for 30 min.
- Coat a 1 mL pipet tip with 1% BSA in PBS, and gently pipet contents of the well up and down to break up the matrix gel. Transfer the organoids to a 1.5 mL microcentrifuge tube placed on ice.
- Rinse each well with 300 µL PBS + 1% BSA and transfer any remaining organoids to the corresponding tubes. Remove Cell Recovery Solution/PBS + BSA from each tube. Add 400 µL of ice-cold PBS, then repeat with another ice-cold PBS rinse.
- Remove PBS and fix organoids with 300 µL ice-cold 4% PFA (in 0.1 M PB) for 20 min, incubating at room temperature. Remove PFA and rinse organoids with 400 µL ice-cold PBS.
- Remove PBS; then, add 400 µL PBS + 1% BSA. Store at 4 °C.
- Immunofluorescence staining
- Rinse organoids in 500 µL PBS + 1% BSA. Then, incubate organoids in blocking solution (5% normal goat or donkey serum, 1% bovine serum albumin, 0.3% Triton X 100 in 1x PBS pH 7.3) for 2 h, rocking gently at room temperature.
- Add the primary antibody solution (primary antibodies diluted in blocking solution) and rock gently for 3 nights at 4 °C.
- Wash organoids 4x for 1 h with 500 µL PBS + 0.2% Triton, rocking gently at room temperature. Add secondary antibody solution (secondary antibodies diluted in blocking solution) and rock organoids overnight, protected from light, at 4 °C.
- Wash organoids 3x for 1 h with 500 µL PBS + 0.2% Triton, protected from light and rocking gently at room temperature. Incubate with DAPI diluted 1:10,000 in 0.1 M PB for 20 min, rocking and covered at room temperature.
- Wash the organoids 3x for 20 min with 0.1 M PB, rocking gently and covered at room temperature.
- Slide mounting of organoids for inverted confocal microscopy.
NOTE: Step-by-step pictures of the slide mounting process are shown in Figure 7.- Create a ~1 mm thick 22 x 22 mm square perimeter of non-toxic modeling clay on a microscope slide.
- Remove 0.1 M PB from microcentrifuge tube, and gently resuspend organoids in 100 µL mounting medium of choice; then, transfer to center of the clay square.
- Fill the clay square until the mounting medium is almost to the top. Then, place 22 x 22 mm square coverslip over clay and press down firmly on the sides of the coverslip to seal. Let it cure according to the manufacturer's instructions (here, room temperature for 1-2 days) and store at 4 °C.
Representative Results
Mice have one CVP, located posteriorly on the tongue, from which LGR5+ stem cells can be isolated (Figure 1A, black box). Injection of an enzyme solution under and around the CVP (Figure 1B) results in slight swelling of the epithelium and digestion of the connective tissue. Sufficient digestion is achieved following a 33 min incubation, which allows easy separation of the CVP epithelium from the underlying tissue. When attempting to peel the CVP epithelium, cuts should be made at a sufficient distance from the CVP to ensure trenches are not disrupted or damaged (Figure 1C). This also enables one to grip the epithelium using forceps without damaging the CVP. Trimming the epithelium surrounding the CVP removes non-target cells and increases the efficiency of the following steps by decreasing the tissue mass being manipulated (Figure 1D). It is important to check that the two trenches (Figure 1C, black arrows) are present before adding CVP epithelium to the microcentrifuge tube; successful peeling of the CVP includes part of the Von Ebner's glands and ducts (Figure 1D, black arrows). If the peeled epithelium does not contain these two opaque structures, the trench epithelium is most likely ruptured due to incomplete digestion of the connective tissue.
To establish proper FACS settings to collect Lgr5-EGFP cells, cells from dissociated CVPs are separately obtained from wild type and Lgr5-EGFP mice. Wild type CVP cells are analyzed first to establish gating parameters, a process in which populations of cells are categorized within the scatterplot output by characteristics of interest24. Here, four parameters were used to ultimately identify cells for plating. The first parameter, forward scatter, filters out particles and debris based on the detected surface area. This parameter removes ~70% of all detected events during the sort (Figure 3). The width parameter further filters events based on size to ensure selection of single cells (singlets). Approximately 90% of events are singlets (Figure 3). The nuclear marker DAPI is taken up by dead but not live cells and thus allows dead cells to be sorted out25. This protocol optimizes cell viability, as over 90% of events are live cells (Figure 3). Lastly, GFP gating parameters are set above the autofluorescence level of wild type cells. Wild type cells are not collected; they are used solely as a gating control for GFP fluorescence. With gating parameters determined from the wild type sample, cells from the Lgr5–EGFP sample are then run through the flow cytometer to be sorted for collection. Gates can be adjusted at the beginning of the Lgr5-EGFP sort to accommodate clear clustering of certain cell populations but should not be significantly changed mid-sort. It was found that the dissociation of three pooled Lgr5-EGFP CVPs yields ~500,000 cells, including an average of 10,000 GFP+ cells.
Proper media and culture conditions are vital for optimal growth and differentiation of organoids. Previous studies utilizing lingual organoids modeled their media components after those from the intestinal organoid field, including Wnt3a, EGF, Noggin, and R-spondin17,18,19,20,21,22. However, when lingual organoids are cultured using a similar method (WENR media), organoids do not grow efficiently (Figure 5A). In human intestinal organoids, drugs A8301 (a TGFß signaling inhibitor) and SB202190 (a p38 MAPKinase signaling inhibitor) are used to promote organoid growth26. Indeed, adding these inhibitors (WENRAS media) induces robust growth of lingual organoids (Figure 5A). Interestingly, removing these inhibitors from the media after 6 days results in higher expression of general TRC marker Kcnq1, suggesting A8301 and SB202190 hinder taste cell differentiation (Figure 5B). Thus, optimal growth and differentiation are obtained by culturing organoids in WENRAS media from days 0-6 and WENR media from days 6-12 (Figure 4, Figure 5), respectively.
Organoid cell type composition can be characterized by qRT-PCR or immunohistochemistry. Mature organoids contain both taste cells, marked by Kcnq1 and KRT8, and non-taste epithelial cells, marked by Krt13/KRT13 (Figure 6, 8A). This suggests isolated LGR5+ cells have a similar potency in vitro as they do in vivo since, in the adult tongue, Lgr5-GFP+ cells produce both taste and non-taste lineages5. Further, Krt13 is expressed at higher levels than all 3 TRC markers (Figure 6), suggesting organoids are predominately composed of non-taste epithelial cells. In fact, relative quantification of gene expression27 indicates Krt13 is expressed 50x higher than general TRC marker Kcnq1 (Student's t-test, p = 0.0004). This is expected, as the tongue has a similar proportion of taste versus non-taste epithelium1. Organoids express all three TRC types (Figure 6, 8B,C). Type I cells (marked by Entpd2) and bitter type II cells (marked by Gnat3) are highly expressed in taste organoids, while sour sensing type III cells (marked by Car4) are less common (Figure 6). TRCs are randomly distributed in organoids (Figure 8) rather than in discrete taste bud structures observed in vivo.

Figure 1: Dissected tongue and peeled CVP epithelium. (A) The tongue is dissected out, and the anterior tongue is removed by cutting just anterior of the intermolar eminence (dashed line), leaving the posterior tongue, which includes the CVP (black box) (B) A needle is inserted just anterior to the intermolar eminence (black arrow), and enzyme mixture is injected below and to the lateral edges of the CVP (black box). (C) Untrimmed peeled epithelium surrounding the CVP trenches (black arrows) and (D) trimmed peeled CVP epithelium. Von Ebner's glands and ducts (D, black arrows) are visible after successful peeling of the trenches. Scale bar: 1 mm. Please click here to view a larger version of this figure.
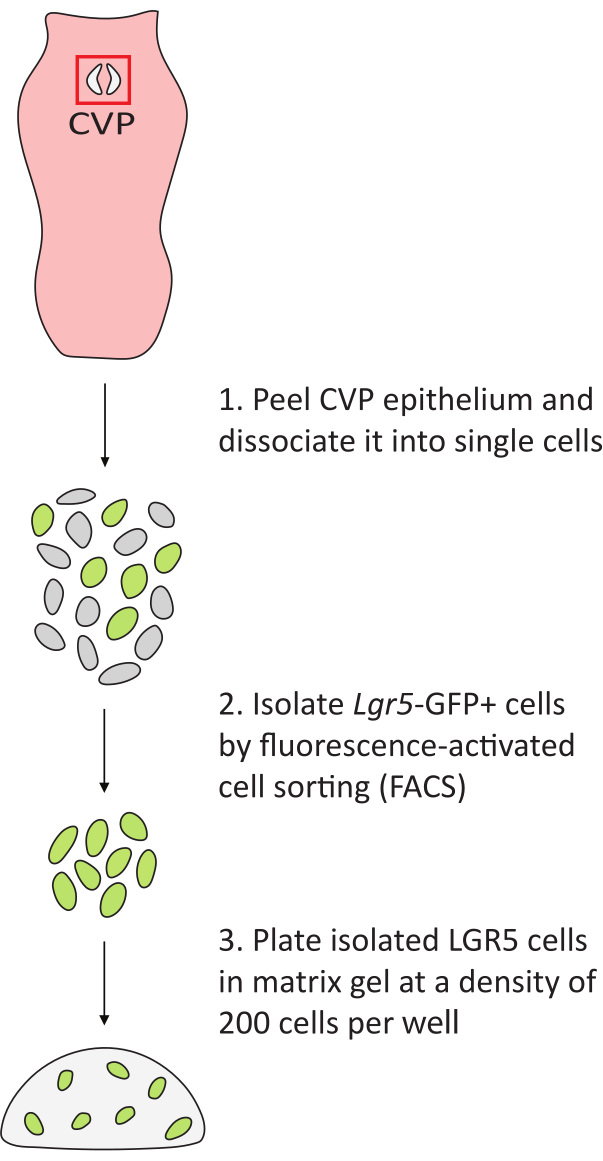
Figure 2: Workflow of lingual organoid generation. The tongue is removed from Lgr5–EGFP mice. The CVP trench epithelia (red box) are peeled from the underlying connective tissue and dissociated into single cells. GFP+ cells are isolated and plated in matrix gel at a density of 200 cells per well in a 48-well plate. Please click here to view a larger version of this figure.
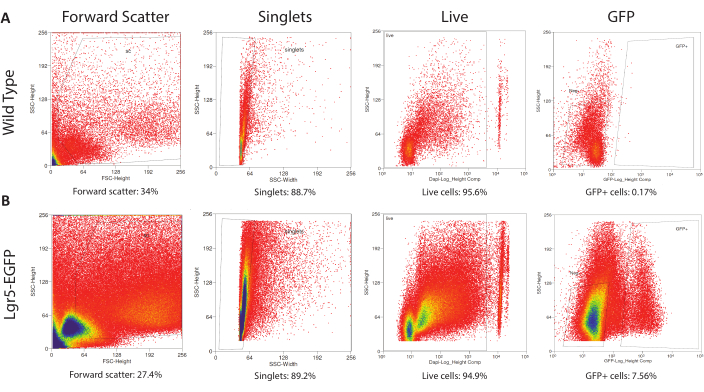
Figure 3: Gating for Fluorescence-Activated Cell Sorting. (A) The control (wild type CVP cells) run determines the FACS gates that eliminate debris and broken cells via forward scatter, identifies singlets via side scatter width, separates DAPIneg live from DAPI+ dead cells, and establishes the autofluorescence level of wild type cells. (B) Previously determined gates are applied to the experimental run (Lgr5EGFP-IRES-CreERT2 CVP cells) to isolate debris-free, single, live, Lgr5-EGFP+ cells. Please click here to view a larger version of this figure.
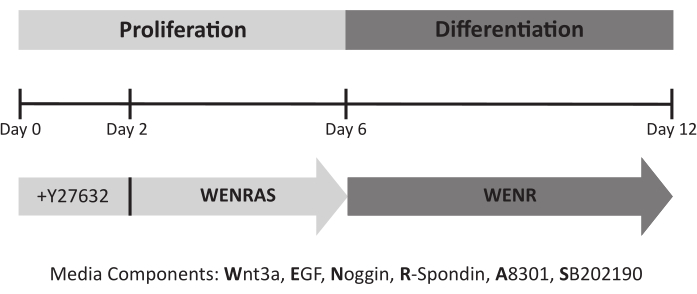
Figure 4: Lingual organoid culture timeline and required media. ROCK inhibitor Y27632 is added to the media for the first 48 h of culture to promote cell survival. During the proliferation phase, organoids are fed conditioned medium containing A8301 and SB202190 (WENRAS) to optimize growth. These drugs are withheld from media (WENR) starting at day 6 to promote differentiation. Please click here to view a larger version of this figure.
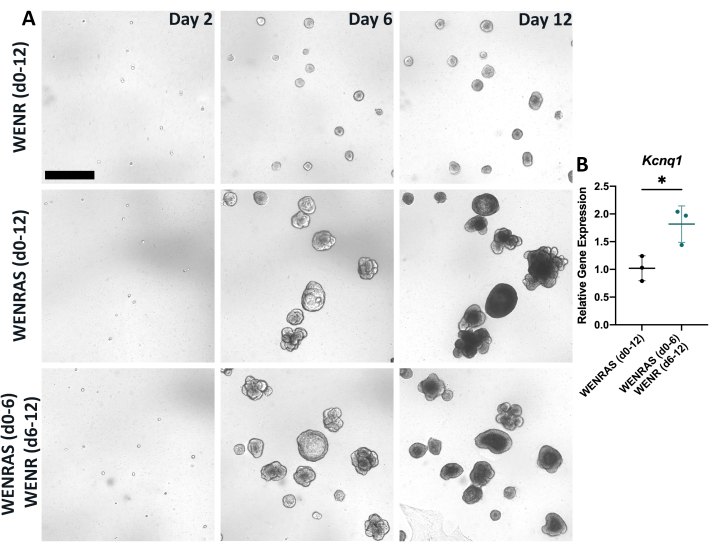
Figure 5: Drugs A8301 and SB202190 affect organoid growth and differentiation. (A) Brightfield images of organoids grown in either WENR media (day 0-12), WENRAS media (day 0-12), or WENRAS (day 0-6), then WENR (day 6-12). Images were captured at day 2, day 6, and day 12 of culture using live imaging software. Scale bar: 400 µm (B) Relative gene expression of a global TRC marker Kcnq1 is significantly reduced when drugs A8301 and SB202190 are present during organoid differentiation. Each point represents one biological replicate, which included three pooled wells. Mean change in relative gene expression (horizontal line) was calculated by averaging three biological replicates. Error bars: ±SD. Please click here to view a larger version of this figure.
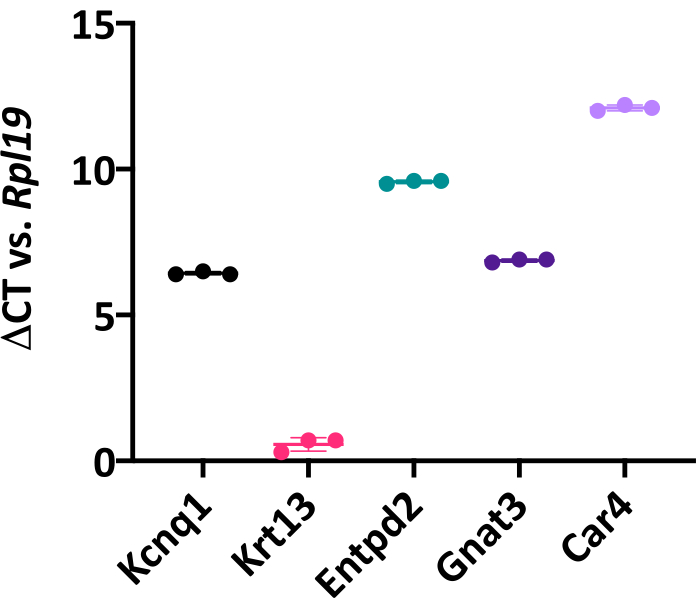
Figure 6: Lingual organoids express canonical taste cell markers. Change in cycle threshold (Ct) value of global TRC marker Kcnq1, non-taste epithelial marker cytokeratin 13 (Krt13), type I TRC marker Entpd2, type II bitter TRC marker Gnat3, and type III sour TRC marker Car4, compared to housekeeping gene Rpl19. Each point represents one biological replicate, which included three pooled wells from a 48-well plate. Mean change in Ct value (horizontal line) for each marker was calculated by averaging three biological replicates. Unpaired Student's t-test, *p < 0.05. Error bars: ± SD. Please click here to view a larger version of this figure.
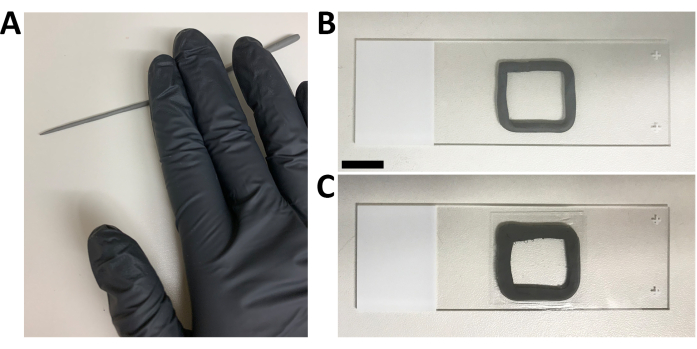
Figure 7: Mounting of lingual organoids for inverted confocal microscopy. (A) An ~1 mm thick string of non-toxic modeling clay is created. (B) The clay is sculpted as an ~20 mm x 20 mm square in the center of a 24 mm x 75 mm microscope slide. (C) A 22 mm x 22 mm square coverslip seals organoids suspended in mounting medium. Scale bar: 10 mm. Please click here to view a larger version of this figure.
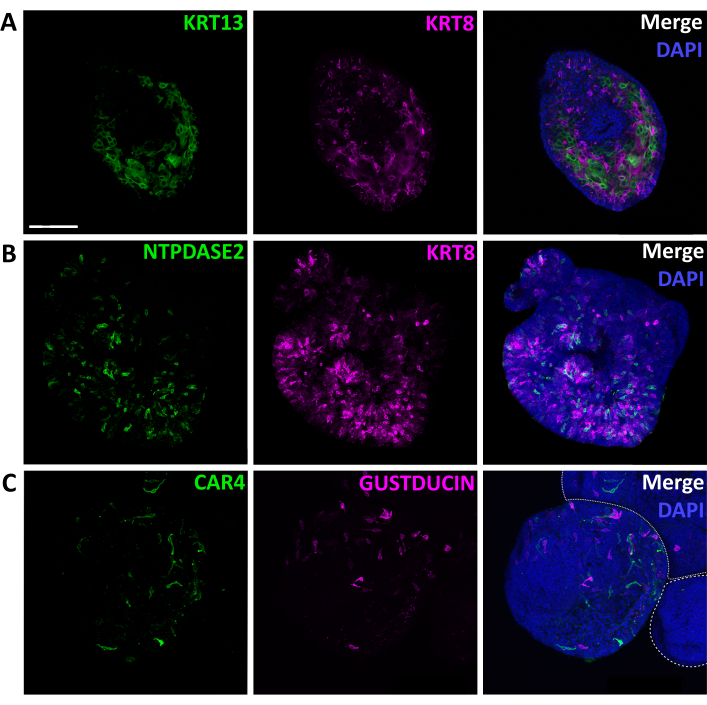
Figure 8: Immunolabeling of intact lingual organoids. Confocal images of fixed, immunostained organoids. (A) Optical section of an organoid stained for non-taste epithelial marker KRT13 (green) and general TRC marker KRT8 (magenta). (B) Maximum projection of a confocal z-stack of one organoid stained for type I glial-like cell marker NTPDase2 (green) and KRT8 (magenta). (C) Maximum projection of a confocal z-stack of two partially shown organoids (white-dashed outlines) and one complete organoid stained for type III sour detecting cell marker CAR4 (green), and bitter detecting type II cell marker GUSTDUCIN (magenta). Scale bar: 100 µm for A, B, and C. Nuclear marker DAPI (blue, right column). Please click here to view a larger version of this figure.
| Gene Name | Forward primer (5'-3') | Reverse primer (5'-3') | Assession Number | ||||||
| Car4 | CTGCTAGGACAAAGGTGAACC | CTCCACTGTGTGTTGATTGTTCT | NM_007607.2 | ||||||
| Entpd2 | GACAAGGAAAATGACACAGGTATCGTGG | GTTCAAGACATTCAACCAGACTC | NM_009849.2 | ||||||
| Gnat3 | ATCCAGGAATCCAAGCCTGC | TGGTTTTCACCCGGGAATGT | NM_001081143.1 | ||||||
| Kcnq1 | TTTGTTCATCCCCATCTCAG | GTTGCTGGGTAGGAAGAG | NM_008434.2 | ||||||
| Krt13 | TCATCTCGGTTTGTCACTGGA | TGATCTTCTCGTTGCCAGAGAG | NM_010662.2 | ||||||
| Rpl19 | GGTCTGGTTGGATCCCAATG | CCCGGGAATGGACAGTCA | NM_009078.2 | ||||||
Table 1: Primer sequences used for quantitative RT-PCR.
Supplementary Table 1: Comparison of published lingual organoid culture media components. Please click here to download this Table.
Discussion
Reported here is an efficient and readily repeatable method for culturing, maintaining, and processing lingual organoids derived from adult mouse taste stem cells. It was found that using three CVPs from 8 to 20-week-old Lgr5–EGFP mice is sufficient to obtain ~10,000 GFP+ cells for experimental use, resulting in 50 wells plated at a density of 200 cells per well in 48-well plates. Removal of CVP trench epithelia is optimized by injecting the lingual epithelium with freshly made Dispase II and type-I Collagenase solution, followed by a 33 min incubation. However, a shorter incubation time, old enzyme aliquots, different lots, or using enzymes from different manufacturers can result in incomplete trench removal. Conversely, a longer incubation time causes over-digestion of the tissue, resulting in loss of taste tissue integrity. Following peeling, further enrichment for CVP trenches was done by trimming away the epithelium surrounding the CVP.
It is critical that all microcentrifuge tubes used during the dissociation and collection process are coated with FBS to prevent tissue and cells from sticking to the plastic walls of the tubes, which significantly reduces recovery of isolated cells (data not shown). Using a light coat of FBS and removing excess liquid from the tubes prior to use minimizes possible inhibitory effects on Trypsin or other enzymes.
Lingual organoids have been grown successfully from dissociated CVP tissue without prior sorting of LGR5+ cells17. Although this method results in the formation of organoids, we have found that a smaller proportion of these organoids contain taste cells compared to those grown from isolated progenitors (data not shown). Flow sorting for Lgr5-EGFP+ cells enriches for taste-competent progenitors, resulting in a higher proportion of taste-replete organoids. Currently, we collect all cells above the threshold of GFP autofluorescence (Figure 3); however, it is possible that GFP brightness levels are associated with variable organoid forming efficiency or taste competency. This hypothesis has not yet been tested but is a promising avenue for future work as it could allow further enrichment of taste cell-containing organoids.
It is well known that plating density affects the efficiency of organoid differentiation, and we recommend that optimal plating density be determined prior to experimentation28. Size and depth of the wells, as well as matrix gel volume, should be considered when establishing plating density. Based on our preliminary work (data not shown), we find that in a 48-well plate, 15 µL of matrix gel and a plating density of 200 cells per well allows efficient organoid expansion and differentiation of all three TRC types. We have found that lingual organoids can be cultured successfully and reproducibly in Reduced Growth Factor Basement Membrane Extract (RGF BME), a synthetic and less expensive matrix gel alternative. Other alternative matrices may also support lingual organoid culture, but further testing is required to investigate this possibility.
During plating, collected cells should be thoroughly but gently resuspended in matrix gel by frequently pipetting up and down to keep the cell suspension homogeneous. The tube containing the cell-matrix gel mixture should also be kept on ice during the entire plating process to prevent matrix gel from gelling on the sides of the tube. These measures ensure an even distribution of cells across wells, yielding more reproducible results when hand-plating cells. Notably, recent developments in microfluidic technologies provide high throughput options for cell dispensing and is a promising tool for future work29.
To assess gene expression in cultured organoids, it was found to be necessary to pool at least three wells for each biological replicate to obtain sufficient RNA and consistency across replicates (Figure 6). It was also determined that immediately lysing harvested organoids according to specific manufacturer's instructions optimizes the quality and quantity of extracted RNA. While not tested here, other storage options such as flash-freezing or resuspension in storage reagents may also preserve RNA yield and quality.
Performing immunohistochemistry on organoids can be challenging, as primary and secondary antibodies must penetrate any residual matrix gel and the entire organoid epithelium. In previous reports, organoids were fixed while still suspended in matrix gel, which subsequently required extremely high concentrations of primary antibody solution to reveal protein expression17,18. Similar to other reports, removing organoids from matrix gel using Cell Recovery Solution prior to fixation was found to increase the efficiency of staining without the need for a high antibody concentration30,31; however, the detection of some taste cell markers, e.g., CAR4, requires a higher concentration of antibody. Further, incubating organoids in primary antisera for 3 nights increases the probability of antibody binding. However, this method may also increase background fluorescence if organoids are insufficiently washed following immunostaining. Importantly, diluted primary antibody solution can be saved and successfully re-used one or more times if stored for less than a week (data not shown). For optimal imaging, organoids are mounted by placing a coverslip on top of a clay perimeter of modeling clay to preserve their 3D structure. Placing the cover glass directly on the slide compresses and breaks organoids, preventing proper analysis.
Organoid culture is a highly applicable, cost-efficient technique. Some applications include, but are not limited to, disease modeling and drug screening and the study of stem cell and developmental biology32. Therefore, it is crucial to standardize organoid models to allow reproducibility across laboratories. In the future, it would be useful to develop a standardized method for passaging lingual organoids that guarantees that taste stem cell potency can be propagated over serial passages, thereby reducing the need for additional animals to generate more organoids. Importantly, while lingual organoids are composed of both taste and non-taste epithelial cells, the organization of these cells differs compared to the in vivo taste system. Discrepancies between organoids and taste epithelium in vivo may be due to the media used for organoid culture and/or because organoids lack interaction with the taste bud microenvironment, including important signals from gustatory nerves and the lamina propria that are required for taste bud formation and maintenance22,33,34,35. Future work to incorporate nerves or vasculature into lingual organoid culture, a strategy currently being adopted in other organoid systems, could allow more accurate modeling of the in vivo taste epithelium36,37.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. Peter Dempsey and Monica Brown (University of Colorado Anschutz Medical Campus Organoid and Tissue Modeling Shared Resource) for providing WNR conditioned media and valuable discussions. We also thank the University of Colorado Cancer Center Cell Technologies and Flow Cytometry Shared Resources, especially Dmitry Baturin, for cell sorting expertise. This work was funded by: NIH/NIDCD R01 DC012383, DC012383-S1, DC012383-S2, and NIH/NCI R21 CA236480 to LAB, and R21DC016131 and R21DC016131-02S1 to DG, and F32 DC015958 to EJG.
Materials
| Antibodies | |||
| Alexa Fluor 546 Donkey anti Goat IgG | Molecular Probes | A11056, RRID: AB_142628 | 1:2000 |
| Alexa Fluor 546 Goat anti Rabbit IgG | Molecular Probes | A11010, RRID:AB_2534077 | 1:2000 |
| Alexa Fluor 568 Goat anti Guinea pig IgG | Invitrogen | A11075, RRID:AB_2534119 | 1:2000 |
| Alexa Fluor 647 Donkey anti Rabbit IgG | Molecular Probes | A31573, RRID:AB_2536183 | 1:2000 |
| Alexa Fluor 647 Goat anti Rat IgG | Molecular Probes | A21247, RRID:AB_141778 | 1:2000 |
| DAPI (for FACS) | Thermo Fischer | 62247 | |
| DAPI (for immunohistochemistry) | Invitrogen | D3571, RRID:AB_2307445 | 1:10000 |
| Goat anti-CAR4 | R&D Systems | AF2414, RRID:AB_2070332 | 1:50 |
| Guinea pig anti-KRT13 | Acris Antibodies | BP5076, RRID:AB_979608 | 1:250 |
| Rabbit anti-GUSTDUCIN | Santa Cruz Biotechnology Inc. | sc-395, RRID:AB_673678 | 1:250 |
| Rabbit anti-NTPDASE2 | CHUQ | mN2-36LI6, RRID:AB_2800455 | 1:300 |
| Rat anti-KRT8 | DSHB | TROMA-IS, RRID: AB_531826 | 1:100 |
| Equipment | |||
| 2D rocker | Benchmark Scientific Inc. | BR2000 | |
| 3D Rotator | Lab-Line Instruments | 4630 | |
| Big-Digit Timer/Stopwatch | Fisher Scientific | S407992 | |
| Centrifuge | Eppendorf | 5415D | |
| CO2 tank | Airgas | CD USP50 | |
| FormaTM Series 3 Water Jackeed CO2 Incubator | Thermo Scientific | 4110 | 184 L, Polished Stainless Steel |
| Incucyte | Sartorius | Model: S3 | Cancer Center Cell Technologies Shared Resource, University of Colorado Anschutz Medical Campus |
| MoFlo XDP100 | Cytomation Inc | Model: S13211997 | Gates Center Flow Cytometry Core, University of Colorado Anschutz Medical Campus |
| Orbital Shaker | New Brunswick Scientific | Excella E1 | |
| Real-Time PCR System | Applied Biosystems | 4376600 | |
| Refrigerated Centrifuge | Eppendorf | 5417R | |
| Spectrophotometer | Thermo Scientific | ND-1000 | |
| Stereomicroscope | Zeiss | Stemi SV6 | |
| Thermal Cycler | Bio-Rad | 580BR | |
| Vortex | Fisher Scientific | 12-812 | |
| Water bath | Precision | 51220073 | |
| Media | |||
| A83 01 | Sigma | SML0788-5MG | Stock concentration 10 mM, final concentration 500 nM |
| Advanced DMEM/F12 | Gibco | 12634-010 | |
| B27 Supplement | Gibco | 17504044 | Stock concentration 50X, final concentration 1X |
| Gentamicin | Gibco | 15750-060 | Stock concentration 1000X, final concentration 1X |
| Glutamax | Gibco | 35050061 | Stock concentration 100X, final concentration 1X |
| HEPES | Gibco | 15630080 | Stock concentration 100X, final concentration 1X |
| Murine EGF | Peprotech | 315-09-1MG | Stock concentration 500 µg/mL, final concentration 50 ng/mL |
| Murine Noggin | Peprotech | 250-38 | Stock concentration 50 µg/mL, final concentration 25 ng/mL |
| N-acetyl-L-cysteine | Sigma | A9165 | Stock concentration 0.5 M, final concentration 1 mM |
| Nicotinamide | Sigma | N0636-100g | Stock concentration 1 M, final concentration 1 mM |
| Pen/Strep | Gibco | 15140-122 | Stock concentration 100X, final concentration 1X |
| Primocin | InvivoGen | ant-pm-1 | Stock concentration 500X, final concentration 1X |
| SB202190 | R&D Systems | 1264 | Stock concentration 10 mM, final concentration 0.4 µM |
| WRN Conditioned media | Received from Dempsey Lab (AMC Organoid and Tissue Modeling Share Resource). Derived from L-WRN (ATCC® CRL-3276™) cells | ||
| Y27632 dihydochloride 10ug | APExBIO | A3008-10 | Stock concentration 10 mM, final concentration 10 µM |
| Other | |||
| 1 ml TB Syringe | BD Syringe | 309659 | |
| 2-Mercaptoethanol, min. 98% | Sigma | M3148-25ML | β-mercaptoethanol |
| 2.0 mL Microcentrifuge Tubes | USA Scientific | 1420-2700 | |
| 48-well plates | Thermo Scientific | 150687 | |
| 5 3/4 inch Pasteur Pipets | Fisherbrand | 12-678-8A | |
| Albumin from bovine serum (BSA) | Sigma Life Science | A9647-100G | |
| Buffer RLT Lysis buffer | QIAGEN | 1015750 | |
| Cell Recovery Solution | Corning | 354253 | |
| Cohan-Vannas Spring Scissors | Fine Science Tools | 15000-02 | |
| Collagenase from Clostridium histolyticum, type I | Sigma Life Science | C0130-1G | |
| Cultrex RGF BME, Type 2, Pathclear | R&D Systems | 3533-005-02 | Matrigel |
| Dispase II (neutral protease, grade II) | Sigma-Aldrich (Roche) | 4942078001 | |
| Disposable Filters | Sysmex | 04-0042-2316 | |
| Dulbecco’s Phosphate Buffered Saline pH 7.4 (1X) (Ca2+ & Mg2+ free) | Gibco | 10010-023 | |
| Dulbecco’s Phosphate Buffered Saline with Ca2+ & Mg2+ | Sigma Life Sciences | D8662-500ML | |
| Dumont #5 Forceps | Fine Science Tools | 11252-30 | |
| EDTA, 0.5M (pH 8.0) | Promega | V4231 | |
| Elastase Lyophilized | Worthington Biochemical | LS002292 | |
| Extra Fine Bonn Scissors | Fine Science Tools | 14084-08 | |
| Fetal Bovine Serum (FBS) | Gibco | 26140-079 | |
| Fluoromount G | SouthernBiotech | 0100-01 | |
| HEPES Solution | Sigma Life Science | H3537-100ML | |
| HyClone Tryspin 0.25% + EDTA | Thermo Scientific | 25200-056 | |
| iScript cDNA Synthesis Kit | Bio-Rad | 1706691 | |
| Modeling Clay, Gray | Sargent Art | 22-4084 | |
| Needle | BD Syringe | 305106 | |
| Normal Donkey Serum | Jackson ImmunoResearch | 017-000-121 | |
| Normal Goat Serum | Jackson ImmunoResearch | 005-000-121 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| PowerSYBR Green PCR Master Mix | Applied Biosystems | 4367659 | |
| RNeasy Micro Kit | QIAGEN | 74004 | |
| Safe-Lock Tubes 1.5 mL | Eppendorf | 022363204 | |
| Sodium Chloride | Fisher Chemical | 7647-14-5 | |
| Sodium Phosphate dibasic anhydrous | Fisher Chemical | 7558-79-4 | |
| Sodium Phosphate monobasic anhydrous | Fisher Bioreagents | 7558-80-7 | |
| SuperFrost Plus Microscope Slides | Fisher Scientific | 12-550-15 | |
| Surgical Scissors – Sharp | Fine Science Tools | 14002-14 | |
| Triton X-100 | Sigma Life Science | T8787-100ML | |
| VWR micro cover glass | VWR | 48366067 | 22x22mm |
References
- Barlow, L. A. Progress and renewal in gustation: new insights into taste bud development. Development. 142, 3620-3629 (2015).
- Liman, E. R., Zhang, Y. V., Montell, C. Peripheral coding of taste. Neuron. 81 (5), 984-1000 (2014).
- Finger, T. E., Silver, W. L. . The neurobiology of taste and smell. , 287-314 (2000).
- Barlow, L. A., Klein, O. D. Developing and regenerating a sense of taste. Current Topics in Developmental Biology. 111, 401-419 (2015).
- Yee, K. K., et al. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 31 (5), 992-1000 (2013).
- Miura, H., Scott, J. K., Harada, S., Barlow, L. A. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 243 (10), 1286-1297 (2014).
- Deshpande, T. S., et al. Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Current Treatment Options in Oncology. 19 (12), 12 (2018).
- Nolden, A. A., Hwang, L. D., Boltong, A., Reed, D. R. Chemosensory changes from cancer treatment and their effects on patients’ food behavior: A scoping review. Nutrients. 11 (10), 2285 (2019).
- Doty, R. L., Shah, M., Bromley, S. M. Drug-induced taste disorders. Drug Safety. 31 (3), 199-215 (2008).
- Kumari, A., et al. Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proceedings of the National Academy of Sciences of the United States of America. 114 (48), 10369-10378 (2017).
- Ermilov, A. N., et al. Maintenance of taste organs is strictly dependent on epithelial hedgehog/gli signaling. PLoS Genetics. 12 (11), 1006442 (2016).
- Gaillard, D., Shechtman, L. A., Millar, S. E., Barlow, L. A. Fractionated head and neck irradiation impacts taste progenitors, differentiated taste cells, and Wnt/β-catenin signaling in adult mice. Scientific Reports. 9, 17934 (2019).
- Nguyen, H. M., Reyland, M. E., Barlow, L. A. Mechanisms of taste bud cell loss after head and neck irradiation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 32 (10), 3474-3484 (2012).
- Ohla, K., et al. Recognizing taste: Coding patterns along the neural axis in mammals. Chemical Senses. 44 (4), 237-247 (2019).
- Chaudhari, N., Roper, S. D. The cell biology of taste. The Journal of Cell Biology. 190, 285-296 (2010).
- Breslin, P. A., Spector, A. C. Mammalian taste perception. Current Biology: CB. 18 (4), 148-155 (2008).
- Aihara, E., et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Scientific Reports. 5, 17185 (2015).
- Ren, W., et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proceedings of the National Academy of Sciences of the United States of America. 111 (46), 16401-16406 (2014).
- Sato, T., Clevers, H. Primary mouse small intestinal epithelial cell cultures. Methods in Molecular Biology. 945, 319-328 (2013).
- Ren, W., et al. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Scientific Reports. 7, 4004 (2017).
- Feng, S., Achoute, L., Margolskee, R. F., Jiang, P., Wang, H. Lipopolysaccharide-induced inflammatory cytokine expression in taste organoids. Chemical Senses. 45 (3), 187-194 (2020).
- Lin, X., et al. R-spondin substitutes for neuronal input for taste cell regeneration in adult mice. Proceedings of the National Academy of Sciences of the United States of America. 118 (2), 2001833118 (2021).
- Miyoshi, H., Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nature Protocols. 8 (12), 2471-2482 (2013).
- Staats, J., Divekar, A., McCoy, J. P., Maecker, H. T. . Immunophenotyping: Methods and Protocols. , 81-104 (2019).
- Perfetto, S. P., et al. Amine-reactive dyes for dead cell discrimination in fixed samples. Current Protocols in Cytometry. , (2010).
- Sato, T., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 141 (5), 1762-1772 (2011).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25 (4), 402-408 (2001).
- Morizane, R., Bonventre, J. V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nature Protocols. 12 (1), 195-207 (2017).
- Ekert, J. E., et al. Recommended guidelines for developing, qualifying, and implementing complex in vitro models (CIVMs) for drug discovery. SLAS Discovery: Advancing Life Sciences R & D. 25 (10), 1174-1190 (2020).
- Dekkers, J. F., et al. High-resolution 3D imaging of fixed and cleared organoids. Nature Protocols. 14 (6), 1756-1771 (2019).
- Fujii, M., et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 23 (6), 787-793 (2018).
- Clevers, H. Modeling development and disease with organoids. Cell. 165 (7), 1586-1597 (2016).
- Castillo-Azofeifa, D., et al. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development. 144 (17), 3054-3065 (2017).
- Vintschgau, M. v., Hönigschmied, J. Nervus glossopharyngeus und schmeckbecher. Archiv für die gesamte Physiologie des Menschen und der Tiere. 14, 443-448 (1877).
- Liu, H. X., et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Biologie du développement. 382 (1), 82-97 (2013).
- Workman, M. J., et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nature Medicine. 23 (1), 49-59 (2017).
- Koning, M., vanden Berg, C. W., Rabelink, T. J. Stem cell-derived kidney organoids: engineering the vasculature. Cell and Molecular Life Sciences: CMLS. 77 (12), 2257-2273 (2020).

