Naked-Eye Detection of Rare Point Mutations in DNA
Summary
This protocol permits the naked-eye identification of point mutated DNA in a 200-fold excess of wild type DNA molecules, by exploiting gold nanoparticles and paramagnetic microparticles.
Abstract
The protocol describes a naked-eye colorimetric test for the detection of somatic point mutations in an excess of wild type DNA. The future foreseen application of the method is the identification of rare mutations in circulating cell-free DNA from liquid biopsies, with a relevance in cancer diagnostics and stratification of oncological patients for personalized therapy. As a proof of concept, the test has been designed to detect the BRAFV600E mutation in the BRAF gene, which is important to identify the sub-group of melanoma patients that can benefit from targeted therapies with BRAF inhibitors. However, this colorimetric test can be easily generalized to other somatic mutations of clinical relevance due to the use of universal detection probes, thus providing strong potential in oncological diagnostics.
The test detects 0.5% of BRAFV600E in an excess of BRAFWT DNA, which matches the sensitivity of some commercial instrumental assays. Such sensitivity is clinically relevant for diagnostic purposes, allowing the early identification of drug-sensitive patients. In contrast to commercial assays based on real-time PCR, this test requires minimal instrumentation and processing, as it can be performed on DNA amplified with a standard PCR (or isothermal techniques) and provides a naked-eye readout with a one-tube reaction of a few steps in only one hour. At present, the test has been used only on synthetic DNA samples. However, the latter have been designed to mimic a real sample amplified from circulating cell-free DNA, to favor the translation of the test to clinical diagnostics.
Introduction
The purpose of the method is to detect underrepresented point mutations in a DNA sample with a minimally instrumented methodology and a naked-eye readout. The final aim is to have a proof-of-principle assay, suitable for future applications in rapid tests for the detection of somatic mutations in circulating cell-free DNA (ccf-DNA) (e.g., from blood biopsy samples) for the early diagnostics and monitoring of cancer1. Cancer-related somatic mutations represent an important cancer biomarker2 and are present in a minor (yet very variable)3 fraction of ccf-DNA, making their identification challenging4. We chose, as a model target, the oncogenic mutation BRAFV600E that causes the constitutive activation of BRAF kinase. This mutation is present in 80% of all BRAF mutated cancers5 and is generally represented in only <1% of circulating tumor DNA6. Identifying patients carrying this mutation is important as it is predictive of the therapeutic response to BRAF inhibitors. Therefore, several methods7,8,9,10 to assess the BRAF mutation status have been developed, with sensitivities ranging from 0.01% to 2%.
The main advantage of this method over the state-of-the-art methods is that its detection is instrument-free (naked-eye), as opposed to instrumental detection of fluorescent molecules by real-time PCR. Another advantage is its efficiency in discriminating one single mutated DNA molecule in an excess of 200 wild type DNA molecules. This discrimination factor of 0.5% is superior11 or matches12 that of some laboratory-based or commercially available kits, based on an instrumental detection and it is, thus, relevant for clinical diagnostic applications. On the other hand, as a laboratory prototype test, the method relies on the manual control of temperature-sensitive steps. However, the number of steps and the total duration of the assay is limited, making its future implementation in automated microfluidic systems conceivable.
This proof-of-concept method has been developed using synthetic DNA molecules. For its efficient translation to the clinics, it should be validated by using real-world samples amplified from patients' blood biopsies. We note that the future application field of the method is not intended to be the direct analysis of unprocessed complex biological matrices, such as bodily fluids. From the latter, DNA needs to be extracted with standard methodologies, and then amplified and purified. Consequently, the starting material for the analysis will always be purified and amplified DNA, which is reasonably comparable, in terms of possible interfering substances, to a synthetic DNA sample, such as that used for the development of this method.
Protocol
1. Synthesis of gold nanoparticle probes
- Synthesize 40 nm citrate capped gold nanoparticles, using two-steps standard seeding growth method as detailed below.
- Synthesize 15 nm citrate capped gold nanoparticles (AuNPs seeds) using the classical Turkevich–Frens method13,14.
- Wash all glassware with aqua regia (HCl:HNO3 in a 3:1 v/v ratio).
- Heat 250 mL of 0.25 mM HAuCl4 to boil while uniformly stirring.
CAUTION: HAuCl4 is corrosive and toxic. - Immediately add 25 mL of 38.8 mM Na3∙citrate.
- Continue boiling and stirring for 30 min, while the solution turns to a red ruby color.
- Remove the solution from the heat and let cool to room temperature while stirring.
- Filter using 0.22 µm PTFE membrane syringe filters.
- Store at 4 °C in a glass bottle.
NOTE: The experiment can be paused here.
- Synthesize 40 nm citrate capped gold nanoparticles (40 nm AuNPs) by seeding growth15.
- Prepare solution A: 390 µL of freshly prepared 0.1 M hydroxylamine sulfate with 13 mL of AuNP seeds in a total volume of 120 mL in H2O.
NOTE: The amount of AuNPs seeds needed to obtain the desired size can vary; it must be titrated for each new AuNPs seed stock.
CAUTION: Hydroxylamine sulfate is corrosive, toxic, mutagenic, and hazardous to the environment. For handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid breathing dust. Do not use with metal spatula or other metal items. For storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep away from metals. - Prepare solution B: 12.25 mL of H2O + 0.25 mL of 0.1 M HAuCl4.
- Load solution B in a syringe and load the syringe in a syringe pump.
- Set the syringe pump parameters: diameter of the syringe in mm; flow rate: 90 mL/h; total volume: 10.80 mL (of which, 0.8 mL are the excess needed for system equilibration, i.e., tube filling).
- Start the syringe pump. After the initial 0.8 mL of solution B have filled the tubing and dropped out to a waste container, position the tube on top of the reaction flask containing solution A (kept under moderate and uniform stirring) and let solution B enter dropwise.
- Cap by adding 2.65 mL of freshly prepared 0.1 M Na3∙citrate, and stir for 5 min.
- Concentrate the obtained 40 nm AuNPs by centrifuging at 2,460 x g for 18 min at 10 °C.
- Remove the supernatant by gently aspirating. Resuspend gently in the residual volume.
- Store at 4 °C.
NOTE: The experiment can be paused here.
- Prepare solution A: 390 µL of freshly prepared 0.1 M hydroxylamine sulfate with 13 mL of AuNP seeds in a total volume of 120 mL in H2O.
- Synthesize 15 nm citrate capped gold nanoparticles (AuNPs seeds) using the classical Turkevich–Frens method13,14.
- Functionalize 40 nm citrate capped gold nanoparticles by standard thiol chemistry16.
- Digest disulfide bond by incubating thiolated probe oligonucleotides (5′ T(30)–(O–CH2–CH2)3–SH 3′) with 10 mM Tris(2-carboxyethil)phosphine (TCEP) at room temperature for 3 h under mild shaking (400 rpm).
- Incubate a large excess of the digested oligonucleotides overnight with 10 nM AuNPs, at room temperature under mild shaking (400 rpm).
- Bring the AuNPs-DNA mixture to 0.3 M NaCl in 10 mM phosphate buffer, pH 7.4, 0.01% SDS, by adding salt stepwise over a 10 h timespan, under mild shaking (400 rpm).
- Incubate overnight at room temperature under mild shaking (400 rpm).
- Wash the DNA-conjugated AuNPs 3 times with 0.25 M NaCl in 10 mM phosphate buffer plus 0.01% SDS, to remove the excess unbound oligonucleotides.
- Store the obtained universal probes-AuNPs at 4 °C until use.
NOTE: The experiment can be paused here. - Determine the density of DNA probes on the AuNPs using a commercial fluorescence-based assay
- Measure the concentration of AuNPs by UV-vis spectroscopy. In order to derive AuNPs concentration from the absorbance data, the Lambert & Beer’s Law (A=εbc) and published extinction coefficients for 40 nm AuNPs16 can be used.
- Characterize AuNPs probes by dynamic light scattering (DLS), and transmission electron microscopy (TEM).
NOTE: The experiment can be paused here.
2. Colorimetric discrimination of BRAFV600E rare mutation
- Prepare target samples for analysis: mixtures of synthetic BRAFV600E DNA and BRAFWT DNA at different ratios (BRAFV600E:BRAFWT 1:10, 1:100, 1:200, BRAFV600E 100%, BRAFwt 100%).
- Equilibrate paramagnetic microparticles by washing them twice with hybridization buffer (HB) (1x PBS pH 7.4, 5% w/v PEG 600) and resuspend them in a volume of HB equal to the starting volume. For washing, put an aliquot of paramagnetic microparticles in a 1.5 mL tube (maximum 50 µL/tube), add 500 µL of HB and mix three times by gently pipetting, apply the magnet, separate the clear supernatant, immediately add 500 µL of HB and repeat the procedure for a second washing step. After removing the supernatant, immediately add the HB to avoid bead drying, as advised by the manufacturer.
- For each sample to be tested, prepare a tube containing 2.5 µL of washed paramagnetic microparticles in HB.
- Functionalize paramagnetic microparticles by incubating 2.5 µL of paramagnetic microparticles with 10 µL of a 10 µM solution of a biotinylated first discriminating probe (DP1), harboring BRAFV600E mutation, for 5 min at room temperature.
- Magnetically separate the microparticles from interfering unbound probes and resuspend them in 12.5 µL of HB. To this aim, apply the magnet to the side of the tube, and wait until the solution is transparent. Remove the solution by carefully pipetting, immediately add HB and gently pipette until the beads are homogeneously resuspended.
- Add to the suspension 10 µL of a 10 µM mixture of the DNA target samples described in step 2.1.
- Incubate for 30 min at room temperature.
- During the incubation, shake samples every 3 min to avoid sedimentation of the paramagnetic microparticles.
- Add to the suspension 10 µL of a 2 µM solution of a second discriminating probe (DP2), designed to have a portion complementary to the target and a poly-A tail.
- Incubate for 15 min at room temperature.
- During the incubation, shake samples every 3 minutes to avoid sedimentation of the paramagnetic microparticles.
- Magnetically separate excess DP2 after incubation.
- Add immediately 300 fmol of AuNPs conjugated with detection probes and incubate at room temperature for 5 min.
- Magnetically separate supernatant containing excess AuNPs and perform the 1st washing step by adding 100 µL of HB at room temperature.
- Magnetically separate the supernatant and perform a 2nd washing step by adding 100 µL of HB.
- Incubate at 52 °C for 5 min.
- Magnetically separate supernatant at 52 °C.
- Resuspend immediately in 12.5 µL of HB for the readout of the colorimetric result.
- Photograph results and store the samples at 4 °C.
Representative Results
This method was used for the detection of BRAFV600E mutation in an excess of BRAFwt synthetic DNA. Figure 1 shows the details of the detection strategy. The assay gives a colorimetric YES/NO result17,18 where red corresponds to a positive result (YES) and yellow to a negative one (NO).
Briefly, streptavidinated paramagnetic microparticles were coated with biotinylated discriminating probes (DP1) harboring BRAFV600E mutation. The target samples to be analysed (a mixture of BRAFwt and BRAFV600E DNA) were added to microparticles. After a brief incubation, a second detection probe (DP2) was added to the tubes, followed by the addition of the colorimetric AuNP probe. BRAFV600E DNA in the sample binds DP1, then DP2 and the AuNP probe in turn binds, forming a sandwich that results in AuNPs decorating the surface of the paramagnetic microparticles (Figure 2) and conferring to the latter a red color (YES result). On the opposite, if the sample tested does not contain BRAFV600E DNA that binds DP1, the hybridization sandwich does not form and AuNP probes are washed away during the last washing step. The surface of the beads thus maintains its pristine yellow color (NO result). As a positive control, a sample containing only BRAFV600E DNA was used. This sample always returned a red (YES) result (Figure 3, tube A). Samples with different allelic fractions were tested to assess the limit of detection of the assay. As shown in Figure 3, the test could clearly discriminate the presence of BRAFV600E allele down to 1:200 dilution (Figure 3D), which corresponds to a fractional abundance of 0.5% (BRAFV600E/BRAFwt). In each test, a sample containing only the BRAFwt allele is always included as a negative control, and it returns a yellow (NO) result (Figure 3E).
These results demonstrate the detection of BRAFV600E as a proof-of-concept with relevance in the clinics. However, by changing the sequence of the oligonucleotide probes, the assay can be adapted to the detection of any other point mutation of interest. Noticeably, the colorimetric AuNP probes are universal for any target, as they recognize and bind to a universal polyA portion of DP2. Another characteristic of the AuNP probes that is important for the sensitivity of the assay is their 40 nm size. This represent an optimal compromise between the colloidal stability of the nanoparticles and their high extinction coefficient, which enhances the sensitivity of the naked-eye detection. The characterization of AuNPs probes, including size, shape and monodispersity, is shown in Figure 4.
| Synthetic DNA targets (ST) | |
| ST-BRAFWT | 5’ ATA GGT GAT TTT GGT CTA GCT ACA GTG AA 3’ |
| ST-BRAFV600E | 5’ ATA GGT GAT TTT GGT CTA GCT ACA GAG AA 3’ |
| Discriminating probes (DP) | |
| DP1- BRAFV600E | 5’ /5BiotinTEG//iSp18/ TTC TCT GTA GC 3’ |
| DP2 | 5’ TAG ACC AAA ATC ACC TAT AAA AAAAAAAAAAAAAAAAAAAAAAAAAAA 3’ |
Table 1. Sequences of synthetic DNA targets and probes
This table has been modified from Udayan et al.1 with permission from The Royal Society of Chemistry.
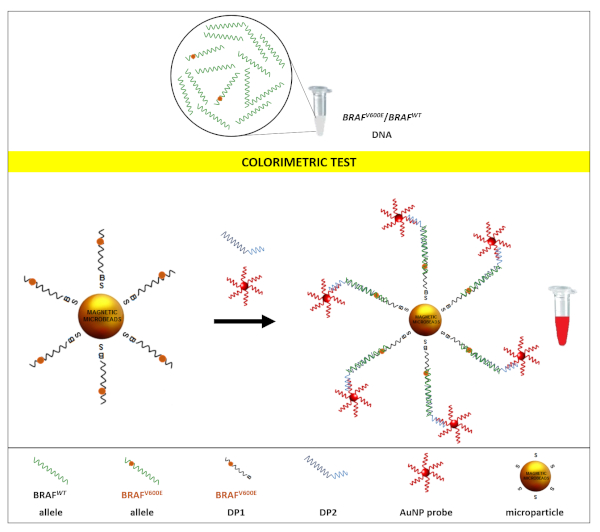
Figure 1. Strategy for the colorimetric detection of BRAFV600E rare mutation. Samples containing mixtures of BRAFV600E and BRAFWT allele in different ratios were analyzed by the colorimetric test. The test employed a sandwich hybridization between the target and two probes, the first one linked to the microparticles surface and specific for the mutant allelic variant (DP1), and the second one complementary to a portion of the target (DP2). The latter also included a polyA tail, which is recognized by the polyT of a third probe, conjugated to AuNPs (AuNPs probe), which provides the colorimetric detection. Red result of the sample indicates the presence of the BRAFV600E allele in the sample. This figure has been republished from Udayan et al.1 with permission from The Royal Society of Chemistry. Please click here to view a larger version of this figure.
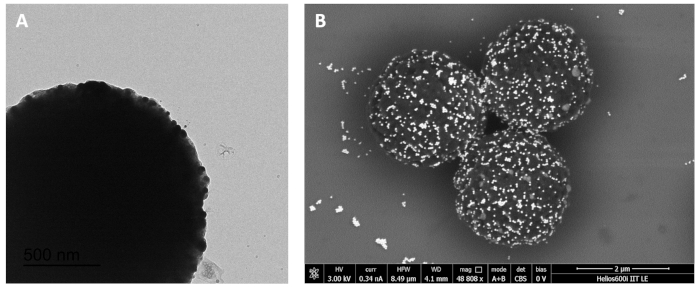
Figure 2. Characterization of paramagnetic microparticles complexed with AuNPs. (A) Transmission electron microscopy (TEM) images of microparticles complexed with AuNPs. AuNPs are visible as small black spheres on the microparticle's surface. (B) Scanning electron microscopy (SEM) images of microparticles complexed with AuNPs. AuNPs are visible as brilliant dots on the surface of the microparticles. This figure has been republished from Udayan et al.1 with permission from The Royal Society of Chemistry. Please click here to view a larger version of this figure.
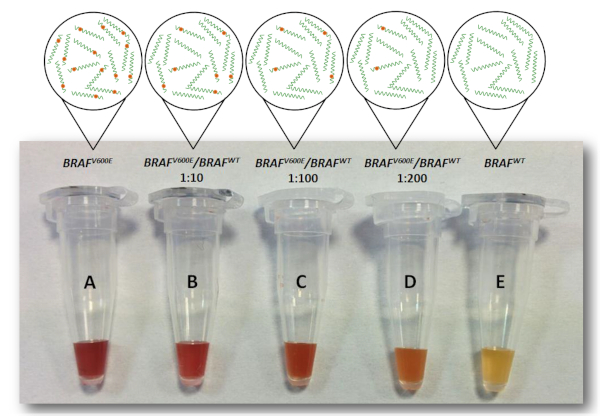
Figure 3. Limit of detection of the colorimetric test. Samples containing different ratios of BRAFV600E and BRAFWT allele (B, C, D), positive control (A, containing only BRAFV600E allele) and negative control (E, containing only BRAFWT allele) were tested with the colorimetric assay. Red samples can be clearly discriminated by naked-eye until 1:200 dilution (D). Thus, the assay was sensitive down to a fractional abundance of BRAFV600E of 0.5%. This figure has been republished from Udayan et al.1 with permission from The Royal Society of Chemistry. Please click here to view a larger version of this figure.

Figure 4. AuNPs characterization. (A) UV-vis spectra of 40 nm AuNPs. (B) DLS characterization. (C) Transmission Electron Microscopy representative image of 40 nm AuNPs. (D) Statistics of size distribution of AuNPs as measured by TEM. This figure has been republished from Udayan et al.1 with permission from The Royal Society of Chemistry. Please click here to view a larger version of this figure.
Discussion
The core aspect of the method is the ability to discriminate a target DNA in the context of an excess of interfering non-target DNA, where target and non-target DNA only differ for one single nucleotide. Thus, the design of the probes and the hybridization conditions are critical to achieve a sensitive discrimination. The assay is designed to use universal colorimetric probes to be adapted to the detection of any point mutations of interest. However, it is possible that some minor optimization of the reaction conditions must be carried out each time a new probe pair is designed for a new mutation.
The only critical step in the method is step 2.17, where magnetic separation must be done at 52 °C. In this step, it is necessary to maintain the temperature of the previous washing step. This is needed because the assay is performed in very small volumes so, if magnetic separation is carried out without temperature control, the temperature will drop down very quickly, causing unspecific binding of non-target molecules to the beads. To ensure that this is not happening, check the color of the negative control, which has to be bright yellow at the end of the assay.
The assay is currently a lab prototype tested on synthetic DNA targets. The translation to the clinics requires the amplification of short portion of genomic DNA containing the target. As the assay needs single stranded DNA, which is readily hybridizable to the probes, it is advisable to amplify the target through a method that yields single strand amplicons. The latter can be obtained either via asymmetric PCR19, or by different isothermal amplification techniques20.
The sensitivity of the assay in detecting BRAFV600E mutation is of ≥0.5%, which is one BRAFV600E copy in 200 interfering BRAFwt copies. This correspond to 500 fmol of the 100 pmol of sample needed for the test, and it is an amount of DNA that can be obtained with a standard PCR. This sensitivity, obtained with a naked-eye readout, is comparable to that obtained with a fluorescence readout by some commercially available rt-PCR assays12, and is clinically relevant6. Moreover, this test does not need a step of allele-specific amplification12.
Given the above, the test could reasonably find future applications in the detection of somatic point mutations in clinical diagnostics.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge Professor Stefano Gustincich (Istituto Italiano di Tecnologia, Genova, IT) for the scientific and financial support. The authors also acknowledge Dr. Maurizio Congedo (Vito Fazzi Hospital, Lecce, IT) and Dr. Paolo Tarantino (Vito Fazzi Hospital, Lecce, IT) for useful scientific discussions. This work was partially supported by the Italian Flagship Project NanoMax.
Materials
| Bench Top Centrifuge- Allegra X 30 | Beckman Coulter | A99473 | |
| DL-Dithiothreitol | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | D0632-25G | |
| Dynabeads M-280 streptavidin paramagnetic microparticles | Invitrogen | 11205D | |
| Hydroxylamine sulfate | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | 379913-25G | |
| KDS 100 Legacy Syringe Pump | kdScientific | 789100 | |
| NanoDrop OneC spectrophotometer | Thermo Fisher Scientific Inc.,Waltham, MA, USA) | ||
| Phosphate Buffered Saline | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | 806552-500ML | |
| Pierce™ TCEP-HCl, No-Weigh™ Format | Thermo Fisher Scientific Inc.,Waltham, MA, USA) | A35349 | |
| Polyethylene glycol 600 | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | 202401 | |
| PTFE 0,22 µm filters, Fluoropore | Millipore | FGLP04700 | |
| Quant-iT™ OliGreen™ ssDNA Assay Kit | Thermo Fisher Scientific Inc.,Waltham, MA, USA) | O11492 | |
| Sodium citrate dihydrate | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | W302600 | |
| Synthetic oligonucleotides | Integrated DNA Technologies, Inc. (IDT DNA) | ||
| Tetrachloroauric(III) acid | Sigma-Aldrich/ Merck KGaA, Darmstadt, Germany | 520918 | |
| Thiolated polyT DNA probes | Integrated DNA Technologies, Inc. (IDT DNA) | ||
| Transmission electron microscopy (TEM) | JEOL JEM 1011 microscope | ||
| Zetasizer Nano S – Dynamic Light Scattering System | Malvern Panalytical |
References
- Udayan, G., Marsella, A., Valentini, P. An ultrasensitive colorimetric test for the detection of somatic rare mutations in DNA. Nanoscale. 12 (5), 2973-2979 (2020).
- Schwarzenbach, H., Hoon, D. S., Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nature Reviews Cancer. 11 (6), 426-437 (2011).
- Diehl, F., et al. Circulating mutant DNA to assess tumor dynamics. Nature Medicine. 14 (9), 985-990 (2008).
- Diefenbach, R. J., Lee, J. H., Rizos, H. Monitoring Melanoma Using Circulating Free DNA. American Journal of Clinical Dermatology. 20 (1), 1-12 (2019).
- Davies, H., et al. Mutations of the BRAF gene in human cancer. Nature. 417 (6892), 949-954 (2002).
- Sullivan, R. J., Flaherty, K. T. Resistance to BRAF-targeted therapy in melanoma. European Journal of Cancer. 49 (6), 1297-1304 (2013).
- Board, R. E., et al. Detection of BRAF mutations in the tumour and serum of patients enrolled in the AZD6244 (ARRY-142886) advanced melanoma phase II study. British Journal of Cancer. 101 (10), 1724-1730 (2009).
- Aung, K. L., et al. Analytical validation of BRAF mutation testing from circulating free DNA using the amplification refractory mutation testing system. Journal of Molecular Diagnostics. 16 (3), 343-349 (2014).
- Ascierto, P. A., et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. Journal of Clinical Oncology. 31 (26), 3205-3211 (2013).
- Shinozaki, M., et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clinical Cancer Research. 13 (7), 2068-2074 (2007).
- Cheng, L., Lopez-Beltran, A., Massari, F., MacLennan, G. T., Montironi, R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Modern Pathology. 31 (1), 24-38 (2018).
- Ashida, A., Sakaizawa, K., Mikoshiba, A., Uhara, H., Okuyama, R. Quantitative analysis of the BRAF (V600E) mutation in circulating tumor-derived DNA in melanoma patients using competitive allele-specific TaqMan PCR. International Journal of Clinical Oncology. 21 (5), 981-988 (2016).
- Turkevich, J., Stevenson, P. C., Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society. 11, 55-75 (1951).
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nature Physical Science. 241 (105), 20-22 (1973).
- Jana, N. R., Gearheart, L., Murphy, C. J. Seeding Growth for Size Control of 5-40 nm Diameter Gold Nanoparticles. Langmuir. 17 (22), 6782-6786 (2001).
- Hurst, S. J., Lytton-Jean, A. K. R., Mirkin, C. A. Maximizing DNA Loading on a Range of Gold Nanoparticle Sizes. Analytical Chemistry. 78 (24), 8313-8318 (2006).
- Valentini, P., et al. Gold-nanoparticle-based colorimetric discrimination of cancer-related point mutations with picomolar sensitivity. ACS Nano. 7 (6), 5530-5538 (2013).
- Valentini, P., et al. Naked-eye fingerprinting of single nucleotide polymorphisms on psoriasis patients. Nanoscale. 8 (21), 11027-11033 (2016).
- Valentini, P., Pompa, P. P. A Universal Polymerase Chain Reaction Developer. Angewandte Chemie International Edition English. 55 (6), 2157-2160 (2016).
- Zhao, Y., Chen, F., Li, Q., Wang, L., Fan, C. Isothermal Amplification of Nucleic Acids. Chemical Reviews. 115 (22), 12491-12545 (2015).

.
