Quantifying the Effects of Antimicrobials on In vitro Biofilm Architecture using COMSTAT Software
Summary
Antimicrobial-induced changes to Pseudomonas aeruginosa biofilm architecture differ among clinical isolates cultured from patients with cystic fibrosis and chronic pulmonary infection. Following confocal microscopy, COMSTAT software can be utilized to quantify variations in biofilm architecture (e.g., surface area, thickness, biomass) for individual isolates to assess the efficacy of anti-infective agents.
Abstract
Biofilms are aggregates of microorganisms that rely on a self-produced matrix of extracellular polymeric substance for protection and structural integrity. The nosocomial pathogen, Pseudomonas aeruginosa, is known to adopt a biofilm mode of growth, causing chronic pulmonary infection in patients with cystic fibrosis (CF). The computer program, COMSTAT, is a useful tool for quantifying antimicrobial-induced changes in P. aeruginosa biofilm architecture by extracting data from three-dimensional confocal images. However, standardized operation of the software is less commonly addressed, which is important for optimal reporting of biofilm behavior and cross-center comparison. Thus, the aim of this protocol is to provide a simple and reproducible framework for quantifying in vitro biofilm structures under varying antimicrobial conditions via COMSTAT. The technique is modeled using a CF P. aeruginosa isolate, grown in the form of biofilm replicates, and exposed to tobramycin and the anti-Psl monoclonal antibody, Psl0096. The step-by-step approach aims to reduce user ambiguity and minimize the chance of overlooking crucial image-processing steps. Specifically, the protocol emphasizes the elimination of subjective variations associated with the manual operation of COMSTAT, including image segmentation and the selection of appropriate quantitative analysis functions. Although this method requires users to spend additional time processing confocal images prior to running COMSTAT, it helps minimize misrepresented biofilm heterogenicity in automated outputs.
Introduction
Biofilms are aggregates of microorganisms oriented in a matrix of self-produced extracellular polymeric substances (EPS). The EPS matrix is very complex, consisting primarily of bacterial cells, water, proteins, polysaccharides, lipids, and nucleic acids1, all of which make biofilms distinctly different from free-living planktonic cells. Biofilm EPS are adherent to each other and various surfaces. The EPS matrix has properties that mediate cell-to-cell exchange of metabolites, genetic material, and compounds used for intercellular signaling and defense2. These properties collectively provide biofilms structural integrity and protection against external stressors, contributing to immune evasion and antimicrobial resistance3.
Pseudomonas aeruginosa is a well-recognized nosocomial pathogen, known to adopt an evasive biofilm growth strategy in response to antimicrobials. A prime example of this occurs in patients with the recessive genetic disorder, cystic fibrosis (CF). Biofilms play a pivotal role in the development of antimicrobial-resistant P. aeruginosa4 and permit the establishment of chronic pulmonary infection in patients with CF, causing accelerated decline in lung function and premature mortality5. Hence, in vitro biofilm studies are performed to test the efficacy of antibiotics and new anti-infective agents against P. aeruginosa isolates obtained from patients with CF6,7. Following biofilm formation, antimicrobials are applied externally to the structure, and confocal laser scanning microscopy (CLSM) is used to generate high-resolution, three-dimensional reconstructions of biofilm segments. It is common practice to then use the computer software, COMSTAT, as a plugin to ImageJ, to quantify changes in biofilm architecture8,9,10,11.
Although COMSTAT is useful for quantifying biofilm structure, the reproducibility and standardization of image analysis is less commonly addressed. For example, the image-processing procedure, performed prior to running COMSTAT, is objective, but contains an element of subjectivity when setting image thresholds12,13. In a similar manner, the COMSTAT program allows the operator to choose from basic to advanced conditions and parameters for image segmentation as well as ten quantitative analysis functions (e.g., thickness distribution, surface area, biomass, dimensionless roughness coefficient). The multitude of user options, compounded with varying operator expertise levels, may result in misguided reporting of biofilm behavior.
Thus, the goal of this protocol is to present a relatively simple method for the quantitative comparison of in vitro biofilm structures using COMSTAT. Herein, three-dimensional images of biofilm segments from a CF P. aeruginosa isolate are captured via CLSM using the chambered coverglass model14—an established technique used to perform reproducible in vitro biofilm experiments. Utilizing COMSTAT as a plugin to ImageJ, this method allows for researchers to quantitatively identify changes in biofilm architecture in the presence of antimicrobials under varying conditions. Overall, this method aims to eliminate subjective variations associated with the manual operation of COMSTAT, thereby facilitating the standardization of protocols across centers.
Protocol
1. Bacterial isolate collection
- Obtain P. aeruginosa isolates from a cohort of pediatric patients with CF undergoing eradication treatment with inhaled tobramycin at SickKids (Toronto). Freeze isolates at -80 °C in glycerol citrate and sub-culture at least three times prior to use.
2. In vitro biofilm formation
NOTE: Use a chambered coverglass method1 for in vitro biofilm formation with modifications. The overall workflow of this model is shown in Figure 1.
- Grow P. aeruginosa isolate overnight at 37 °C on blood agar prepared with tryptic soy agar and 5% sheep blood (see Table of Materials).
- Inoculate 1–2 bacterial colonies from the blood agar into 4 mL of lysogeny broth (LB). Grow overnight at 37 °C on a shaker set to 225 rpm.
- Prepare a 1:100 dilution of overnight inoculum by adding 40 μL of the culture in 4 mL of fresh LB. Grow for 3–4 h at 37 °C on a shaker set to 225 rpm, to achieve an optical density of approximately 0.1 at 600 nm (OD600) (early log phase).
- Transfer 220 μL of the inoculum to each well of an 8-chambered coverglass slide. Incubate undisturbed at 37 °C for 24 h.
- Slowly remove the medium from each well to prevent the biofilms from detaching at the base.
NOTE: Tilt the slide forward at a 45° angle and aspirate the medium from the bottom corners of each chambered well without touching the base with pipette tip. - Prepare and slowly add 100 μL of 56 μg/mL fluorescent labelled monoclonal antibody (mAb) (see Table of Materials) to the side of the designated chambered wells. Incubate at room temperature (RT) for 1 h to allow antibody attachment to the bacterial antigen epitope.
NOTE: Prior to use, dilute the fluorescent labelled (red) mAb, Psl0096, in LB to obtain a final concentration of 56 μg/mL. Psl0096 is an anti-Psl mAb (optimized affinity derivative of Cam003), which binds to the class I Psl epitope—a key EPS matrix component of P. aeruginosa biofilms involved in initial cell attachment and structural integrity15. - Prepare and slowly add 100 μL of a 1000 μg/mL antibiotic solution (see Table of Materials) to the side of the designated chambered wells. Incubate undisturbed at 37 °C for 24 h.
NOTE: Prior to use, dilute a 50 mg/mL stock of tobramycin antibiotic in LB to obtain a final concentration of 1000 μg/mL.
3. Biofilm fluorescent staining
- Prepare a 0.01 mM solution of a live-cell-staining fluorescent dye. Slowly remove the medium from the chambered wells and add 200 μL of the dye mixture to each well. Incubate at RT in the dark for 45 min.
NOTE: Prior to use, prepare the live cell staining (green) fluorescent dye by adding 4 μL of a 5 mM stock to 2 mL of LB. - Slowly remove the medium from each well, and wash 2x with 200 μL of fresh LB.
- Add 200 μL of fresh LB to each well and proceed to examination via confocal microscopy.
4. Image acquisition by confocal microscopy
NOTE: The image-processing and COMSTAT analysis procedure is presented in Figure 2. Acquire images of wells on the same day of biofilm staining. If delay in visualization exceeds 1 h, refrigerate the chambered coverglass in the dark until further processing.
- Acquire images of wells using a confocal microscope system (see Table of Materials) with appropriate laser excitation wavelengths and filter sets for acquisition.
NOTE: Here, excite the fluorescent labeled (red) mAb and live cell (green) stain using 561 and 491 nm excitation wavelengths, respectively. - Capture layered z-stack images (from the substratum to the top of each biofilm segment) in increments of 0.3 μm with a 20–25x water immersion lens. Take a at least 6 image stacks per well.
NOTE: Here, visualize the images using a high-resolution camera with a 25x water immersion lens and processed using image analysis software (see Table of Materials). Keep software setup and digital imaging parameters (i.e., brightness and sensitivity) constant for all acquisitions in a single experiment. - Save images as OME-TIFFs for COMSTAT analysis.
NOTE: Ensure that OME-TIFFs are saved separately for each channel (i.e., red and green). This step varies depending on the image analysis software used. - Repeat steps 2.1–4.3 to capture images from a total of 3 biological replicates (i.e., 3 independent experiments) per bacterial isolate.
5. COMSTAT analysis
NOTE: Analyze images quantitatively using the freely available computer program, COMSTAT16,17, rewritten as a plugin (Comstat2) to ImageJ. Read the general instructions for analyzing image stacks of biofilms within the downloaded package. This contribution provides a summarized protocol, with selected ImageJ processing steps and COMSTAT features recommended for quantifying the effects of antimicrobials on biofilm formation.
- Download the Comstat2 package from http://www.comstat.dk/. Within the installed folder, locate ImageJ and run it.
- Create a source folder on desktop and add a single OME-TIFF to the folder.
- Open OME-TIFF from the source folder, and delete any empty layers containing no biomass. These layers will be either the first or last few layers of the z-stack.
NOTE: Microscope-defined z-stack boundaries are sometimes overestimated by users. Deleting these empty layers establishes a more refined z-stack boundary for COMSTAT analysis. - Import OME-TIFF in ImageJ by selecting File | Import | Image Sequence. Locate the source folder, highlight without opening it, and click Select. A ‘Sequence Options’ window will appear. Select OK.
NOTE: To import additional images in ImageJ, first remove the previous OME-TIFF from the source folder, then add the new OME-TIFF to the folder, and repeat steps 5.3 and 5.4. - Flip the orientation of the biofilm by selecting Image | Transform | Flip Z to position the substratum as the first (topmost) stack.
NOTE: COMSTAT algorithms read biofilms in the z-direction from top (stack 1) to bottom. Depending on the confocal microscope system used, the OME-TIFF output can be inverted. Thus, it is important to reverse the order of slices by positioning the substratum as image stack 1 to prevent output data from becoming flawed. - Define image properties by selecting Image | Properties. A ‘Source’ window will appear.
- Specify ‘Unit of Length’ as ‘micron’.
- Mathematically determine ‘Pixel width’ and ‘Pixel height’ using the following equation:
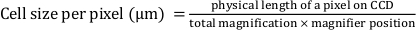 (1)
(1)
Here, ‘Pixel width’ and ‘Pixel height’ are defined as 0.427, where the physical length of a pixel on the charge-coupled device (CCD) is 16 μm; total magnification is 25x; and magnifier position is 1.5x.
NOTE: The equation used to calculate cell size per pixel may vary depending on the microscope camera manufacturer. Alternately, ‘Pixel width’ and ‘Pixel height’ can be defined by spatial calibration (refer to https://imagej.net/Spatial_Calibration). - Define ‘Voxel depth’ as 0.3 (i.e., incremental space between each z-stack layer). Select OK in the ‘Source’ window.
- Adjust the image threshold by selecting Image | Adjust | Threshold. A ‘Threshold’ window will appear.
NOTE: Objects in the image will appear red with a greyscale background. Alternatively, threshold can be adjusted in black-and-white, by using the drop-down menu in the ‘Threshold’ window to change ‘Red’ to ‘B&W’.- In the image window, adjust the slider to the far right (i.e., topmost layer of the biofilm). To remove background noise, use the ‘Threshold’ window, which displays a histogram of the image, to manually set the maximum and minimum threshold values. First, set the maximum threshold value by adjusting the lower slider as far right as possible. Second, use the upper slider to adjust the minimum threshold value, which segments the image into two separate phases: red biomass and greyscale background (Figure 3).
NOTE: The in vitro biofilm formation and fluorescent microscopy procedure described herein generates OME-TIFFs in the ideal case, allowing for images to be segmented into two distinct phases by means of a simple histogram threshold method. However, in some cases, the histogram distinction between the different phases is not as clear. This can be due to the presence of extensive background noise, varying background intensities, or a low intensity contrast between biomass and background. In such cases, users should adopt an enhanced segmentation procedure18,19.- Alternatively, adjust thresholds algorithmically for individual images using the left drop-down menu set as ‘Default’ in the ‘Threshold’ window. This feature provides 17 different algorithmic threshold options to choose from (refer to https://imagej.net/Auto_Threshold). Select the most applicable option, then ‘Auto’ to set threshold.
- When threshold values are adjusted, use the slider in the image window to scroll through each layer to ensure that background noise is sufficiently removed throughout.
- Select Set in the ‘Threshold’ window to first fix the lower threshold value. A ‘Set Threshold Levels’ window will appear. Select OK. Select Set again and repeat this step to fix the maximum threshold value.
NOTE: Each time ‘Set’ is selected, the lower slider may adjust automatically. In such cases, manually re-adjust the slider (or select ‘Auto’ if using one of the algorithmic thresholds) and repeat steps 5.6.2–5.6.3. The main idea is that whenever the sliders are re-adjusted automatically, ‘Set’ should be selected an additional two times afterwards to ensure that both the upper and lower thresholds are fixed. - Select Apply and a ‘Convert Stack to Binary’ window will appear. Select OK, and then exit the ‘Threshold’ window.
- Save the newly adjusted OME-TIFF by selecting Plugins | Bio-Formats | Bio-Formats Exporter|. Enter a new file name and save as OME-TIFF in the source folder. A ‘Bio-Formats Exporter – Multiple Files’ window will appear. Select OK. A ‘Bio-Formats Exporter Options’ window will appear. Select OK.
NOTE: Ensure that only the newly adjusted, black-and-white OME-TIFF(s) are saved in the source folder before proceeding to COMSTAT analysis. Remove all original OME-TIFFs from the folder.
- In the image window, adjust the slider to the far right (i.e., topmost layer of the biofilm). To remove background noise, use the ‘Threshold’ window, which displays a histogram of the image, to manually set the maximum and minimum threshold values. First, set the maximum threshold value by adjusting the lower slider as far right as possible. Second, use the upper slider to adjust the minimum threshold value, which segments the image into two separate phases: red biomass and greyscale background (Figure 3).
- Run COMSTAT by selecting Plugins | Comstat2 |. An ‘About’ window will appear. Select OK. Three windows will appear.
- In the ‘Observed Directories’ window (top right), select Add. Locate the source folder, highlight without opening it, and select Choose. An ‘Images in Directories’ window will appear (top left) that lists the OME-TIFFs to be analyzed via COMSTAT.
- On the ‘Comstat 2.1’ window (bottom right), de-select ‘Automatic thresholding (Otsu’s method)’ to ensure that the software uses threshold values previously set up for individual OME-TIFFs. Also, de-select Connected Volume Filtering (CVF) to ensure that very thin parts of the biofilm as well as free floating cells or biomass found within voids of the biofilm structure are included in the analysis.
NOTE: Here, de-select CVF because COMSTAT analysis is performed on very early biofilms (24 h initial growth) and remaining planktonic cells/colonies post antimicrobial treatment. For mature biofilms, select CVF to ensure that only biomass connected to the biofilm structure is quantified. - On the ‘Comstat 2.1’ window (bottom right), select desired features for quantitative analysis. Here, select Bio Mass, Thickness Distribution, and Surface Area. Select Go to run the program. In the ‘Log’ window (bottom left), the output data are shown processing until ‘Done with selected functions/images!’ appears. Record the COMSTAT measurements. These measurements are also automatically saved as TXT files in the source folder.
Representative Results
A P. aeruginosa isolate cultured from an infected patient with CF is used to demonstrate the strengths of this approach in accurately quantifying antimicrobial-induced changes in in vitro biofilm architecture. The overall workflow of this model is represented in Figure 1. The image-processing and COMSTAT analysis procedure in ImageJ is shown in Figure 2. A simple histogram thresholding approach for image segmentation in ImageJ, applied to a CLSM z-stack image (saved as OME-TIFF), is shown in Figure 3. Biofilm structural changes caused by tobramycin and anti-Psl mAb, Psl0096, is shown in Figure 4. Representative confocal images of biofilm segments separated into live cell (green) and antibody (red) channels are shown in Figure 4A. Corresponding data of three COMSTAT parameters, including average biofilm thickness, biomass, and surface-to-biovolume ratio are shown in Figure 4B-D. Overall, COMSTAT data demonstrate significant differences among biofilm structures compared to control wells. With tobramycin, a clear reduction in average thickness and biomass can be observed in Figure 4B and Figure 4C, respectively. However, in the presence of the anti-Psl mAb, Psl0096, the P. aeruginosa biofilm is resistant to tobramycin. When examining surface-to-biovolume ratio (Figure 4D), a significant reduction is observed with anti-Psl mAb, Psl0096 (p < 0.0001), in both tobramycin-exposed and unexposed biofilms, representing the formation of aggregates (Figure 4A).
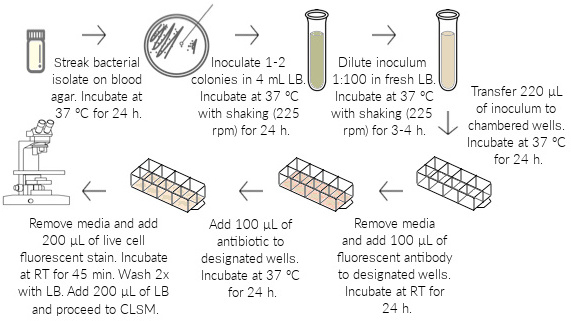
Figure 1: In vitro biofilm formation. A Pseudomonas aeruginosa isolate is grown on blood agar and inoculated in LB overnight. The overnight culture is diluted 1:100 and grown to early log phase (3–4 h incubation) to obtain a final OD600 of approximately 0.1. The OD-adjusted inoculum is seeded into each well of an 8-chambered coverglass and grown for 24 h, allowing biofilms to attach and mature at the base of the wells. Media is carefully removed from the wells, and a fluorescent labelled (red) mAb is added to the designated wells. The chambered coverglass is incubated for 1 h, allowing the antibody to bind to the bacterial antigen epitope. The antibiotic is added to designated wells, and biofilms are grown for 24 h. Medium is removed from the wells, and a live-cell fluorescent (green) stain is added. Following 45 min of incubation, the wells are washed 2x with fresh LB, and confocal imaging is performed in LB.
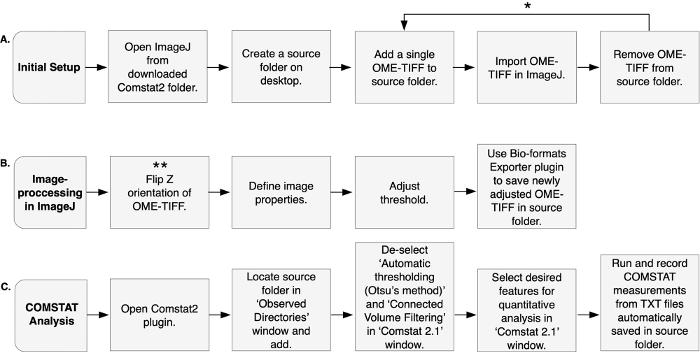
Figure 2: Image-processing and COMSTAT analysis. Comstat2, as a plugin to ImageJ, is used for the quantitative analysis of in vitro biofilm architecture. (A) Initial setup is performed by first opening the ImageJ program found within the downloaded Comstat2 package. It is then recommended to create a source folder, from which a single OME-TIFF can be added and imported into ImageJ. Once imported, the OME-TIFF is deleted from the source folder. * These steps can be repeated to add additional OME-TIFFs, allowing the operator to process multiple images at a time. (B) Image-processing is performed entirely in ImageJ. The orientation of OME-TIFF is flipped in the z-direction, assigning the first layer of biofilm substratum as stack 1. ** This step is only necessary if the OME-TIFF output from the confocal microscope is inverted. Image properties are defined, and the threshold of OME-TIFF is adjusted manually. The Bio-formats Exporter plugin is used to save the newly adjusted OME-TIFF in source folder. (C) COMSTAT analysis is performed using the Comstat2 plugin. The source folder containing the OME-TIFF(s) is added. Specific features that may flaw results are de-selected, while desired features for quantitative analysis are selected. ‘Run’ is selected to initiate analysis. Once completed, measurements are recoded from TXT files automatically saved in the source folder. Please click here to view a larger version of this figure.
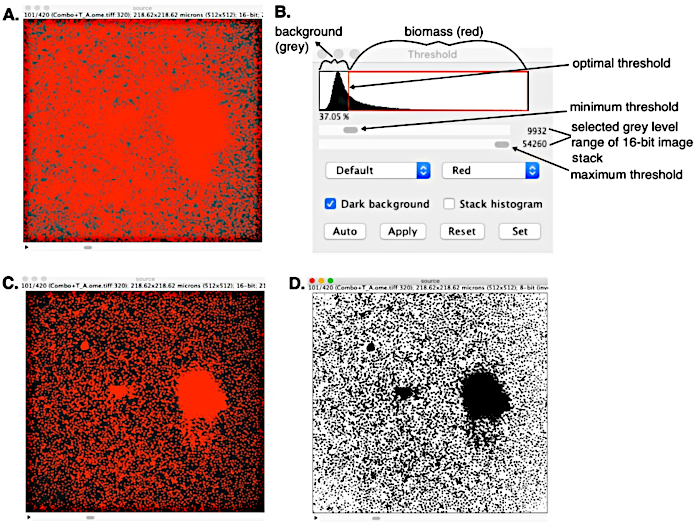
Figure 3: Simple histogram thresholding of a 16-bit CLSM OME-TIFF in ImageJ. (A) OME-TIFF before thresholding with background noise and two distinct phases: red biomass and greyscale background. (B) Threshold window used to remove background noise and set optimal threshold by converting lower intensity pixels (values less than the minimum set threshold) into black background. Maximum threshold value set to establish gray level range to be converted to red biomass. (C) Quality segmented z-stack image generated, and (D) converted stack to a binary black-and-white OME-TIFF that is readable to COMSTAT algorithms. Please click here to view a larger version of this figure.
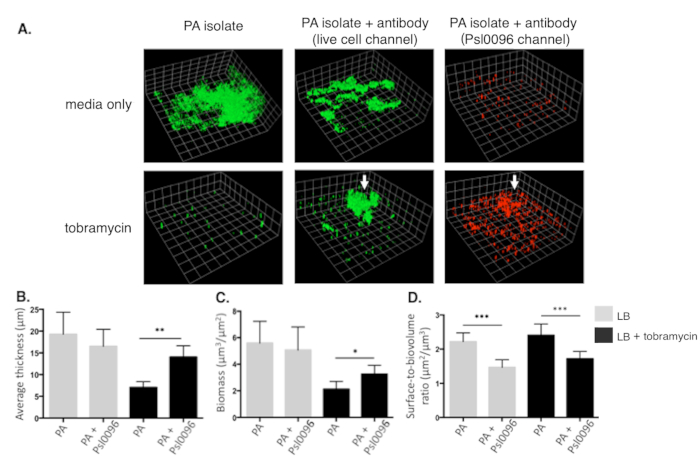
Figure 4: Representative CLSM images and COMSTAT comparison of fluorescent labelled CF Pseudomonas aeruginosa biofilms under varying antimicrobial conditions. (A) Three-dimensional images of 48 h biofilms grown in chambered coverglass. Live-cell (green) channel of biofilm grown in LB media alone (top left) and with 1000 μg/mL tobramycin in LB (bottom left), representing antibiotic effect on control wells. Live-cell channel of biofilm exposed to anti-Psl mAb, Psl0096, in the absence (top middle) and presence (bottom middle) of tobramycin, showing the formation of aggregates and tobramycin resistance. Corresponding Psl0096 (red) channel of identical biofilm is shown in the absence (top right) and presence (bottom right) of tobramycin. White arrows indicate high Psl-antibody binding localized in the same region of aggregates shown in the live cell channel. Quantitative comparison of COMSTAT parameters are displayed in a series of bar graphs, including (B) average thickness (entire well), (C) biomass, and (D) surface-to-biovolume ratio of the isolate. One scale unit is equivalent to 19.68 μm. Each bar represents average of multiple images (n=18) from 3 biologically independent experiments, in the absence (grey bars) and presence (black bars) of tobramycin, with standard error bars of the mean. *p <0.01 **p <0.001 ***p < 0.0001 by Mann-Whitney test. Please click here to view a larger version of this figure.
Discussion
There is no prescribed method for quantitatively comparing three-dimensional images of in vitro biofilm structures, and procedures described in this context are often difficult to standardize due to inter-operator variability20. Thus, this protocol offers a simple and reproducible framework for COMSTAT applications seeking to quantify changes in in vitro biofilm architecture under varying antimicrobial conditions. The strengths of this technique are modeled using a CF P. aeruginosa isolate, grown in the form of biofilm replicates, and exposed to tobramycin and the anti-Psl mAb, Psl0096.
One of the most critical steps outlined in this protocol, as well as in related work, is image thresholding13,21. The image thresholding procedure is a segmentation technique, which reduces greyscale images into a readable black-and-white format for COMSTAT algorithms. During this process, it is important to effectively differentiate which greyscale pixels should be considered biomass (black) versus background noise (white), otherwise the threshold value will change the volume and morphology assigned to an individual biofilm image22. Thresholding can be performed in many different ways in ImageJ: manually or algorithmically by one of 17 options; set for individual images or by applying a fixed value (determined by the user) to a group of images17; applied to the entire image (global); or algorithmically applied to specific regions (local)23,24 of an 8-bit image. The chosen method for thresholding and how it is performed is up to the discretion of the operator, which can introduce significant errors in biofilm image analysis and lead to vastly different results.
In the present protocol, global thresholding was performed manually to segment individual, 16-bit z-stack images. Although algorithmic methods have advantages over manual, including elimination of user intervention13,22 and reduced time spent on image-processing (especially for large data sets)25, they do not account for the wide characteristic variability of images from heterogenous samples, as observed among P. aeruginosa isolates26,27,28. Previous work suggests that to numerically correlate biofilm activity with architectural changes, it is necessary to threshold images in a way that emulate biofilm heterogeneity29,30. Available algorithms in ImageJ for thresholding are considered less suitable given their inconsistencies with expert-determined values30, and technological limitations, including an inability to segment discrete geometric properties22. These algorithms are also limited because very few studies have evaluated their strengths and weaknesses with respect to manual operations. Without a consensus on the best algorithm for thresholding, manual eye-based methods in ImageJ remain most suitable13,17.
Apart from thresholding, CVF is another segmentation process that can be used to remove additional background noise from three-dimensional biofilm images (e.g., planktonic elements that are not attached to the biofilm structure). However, when analyzing early biofilms, utilizing CVF runs the risk of generating null or unexpectedly low COMSTAT outputs because the function assumes that all biofilms grow in a connected fashion from the substratum31. A biofilm may consist of spatial structures, such as voids or channels filled with fluids and floating cells32,33, which CVF inaccurately excludes from the analysis. In the study described herein, de-selection of CVF is important, especially because COMSTAT is performed to quantify the remaining planktonic cells/colonies post antimicrobial treatment. Similarly, under certain conditions, different bacterial strains produce very thin areas within the biofilm that are undetected by the CVF feature due to the set voxel depth16 (in this case, 0.3 μm), and are subsequently removed from the analysis. Therefore, the present study de-selects CVF to prevent null outputs or undercalculations of biomass.
A final critical step of the protocol involves selecting quantitative analysis functions that best represent morphological changes to biofilm architecture. Here, biomass, thickness distribution, and surface area are selected. COMSTAT parameters that quantify biomass and thickness are widely used in studies that describe structural changes to P. aeruginosa biofilms8,9,10,11,14,27. Although less frequently interpreted, surface-to-biovolume ratio (among the surface area outputs) is found herein to be most effective in quantifying aggregation—an important mechanism of antimicrobial resistance, preventing anti-infective agents from penetrating the full depth of biofilms5. This observation is consistent with previous work, which determined a negative correlation of surface-to-biovolume ratio to be indicative of densely clustered bacterial cells34,35. Furthermore, a closely related study reported a 2.6-fold higher surface-to-biovolume ratio among flat formed, undifferentiated PA01 biofilms compared to an overproducing-Psl strain, which visually formed aggregates described as hyper-biofilm structures36. Notably, because of the inherent complexity of biofilms, it is typical to generate variable COMSTAT results, regardless of chosen analysis function and even if experimental conditions are kept constant. Therefore, all quantitative measurements of CLSM images should be performed with a minimum of 3 biological replicates and a consistent number of z-stack images per condition, accompanied by statistical analysis.
Overall, a key approach in quantifying CLSM images via COMSTAT is in the development of a standardized framework for software operation and analysis. To our knowledge, such an integration does not exist, but rather there is a surplus of biofilm cultivation frameworks, image segmentation techniques, and operational parameters applied to different studies. In the absence of a gold standard, this protocol offers an important step toward transparency across laboratories involved in determining the effects of antimicrobials against in vitro biofilm formation. A limitation of this technique is that it does not include a dual fluorescent staining procedure to differentiate live cells from dead cells. Thus, future applications of this method may wish to incorporate a viability assay, as an internal control to determine viable cell numbers. Likewise, a crystal violet assay may be useful for correlating relative cell density with microscopy results. With the rise of new mathematical modeling and algorithmic plugins for image segmentation, it may also be worth investigating different ways to refine this protocol to better depict the heterogenicity of biofilms. This may include scripts for capturing the irregularities of biofilm matrices, spatial coherence of segmented voxels and channels, and localized thresholds. In terms of clinical application, this protocol may be useful for measuring the therapeutic success of different antimicrobials used to treat biofilm infections. It may also be valuable for testing various strategies used to disrupt biofilms to make them more susceptible to antimicrobial treatment.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Cystic Fibrosis Foundation for providing funding for this research.
Materials
| Anti-Psl mAb, Psl0096 | Medimmune | ||
| Blood Agar (TSA with 5 % Sheep Blood) Medium | Fisher Scientific | R01200 | |
| Eight-well Chambered Coverglass w/ non-removable wells | Thermo Fisher Scientific | 155411 | |
| Invitrogen SYTO 9 Green Fluorescent Nucleic Acid Stain | Thermo Fisher Scientific | S34854 | |
| LB BROTH (LENNOX), Liquid Autoclave Sterilized | BioShop Canada | LBL666 | |
| Tobramycin, 900 µg/mg | Alfa Aesar by Thermo Fisher Scientific | J66040 | It is recommended to perform a minimal inhibitory concentration (MIC) test for every batch made to ensure quality control of antimicrobial potency |
| Quorum Volocity 6.3 | Quorum Technologies | Image analysis software |
Referencias
- Flemming, H. C., Wingender, J. The biofilm matrix. Nature Reviews Microbiology. 8, 623-633 (2010).
- Flemming, H. C., et al. Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology. 14, 563-575 (2016).
- Rybtke, M., Hultqvist, L. D., Givskov, M., Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: community structure, antimicrobial tolerance and immune response. Journal of Molecular Biology. 427, 3628-3645 (2015).
- Wendel, A. F., Ressina, S., Kolbe-Busch, S., Pfeffer, K., MacKenzie, C. R. Species diversity of environmental GIM-1-producing bacteria collected during a long-term outbreak. Applied and Environmental Microbiology. 82, 3605-3610 (2016).
- Costerton, J. W., Stewart, P. S., Greenberg, E. P. Bacterial biofilms: a common cause of persistent infections. Science. 284, 1318-1322 (1999).
- Powell, L. C., et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms and Microbiomes. 4, 1-10 (2018).
- Ciofu, O., Tolker-Nielsen, T., Jensen, P. &. #. 2. 1. 6. ;., Wang, H., Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Advanced Drug Delivery Reviews. 85, 7-23 (2015).
- Landry, R. M., An, D., Hupp, J. T., Singh, P. K., Parsek, M. R. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Molecular Microbiology. 59, 142-151 (2006).
- Beaudoin, T., et al. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms and Microbiomes. 3, 1-9 (2017).
- Rojo-Molinero, E., et al. Sequential treatment of biofilms with aztreonam and tobramycin is a novel strategy for combating Pseudomonas aeruginosa chronic respiratory infections. Antimicrobial Agents and Chemotherapy. 60, 2912-2922 (2016).
- Hentzer, M., et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. Journal of Bacteriology. 183, 5395-5401 (2001).
- Tolker-Nielsen, T., Sternberg, C. Growing and analyzing biofilms in flow chambers. Current Protocols in Microbiology. 21, 1-17 (2011).
- Luo, T. L., et al. A Sensitive thresholding method for confocal laser scanning microscope image stacks of microbial biofilms. Scientific Reports. 8, 1-14 (2018).
- Beaudoin, T., Kennedy, S., Yau, Y., Waters, V. Visualizing the effects of sputum on biofilm development using a chambered coverglass model. Journal of Visualized Experiments. (118), e54819 (2016).
- DiGiandomenico, A., et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. Journal of Experimental Medicine. 209, 1273-1287 (2012).
- Heydorn, A., et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 146, 2395-2407 (2000).
- Vorregaard, M. Comstat2 – a modern 3D image analysis environment for biofilms, in Informatics and Mathematical Modelling. Technical University of Denmark. , (2008).
- Hashemi, M. A., Khaddour, G., François, B., Massart, T. J., Salager, S. A tomographic imagery segmentation methodology for three-phase geomaterials based on simultaneous region growing. Acta Geotechnica. 9, 831-846 (2014).
- Rogowska, J. Overview and fundamentals of medical image segmentation. Handbook of Medical Image Processing and Analysis. , 73-90 (2009).
- Webb, D., et al. Assessing technician effects when extracting quantities from microscope images. Journal of Microbiological Methods. 53, 97-106 (2003).
- Azeredo, J., et al. Critical review on biofilm methods. Critical Reviews in Microbiology. 43, 313-351 (2017).
- Xavier, J. B., et al. Objective threshold selection procedure (OTS) for segmentation of scanning laser confocal microscope images. Journal of Microbiological Methods. 47, 169-180 (2001).
- Arena, E. T., et al. Quantitating the cell: turning images into numbers with ImageJ. Wiley Interdisciplinary Reviews: Developmental Biology. 6, 260 (2017).
- Daims, H., Wagner, M. Quantification of uncultured microorganisms by fluorescence microscopy and digital image analysis. Applied Microbiology and Biotechnology. 75, 237-248 (2007).
- Yerly, J., Hu, Y., Jones, S. M., Martinuzzi, R. J. A two-step procedure for automatic and accurate segmentation of volumetric CLSM biofilm images. Journal of Microbiological Methods. 70, 424-433 (2007).
- Lee, B., et al. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Journal of Clinical Microbiology. 43, 5247-5255 (2005).
- Stapper, A. P., et al. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. Journal of Medical Microbiology. 53, 679-690 (2004).
- Reichhardt, C., Parsek, M. Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Frontiers in Microbiology. 10, 677 (2019).
- Yang, X., Beyenal, H., Harkin, G., Lewandowski, Z. Quantifying biofilm structure using image analysis. Journal of Microbiological Methods. 39, 109-119 (2000).
- Yang, X., Beyenal, H., Harkin, G., Lewandowski, Z. Evaluation of biofilm image thresholding methods. Water Research. 35, 1149-1158 (2001).
- Ross, S. S., et al. Quantification of confocal images of biofilms grown on irregular surfaces. Journal of Microbiological Methods. 100, 111-120 (2014).
- Ma, L., et al. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathogens. 5, (2009).
- Mah, T. F., et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 426, 306-310 (2003).
- Srinandan, C. S., Jadav, V., Cecilia, D., Nerurkar, A. S. Nutrients determine the spatial architecture of Paracoccus sp. biofilm. Biofouling. 26, 449-459 (2010).
- Ramos, I., Dietrich, L. E., Price-Whelan, A., Newman, D. K. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Research in Microbiology. 161, 187-191 (2010).
- Ma, L., Jackson, K. D., Landry, R. M., Parsek, M. R., Wozniak, D. J. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. Journal of Bacteriology. 188, 8213-8221 (2006).

