大鼠胰腺组织快速、经济高效的RNA提取
Summary
分离的RNA的纯度和完整性是RNA依赖性测定中的重要一步。在这里,我们提出了一个实用、快速和廉价的方法,从少量未受损的胰腺组织中提取RNA。
Abstract
无论提取方法如何,组织和细胞系的优化RNA提取分四个阶段进行:1) 均质化,2) RNA中蛋白质的有效变性,3) 核糖核糖核素灭活,以及 4) 去除DNA、蛋白质和碳水化合物中的污染。然而,当组织中有高水平的RNA时,保持RNA的完整性是非常费力的。自发的自解使得在不损害胰腺组织的情况下从胰腺组织中提取RNA变得非常困难。因此,在提取过程中需要一种实用的RNA提取方法来维持胰腺组织的完整性。通过在不到2分钟内获得20-30毫克大鼠胰腺组织并提取RNA,对现有方案进行了实验和比较研究。结果通过电泳评估。实验进行了三次,以概括结果。当RNA提取试剂用作试剂时,在-80°C下将胰腺组织浸入RNA稳定试剂中24小时,产生高完整性RNA。所得结果与具有自旋柱结合的商业套件所得结果相当。
Introduction
结构基因数据可以通过基因表达转录到功能产品。RNA分析用于发现不同条件下基因表达的差异。提取核酸的方法有很多,如下:硫化核酸,通过苯酚-氯仿提取,纤维素基色谱,二氧化硅基提取,和核子交换1,2。,2
正确检测基因表达受从组织分离的RNA的完整性影响;因此,在进行进一步测试之前,评估从组织分离的RNA的完整性至关重要,因为对低质量RNA的补充分子测试可能会危及诊断应用结果。因此,具有不同诊断应用的分子生物学测试需要高完整性RNA:定量RT-PCR、微阵列、核糖核酸酶保护测定、北方印迹分析、RNA映射和cDNA库结构3、4。,4
长期保存后,RNA变得相当不稳定。超过10 kb的长mRNA片段特别容易降解5,5,6。因此,研究人员必须考虑影响纯化RNA完整性的各种因素。RNA的纯度必须受到保护,防止RNA、蛋白质、基因组DNA和酶抑制剂污染。此外,RNA 与 UV (260/280) 的最佳和可接受的吸收比必须在 1.8-2.0 范围内,与电泳的碎片最小。最近开发的实验室技术使科学家能够更实际地评估分子分析样本的完整性,。
从胰腺组织中提取未受损的RNA比从其他类型的组织中提取未受损的RNA要困难得多,因为核糖核糖核酸(RNases)的量很高。然而,现有的提取方法,即胰腺组织从腹腔快速弹出和低温均质,以,阻碍RNases,已被证明是无效的7,8,9,10,11,12,13,14。89,10,11,12,13,14
本比较实验研究的目的是修改和比较现有方法,以确定最有效的方法。为此,对RNA提取的各种方案进行了修改和比较。它特别旨在确定最昂贵的方法,需要最低数量的胰腺组织。
Protocol
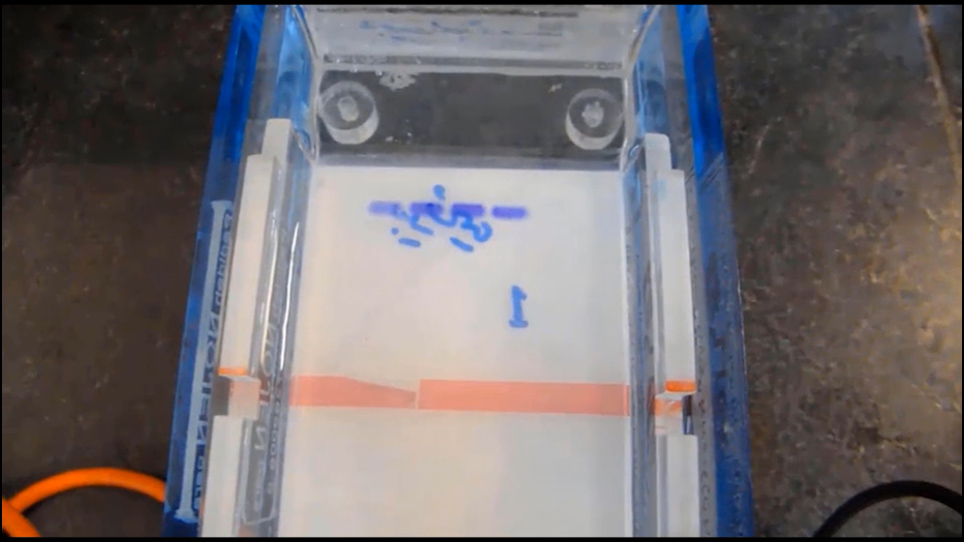
Representative Results
Discussion
在分子生物学中,获得高质量的RNA至关重要。核糖核酸酶在细胞和组织中的存在会迅速降解RNA,使提取变得复杂。RNases 是稳定的酶功能,没有任何共同因素。少量的RNase足以破坏RNA。当大鼠胰腺组织从腹腔中取出时,需要用强洗涤剂对手术器械进行消毒,彻底冲洗,并在240°C下将其放入烤箱至少4小时,在手术前灭活RNases。鉴于RNase水平在胰腺中非常高,手术地点用NaOH和轻度漂白剂进行灭菌,以停…
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
本研究由设拉子医科大学提供财政支持(第93-01-01-7178-03-07-2014号格兰特)。我们感谢设拉子医科大学医学电子学习系、虚拟学校和电子学习卓越中心的佐莫罗迪安和罗斯塔米先生编辑了视频。
Materials
| Agarose | Merck | 116801 | Germany |
| Atoclave | Teb Zaim | Iran | |
| Centrifuge | Sigma | Germany | |
| Chloroform | Merck | 107024 | Germany |
| Diethylpyrocarbonate (DEPC)-treated water | Sigma | Germany | |
| EDTA | sigma | 60-00-4 | Germany |
| Electrophoresis tank | Payapajoohesh | Iran | |
| Eppendorf microTube | Extragene | Taiwan | |
| EtBr | sigma | E 8751 | Germany |
| Ethanol | Merck | 81870 | Germany |
| Falcon Tube | Extragene | Taiwan | |
| Formaldehyde | Merck | 344198 | Germany |
| Formamide | Merck | 344206 | Germany |
| Homogenizer-sunicator | Microson XL 2000 | USA | |
| Isopropanol | sigma | 19516 | Germany |
| Ketamine hydrochloride | sigma | 1867-66-9 | Germany |
| Laminar Flow Hood | Jal Tajhiz | Iran | |
| Mgnetic stirrer | Labrotechnik | USA | |
| Microcentrifuge | Eppendorf | Germany | |
| Micropipette Tips | Extragene | Taiwan | |
| MOPS | sigma | 85022106 | Germany |
| Na AC | Merck | 567422 | Germany |
| NaOH | Merck | 109137 | Germany |
| Oven | Teb Zaim | Iran | |
| PH meter | Knick | Germany | |
| RNA Later/RNA stabilization reagent | Qiagen | 76104 | USA |
| Surgical instrument | Agn Thos | German made | |
| Syringes | AvaPezeshk | Iran | |
| TriPure reagent/RNA extraction reagent | Roche | 11667157001 | USA |
| Vortex | Labinco | Netherland | |
| Water bath | Memmert | Germany | |
| zylazine | sigma | 7361-61-7 | Germany |
Referencias
- McCarthy, B., Hoyer, B. Identity of DNA and diversity of messenger RNA molecules in normal mouse tissues. Proceedings of the National Academy of Sciences. 52 (4), 915-922 (1964).
- Tan, S. C., Yiap, B. C. DNA, RNA, and protein extraction: the past and the present. BioMed Research International. 2009, (2009).
- Peirson, S. N., Butler, J. N. RNA extraction from mammalian tissues. Circadian Rhythms: Methods and Protocols. , 315-327 (2007).
- Skidmore, A. F., Beebee, T. J. Characterization and use of the potent ribonuclease inhibitor aurintricarboxylic acid for the isolation of RNA from animal tissues. Biochemical Journal. 263 (1), 73-80 (1989).
- Mukhopadhyay, T., Roth, J. A. Isolation of total RNA from tissues or cell lines: visualization in gel. RNA Isolation and Characterization Protocols. , 55-59 (1998).
- Raeymaekers, L. Quantitative PCR: theoretical considerations with practical implications. Analytical Biochemistry. 214 (2), 582-585 (1993).
- Sparmann, G., Jäschke, A., Loehr, M., Liebe, S., Emmrich, J. Tissue homogenization as a key step in extracting RNA from human and rat pancreatic tissue. Biotechniques. 22 (3), 408 (1997).
- Kiba, T., et al. High-quality RNA extraction from rat pancreas for microarray analysis. Pancreas. 35 (1), 98-100 (2007).
- Gill, S. S., Aubin, R. A., Bura, C. A., Curran, I. H., Matula, T. I. Ensuring recovery of intact RNA from rat pancreas. Molecular Biotechnology. 6 (3), 359-362 (1996).
- Hernandez, G. E., Mondala, T. S., Head, S. R. Assessing a novel room temperature RNA storage medium for compatibility in microarray gene expression analysis. Biotechniques. 47 (2), 667 (2009).
- Mullin, A. E., Soukatcheva, G., Verchere, C. B., Chantler, J. K. Application of in situ ductal perfusion to facilitate isolation of high-quality RNA from mouse pancreas. Biotechniques. 40 (5), 617 (2006).
- Li, D., et al. A modified method using TRIzol reagent and liquid nitrogen produces high-quality RNA from rat pancreas. Applied Biochemistry and Biotechnology. 158 (2), 253-261 (2009).
- Griffin, M., Abu-El-Haija, M., Abu-El-Haija, M., Rokhlina, T., Uc, A. A simplified and versatile method for obtaining high quality rna from pancreas. Biotechniques. 52 (5), 332 (2012).
- Jun, E., et al. Method optimization for extracting high-quality RNA from the human pancreas tissue. Translation Oncology. 11 (3), 800-807 (2018).
- Green, M. R., Sambrook, J. J. How to win the battle with RNase. Cold Spring Harbor Protocols. (2), 101857 (2019).
- Li, D. -. S., Yuan, Y. -. H., Tu, H. -. J., Dai, L. -. j. A protocol for islet isolation from mouse pancreas. Nature Protocols. 4 (11), 1649 (2009).
- Armstrong, J. A., Schulz, J. R. J. Agarose gel electrophoresis. Current Protocol: Essential Laboratory Techniques. (1), 1-20 (2008).
- Aranda, P. S., LaJoie, D. M., Jorcyk, C. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis. 33 (2), 366-369 (2012).
- Sambrook, J., Fritsch, E. F., Maniatis, T. Molecular cloning: a laboratory manual. Cold spring harbor laboratory press. , (1989).
- Potenza, N., et al. Hybridase activity of human ribonuclease-1 revealed by a real-time fluorometric assay. Nucleic Acids Research. 34 (10), 2906-2913 (2006).
- Jackson, D., Lewis, F., Taylor, G., Boylston, A., Quirke, P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. Journal of Clinical Pathology. 43 (6), 499-504 (1990).
- Quesada, I., Tudurí, E., Ripoll, C., Nadal, &. #. 1. 9. 3. ;. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology. 199 (1), 5-19 (2008).
- Quertinmont, E., Nicaise, C., Gustot, T., Deviere, J. Tissue Homogenization with the MagNA Lyser Instrument for Total RNA Extraction Using the TriPure Reagent. Liver (mg). 100 (100), 100 (2004).
- Dastgheib, S., Irajie, C., Assaei, R., Koohpeima, F., Mokarram, P. Optimization of RNA extraction from rat pancreatic tissue. Iranian Journal of Medical Sciences. 39 (3), 282 (2014).