Pneumococcus Infection of Primary Human Endothelial Cells in Constant Flow
Summary
This study describes the microscopic monitoring of pneumococcus adherence to von Willebrand factor strings produced on the surface of differentiated human primary endothelial cells under shear stress in defined flow conditions. This protocol can be extended to detailed visualization of specific cell structures and quantification of bacteria by applying differential immunostaining procedures.
Abstract
Interaction of Streptococcus pneumoniae with the surface of endothelial cells is mediated in blood flow via mechanosensitive proteins such as the Von Willebrand Factor (VWF). This glycoprotein changes its molecular conformation in response to shear stress, thereby exposing binding sites for a broad spectrum of host-ligand interactions. In general, culturing of primary endothelial cells under a defined shear flow is known to promote the specific cellular differentiation and the formation of a stable and tightly linked endothelial layer resembling the physiology of the inner lining of a blood vessel. Thus, the functional analysis of interactions between bacterial pathogens and the host vasculature involving mechanosensitive proteins requires the establishment of pump systems that can simulate the physiological flow forces known to affect the surface of vascular cells.
The microfluidic device used in this study enables a continuous and pulseless recirculation of fluids with a defined flow rate. The computer-controlled air-pressure pump system applies a defined shear stress on endothelial cell surfaces by generating a continuous, unidirectional, and controlled medium flow. Morphological changes of the cells and bacterial attachment can be microscopically monitored and quantified in the flow by using special channel slides that are designed for microscopic visualization. In contrast to static cell culture infection, which in general requires a sample fixation prior to immune labeling and microscopic analyses, the microfluidic slides enable both the fluorescence-based detection of proteins, bacteria, and cellular components after sample fixation; serial immunofluorescence staining; and direct fluorescence-based detection in real time. In combination with fluorescent bacteria and specific fluorescence-labeled antibodies, this infection procedure provides an efficient multiple component visualization system for a huge spectrum of scientific applications related to vascular processes.
Introduction
The pathogenesis of pneumococcus infections is characterized by a multifaceted interaction with a diversity of extracellular matrix compounds and components of the human hemostasis, such as plasminogen and VWF1,2,3,4,5,6,7,8. The multidomain glycoprotein VWF serves as key regulator of a balanced hemostasis by mediating thrombocyte recruitment and fibrin incorporation at the site of vascular thrombus formation9. The importance of functional, active VWF for bleeding control and wound healing is demonstrated by von Willebrand’s disease, a common inherited bleeding disorder10.
Globular VWF circulates in the human blood system at a concentration of up to 14.0 µg/mL11,10. In response to vascular injury, the local release of VWF by endothelial Weibel Palade Bodies (WBP) is markedly increased11,12. Previous studies show that pneumococcus adherence to human endothelial cells and its production of the pore-forming toxin pneumolysin significantly stimulates luminal VWF secretion13. The hydrodynamic forces of the blood flow induce a structural opening of the mechanoresponsive VWF domains. At flow rates of 10 dyn/cm2 the VWF multimerizes to long protein strings of up to several hundred micrometers in length that remain attached to the subendothelium10,12.
To understand the function of multimerized VWF strings generated under shear stress in the interaction of pneumococcus with the endothelial surface, a microfluidic-based cell culture infection approach was established. A microfluidic device with a software-controlled air-pressure pump system was used. This enabled a continuous, unidirectional recirculation of cell culture medium with a defined flow rate. Thereby, the system applied a defined shear stress on the surface of endothelial cells, which remained attached inside specialized channel slides. This approach enabled the simulation of the shear force within the blood stream of the human vascular system, in which VWF strings are generated on differentiated endothelial cells under defined constant flow conditions. For this purpose, the endothelial cells were cultivated in specific channel slides (see Table of Materials), which were adapted for microscopic analyses during flow. The microfluidic pump system provided the highly defined and controlled shear stress situation required for the formation of extended VWF strings on the confluent endothelial cell layer. After the stimulation of VWF-secretion of confluently grown human umbilical vein endothelial cells (HUVEC) by histamine supplementation, the string formation was induced by applying a shear stress (ԏ) of 10 dyn/cm2. The shear stress is defined as the force acting on the cell layer. It is calculated approximately according to Cornish et. al.14 with equation 1:

Where ԏ = shear stress in dyn/cm2, η = viscosity in (dyn∙s)/cm2, h =half of channel height, w = half of channel width, and Φ = flowrate in mL/min.
The result of equation 1 depends on the different heights and widths of the different slides used (see Table of Materials). In this study a Luer channel slide of 0.4 µm resulting in a chamber slide factor of 131.6 was used (see formula 2).

Viscosity of the medium at 37 °C is 0.0072 dyn∙s/cm² and a shear stress of 10 dyn/cm² was used. This resulted in a flow rate of 10.5 mL/min (see formula 3).
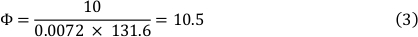
Here, the adaptation and advancement of a microfluidic cell culturing procedure using a unidirectional laminar flow system for the investigation and visualization of bacterial infection mechanisms in the host vasculature is described in detail. The generation of VWF strings on endothelial layers can also be stimulated by using other pump systems that are able to apply a continuous and steady shear stress15.
After cultivation of primary endothelial cells to confluence in flow and stimulation of VWF string formation, pneumococci expressing red fluorescence protein (RFP)16 were added to the endothelial cell layer under constant microscopic control. The attachment of bacteria to VWF strings on the surface of endothelial cells was microscopically visualized and monitored for up to three hours in real time by using VWF-specific fluorescent-labelled antibodies. With this approach, the role of VWF as an adhesion cofactor promoting bacterial attachment to the vascular endothelium was determined8.
In addition to the microscopic visualization of protein secretion and conformational changes, this method could be used to monitor single steps of bacterial infection processes in real time and to quantify the amount of attached bacteria at different time points of infection. The specific software-controlled pump system also provides the possibility to culture the endothelial cells in defined constant flow conditions for up to several days and enables a defined pulsed medium flow incubation. Moreover, this method can be applied using different cell types. Adapting the staining protocol also enables the detection and visualization of bacteria internalized into eukaryotic cells.
This manuscript describes this advanced experimental protocol that can be used as a defined, reliable, and reproducible approach for an efficient and versatile characterization of pathophysiological processes.
Protocol
The microfluidic cell cultivation was performed with commercial primary human umbilical vein endothelial cells (HUVEC). The company isolated the cells with informed consent of the donor. This study was approved by the Ethics Committee of Doctors Chamber of the Federal State Baden-Wuerttemberg with the reference number 219-04.
NOTE: See Table of Materials for protocol supplies.
1. Precultivation of Primary Endothelial Cells
- Thaw a frozen glycerol vial containing 1 x 105 primary HUVEC from three different donors gently at 37 °C and seed the cells in 7 mL of prewarmed endothelial cell growth medium (ECGM, ready to use with supplements) in a 25 cm2 cell flask.
NOTE: The primary endothelial cells lose differentiation capacity after more than 5 proliferation cycles. Therefore, only cells with less than 5 passages can be used if high grades of cell differentiation are required. - Cultivate the cells at 37 °C in 5% CO2 atmosphere for 60 min to allow surface attachment and exchange the ECGM cell culture medium to get rid of residue from cryoconservation.
- Continue culturing the cells at 37 °C in 5% CO2 atmosphere until they form a subconfluent cell layer.
NOTE: The HUVEC must not grow to a confluent layer since the tight cell-cell contacts prevent formation of a stable cell layer later in flow.
2. Precultivation of Streptococcus pneumoniae
CAUTION: Streptococcus pneumoniae is a biosafety level 2 agent and is only allowed to be cultured in biosafety level 2 laboratories. Use a clean bench classified for safety level 2 for all bacterial treatments, strictly avoid aerosol formation, and use a centrifuge with aerosol protection for sedimentation of bacteria.
- Inoculate a Columbia blood agar plate with Streptococcus pneumoniae clinical isolate ATCC11733 derived from a glycerol stock constantly stored at -80 °C and cultivate the agar plate overnight at 37 °C and 5% CO2.
- Prepare 40 mL of Todd Hewitt liquid broth supplemented with 1% yeast extract (THY) and 15 mL of sterile phosphate-buffered saline (PBS) pH 7.4, for bacterial cultivation and washing steps.
- Use a sterile tube for bacterial cultivation and inoculate the liquid culture broth with bacterial mass. Control the amount of inoculation by photometric measurement of 1 mL aliquots at 600 nm against non-inoculated liquid broth as reference. Fill in bacterial mass into the liquid broth until it reaches an optical density at 600 nm (OD600) of 0.15.
- Incubate the inoculated liquid broth without shaking at 37 °C and 5% CO2 and determine the OD600 every 30 min by measuring 1 mL aliquots using plastic cuvettes.
- As soon as the bacterial culture has reached an OD600 of 0.4, which corresponds to the exponential growth phase, centrifuge the bacterial culture suspension for 10 min at 1,000 x g at room temperature (RT).
NOTE: Do not allow a pneumococcus culture to reach an OD600 of more than 1.0, because a high pneumococcus culture density is known to trigger bacterial autolysis, which might affect overall bacterial fitness. - Resuspend the bacterial sediment gently with 10 mL PBS and sediment again for 10 min at 1,000 x g at RT.
- Resuspend the washed bacterial sediment gently in 1 mL PBS and determine the OD600 of 10 µL of the bacterial suspension using 1 mL of PBS as a reference.
- Adjust the amount of bacteria in PBS to an OD600 of 2.0. According to formerly determined bacterial counting, an OD600 of 2.0 corresponds to 2 x 109 colony forming units (CFU). Immediately proceed with the infection procedure to prevent bacterial autolysis.
3. Endothelial Cell Cultivation of HUVEC Under Microfluidic Conditions
- Detach the primary endothelial cells from the cell culture flask by controlled proteolysis. Perform the following steps in a sterile environment using a clean bench. Prepare a volume of 15 mL of sterile PBS for the washing steps.
- Remove the ECGM from a subconfluently-grown HUVEC layer and wash the cell layer with 10 mL PBS using a serological pipette to get rid of the cell culture medium.
- Incubate the washed HUVEC with 3 mL of 37 °C prewarmed cell dissociation solution for cell detachment for 5 min at 37 °C. Observe the proteolytic cell detachment by microscopic monitoring each minute.
- Pipette the detached cell suspension into a tube containing 7 mL of ECGM supplemented with 2% fetal calf serum (FCS) for stopping proteolysis and sediment the cells for 3 min at 220 x g at RT.
- Remove the supernatant and resuspend the HUVEC in 250 µL of ECGM supplemented with 5% FCS and 1 mM MgSO4. Use 10 µL of the cell suspension for cell counting using a Neubauer cell counting chamber and adjust the cell count to 4 x 106 cells/mL ECGM supplemented with 5% FCS and 1 mM MgSO4.
NOTE: For each flow experiment 30 mL of ECGM medium supplemented with 5% FCS and with 1 mM MgSO4 will be required. From here on this medium composition is named ECGMS-medium. The increase of FCS concentration in the culture medium from 2% to 5% supports cell attachment and cell viability of HUVEC seeded in the channel slide. The medium supplements FCS and MgSO4 substantially stabilize the cell attachment of HUVEC under shear stress conditions.
- Seed and cultivate the HUVEC in a channel slide. Work in a sterile environment using a clean bench. The cells will be cultivated under shear stress for 2 days followed by infection with bacteria and microscopic monitoring for another 2 h.
- Equilibrate a channel slide, a perfusion set 1.6 mm in diameter and 50 cm in length, an aliquot of the ECGMS-medium, and a Luer channel slide 0.4 µm in height, for 24 h in an incubator with 5% CO2 atmosphere at 37 °C to reduce the number of air bubbles.
NOTE: This procedure is recommended to degas the plastic equipment and to prewarm the medium, the perfusion tubes, and the reservoirs. If materials or liquids have been stored at RT or in the refrigerator, gases dissolved in the plastic and liquids will be released when heated up in the incubator during the experiment. Gas bubbles will then appear. Degassing all plastic components before the experiment will eliminate this effect. Each time the system is taken out of the incubator, the process of gas absorption begins again. Therefore, work quickly at RT and never leave the fluidic unit outside the incubator for longer time periods. - Use a pipette to inject 100 µL of a 2% sterile-filtered porcine gelatin solution in PBS solution into one of the reservoirs of a temperature-equilibrated channel slide. Incubate the gelatin-solution for 1 h at 37 °C and rinse the channel of the slide with 1 mL PBS under sterile conditions using a 1 mL Luer syringe.
- Place the gelatin-coated channel slide on a thin polystyrene or styrofoam plate to prevent a drop in the slide temperature. Add 100 µL of the 4 x 106/mL HUVEC suspension with a 1 mL Luer syringe into the slide.
NOTE: Placing the channel slide on the cold metal surface of the clean bench could decrease the temperature of the slide bottom, thereby generating cold stress to the endothelial cells. During cell pipetting hold the slide a bit upwards to let air bubbles rise and disappear from inside the slide. - Incubate the channel slide with the HUVEC for 60 min at 37 °C and 5% CO2 and fill up the medium reservoirs at both ends of the channel slide with 60 µL ECGMS-medium each. Incubate for 1 h at 37 °C and 5% CO2.
- Equilibrate a channel slide, a perfusion set 1.6 mm in diameter and 50 cm in length, an aliquot of the ECGMS-medium, and a Luer channel slide 0.4 µm in height, for 24 h in an incubator with 5% CO2 atmosphere at 37 °C to reduce the number of air bubbles.
- Adjust the microfluidic pump and the software settings.
- Connect the equilibrated perfusion set to the pump unit, fill with 13.6 mL of ECGMS-medium, and start the pump control software. Select the adequate perfusion set and type of chamber slide using the scroll down windows in the menu of the fluidic unit set up. Choose 0.007 (dyn∙s/cm2) in the software for medium viscosity. (Refer to the pressure pump software settings marked with red arrows in Supplementary Figure 1).
- Outside of the incubator, connect a glass bottle filled with drying silica beads to the air pressure tubing (refer to Figure 1, Inset 3). The air of the pressure pump circulates between the perfusion reservoirs and the pump and must be dry before reentering the pump device. Select Flow Parameters in the software menu, set the pressure to 40 mbar, and flush the pump tubes with the liquid medium by starting the continuous medium flow. (These settings are also indicated by red arrows in Supplementary Figure 1).
- Program the desired shear stress cycles of flow cultivation. Start with 5 dyn/cm2, control balanced reservoir pumpinng and assure that no air bubbles are circulating in the pump system.
NOTE: The wall shear stress in a channel slide depends on the flow rate and the viscosity of the perfusion medium. If using another pump system, please refer to the equations described in the introduction to set a flow rate generating the desired shear stress level. The described settings correspond to a flow rate of 5.42 mL/min. (An exemplary screen shot showing the adequate flow parameter settings in the pressure pump software is shown in Supplementary Figure 2). - Stop the flow circulation in the pump control software and hold the medium flow in the perfusion tubing by clamping the tubes near the Luer connections. Connect the channel slide, thereby avoiding air bubbles, and place the fluidic unit with the connected channel slide in a CO2 incubator at 37 °C and 5% CO2. Start the shear stress at 5 dyn/cm2 for 30 min to smoothly adapt the cells to the forces generated by the shear stress before accelerating the shear stress level (see Supplementary Figure 3).
NOTE: Take care that no air bubbles remain in the tube system or in the slide after connectinng to the tube system because the movement of air bubbles in the flow might lead to cell detachment. - Accelerate the shear stress to 10 dyn/cm2 (which correspond to 10.86 mL/min in this flow setting) and incubate the channel slide in continuous shear stress for 48 h in a small CO2 incubator at 37 °C and 5% CO2 to allow cell differentiation (the respective software settinngs are indicated with red arrows in Supplementary Figure 4).
NOTE: HUVEC cells tend to detach from the channel surface if flow cultivation is directly started at 10 dyn/cm2. The cells remain attached to the chamber surface if flow cultivation is started with less shear stress using 5 dyn/cm2 for a minimum of 30 min followed by increasing the shear stress slowly up to the desired 10 dyn/cm2. A shear stress of 10 dyn/cm2 is the minimum value in this perfusion setting required for VWF string formation. - After 24 h of microfluidic cell cultivation, stop the medium flow with the pump control software exactly when a balanced medium level is reached in both medium reservoirs. Place the fluidic unit in a clean bench and remove 10 mL of the circulated cultivation medium of the perfusion reservoirs using a 10 mL serological pipette. Add 10 mL of ECGMS-medium into the reservoirs to renew the medium, place the fluidic unit back into the CO2 incubator at 37 °C and 5% CO2, and restart the fluidic cultivation using the pump control software.
NOTE: The function of the pressure pump can suddenly be disrupted by laboratory machines such as large centrifuges, which might create a strong magnetic field disturbance. This sudden disruption might lead to cell detachment. Take care that such machines are not active near the pressure pump during the experiment. - Start the prewarming of the temperature incubation chamber covering the stage of the fluorescence microscope to 37 °C for temperature equilibration 24 h before the microscopic visualization. After the microscope is prewarmed, start the microscope software control and adjust the principal settings for the fluorescence microscopic monitoring by selecting the appropriate filter settings (540 nm/590 nm for detection of the RFP-expressing bacteria and 470 nm/515 nm for detection of fluorescein emission of the FITC-conjugated VWF-specific antibodies). Prewarm an additional heating chamber for incubation of the fluidic unit at 37 °C.
NOTE: During the infection analyses and microscopic monitoring the temperature of the channel slide and the circulating medium should not decrease substantially, because this would generate cold stress on the cells. In general, the size of the temperature chambers covering the microscope stage is not enough to cover the whole fluidic unit. Therefore, the use of an additional heating chamber prewarmed to 37 °C is recommended. - For microscopic visualization, place the fluidic unit into the 37 °C prewarmed heating chamber and place the channel slide on a stage of the 37 °C prewarmed microscope.
NOTE: For microscopic visualization, the fluidic unit and the channel slide needed to be removed from the CO2 incubator due to the limited length of the perfusion tubing. If infection times and microscopic monitoring longer than 180 min are required outside the 5% CO2 atmosphere for pH buffering, a pH-buffered medium should be used for microfluidic cultivation. - Control the cell morphology and the integrity of the HUVEC layer prior to injection of histamine and bacteria to the flow circulation throughout the time of the flow experiment and after finishing the flow experiment using the bright field mode of the microscope.
4. Induction of VWF-release and Visualization of Multimerized VWF Strings
- Maintain the flow setting, because a shear stress of 10 dyn/cm2 is required to trigger the multimerization of VWF to long strings of up to 200 MDa. Induce the release of VWF from endothelial WPB by injecting 136 µL of a 100 mM histamine stock solution into the ECGMS-medium circulating in the perfusion tubing using an injection port. The final concentration of histamine in the flow medium will be 1 mM. If no injection port is available, the histamine can be added alternatively by pipetting into the medium of the pump reservoirs.
- For immunofluorescence detection of multimerized VWF strings, stop the flow when a balanced medium level in the reservoirs is reached, and inject 20 µg of a VWF-specific FITC-conjugated antibody in a volume of 200 µL PBS (pH 7.4) into the circulating 13.6 mL of ECGMS-medium using an injection port. If no injection port is available, the antibody can be added alternatively by pipetting into the medium of the pump reservoirs. This results in a final antibody concentration of 1.3 µg/mL.
- For microscopic scanning of several fields of view in a short time, use the fluorescence unit of the microscope with a Xenon fluorescence device at 30% power and an epifluorescence camera. Monitor the shape and morphology of the HUVEC layer with the bright field mode to select representative cells suitable for the VWF-strings visualization.
- For visualization of green fluorescent VWF strings, select a 63x/1.40 oil objective and a 470 nm detection filter in the fluorescence unit menu of the microscope software (LasX). Create snapshots of Z-stacks of at least 50 representative field views, each containing approximately 10 morphologically intact HUVEC. For quantification of the green fluorescent VWF strings at different time points, scan several fields of view.
5. Microscopic Evaluation of Bacterial Attachment to VWF-strings in Flow in Real Time
- Quantify pneumococcal attachment to the VWF-strings generated on HUVEC cell surfaces via immunofluorescence detection.
- Hold the flow and inject 1.35 x 108 CFU/mL RFP-expressing pneumococci in a maximum volume of 1 mL into the ECGMS-medium using the injection port. Alternatively, pipette the bacteria into the medium in the pump reservoir. Restart the shear stress at 10 dyn/cm2 to let the bacteria circulate within the pump system.
- Select a 63x oil-immersion objective for microscope magnification and adjust the fluorescence filter settings in the microscope software to the RFP-channel (540 nm detection filter) for detection of RFP-expressing pneumococci.
- For quantification of bacterial attachment to the VWF strings, stop the flow and create snapshots of Z-stacks of at least 30 representative field views, each containing approximately 10 morphologically intact HUVEC, and count the amount of pneumococci.
- Use the ANOVA one-factorial statistics algorithm in order to evaluate the data, followed by a post hoc two-tailed unpaired sample test for detailed statistical comparison. P values of <0.05 were considered statistically significant.
6. Microscopic Evaluation of Bacterial Attachment to VWF-strings After Sample Fixation
- Sample the fixation prior to immunofluorescence staining.
- Stop the flow, remove 10 mL of ECGMS-medium from the pump reservoirs, and add 10 mL of PBS supplemented with 5% paraformaldehyde (PFA). Let the PFA solution circulate for 10 min at a shear stress of 10 dyn/cm2.
- Disconnect the channel slide from the pump unit.
- Block unspecific binding sites on the cell surface and perform immunodetection of VWF strings and attached bacteria.
- Prepare 4 mL of a washing solution containing 100 mM Na2CO3 (pH 9.2) supplemented with 4% sucrose for all washing steps. Prepare 1 mL of a blocking solution containing 100 mM Na2CO3 (pH 9.2) supplemented with 4% sucrose and 2% bovine serum albumin (BSA) for blocking of unspecific binding sites.
- Wash the PFA-incubated channel slide 3x using a 1 mL Luer syringe to inject 200 µL of the washing solution and incubate the slide for 120 min at RT with 200 µL blocking solution.
- Prepare 4 mL of another blocking solution containing 100 mM Na2CO3, (pH 9.2) supplemented with 4% sucrose and 0.5% BSA for the dilution of the antibodies. Use 200 µL of this blocking solution to dilute the pneumococcus-specific rabbit antiserum 1:100. Use 200 µL of this blocking solution to dilute the VWF-specific mouse antibody 1:50 to make a VWF-specific antibody concentration of 4 µg/mL. Dilute the AlexaFluor488-conjugated secondary antibody from a 2 mg/mL stock solution 1:100 in 200 µL of PBS (pH 7.4) to generate a final concentration of 20 µg/mL.
NOTE: In the described immunofluorescence settings, the antibody detection delivered optimal results when the antibodies were diluted in the above-mentioned alkaline carbonate buffer. Based on previous results, the recommended blocking solutions and the amount of antibodies are suitable for many applications. However, different experiments might require individual optimization of the antibody combination, antibody concentration, incubation time, and constitution of the blocking buffer. As an alternative, a phosphate-buffered system with a neutral pH range might be suitable or even preferred as an incubation buffer. In case of weak fluorescence signals, the concentration of secondary antibody should be increased. If too much unspecific fluorescence background noise is detected, the amount of blocking substances should be increased. - For VWF-immunofluorescence staining, wash the channel slide using a 1 mL Luer syringe by injecting 200 µL of the washing solution 3x and incubate the slide with the 1:50 diluted VWF-specific antibody for 30 min at RT. Afterwards, wash the channel slide again 3x with 200 µL of the washing solution and incubate the slide with the 1:100 diluted AlexaFluor488-conjugated mouse-specific antibody for 30 min at RT. Finally, wash the channel slide again 3x with 200 µL of the washing solution.
NOTE: The AlexaFluor-fluorophores are sensitive to bleaching. Therefore, the slide should be protected by keeping it in a dark chamber during the incubation steps with the fluorophore-conjugated antibodies. - For immunodetection of the pneumococci, incubate the slide with a 1:100 diluted pneumococcus-specific rabbit antibody for 30 min at RT. Afterwards, wash the channel slide 3x with 200 µL of the washing solution and incubate the slide with 1:100 diluted AlexaFluor568-conjugated rabbit-specific antibody for 30 min at RT. Wash the channel slide again 3x with 200 µL of the washing solution.
- To stain the cellular actin cytoskeleton with fluorescent phalloidin, permeabilize the HUVEC by incubation with 120 µL of 0.1% Triton X-100 for 5 min at RT. Wash the channel slide 3x with 200 µL of the washing solution and incubate the slide with 120 µL of 1:1,000 diluted AlexaFluor350-conjugated phalloidin. This incubation step will visualize the polymerized actin cytoskeleton and allow monitoring of the cell shape and possible stress-induced morphological changes.
- Wash the channel slide 3x with 200 µL of the washing solution. Finally, wash the slide 4x with 200 µL ddH2O and visualize the green fluorescent VWF strings, the red fluorescent bacteria, and the blue fluorescent actin cytoskeleton using the appropriate filter settings on the fluorescence microscope.
Representative Results
Culturing primary HUVEC in a constant unidirectional flow results in the formation of a confluent and tightly packed cell layer that promotes the generation of cellular WPBs filled with the mechanosensitive VWF13,14. This protocol describes the use of an air pressure pump-based, pulseless recirculation system for infection analysis that requires mimicking the shear stress situation in the human blood flow.
This system enables a defined, software-controlled setting of flow conditions. The flow scheme in Figure 1 illustrates the principal workflow, starting with the precultivation of primary endothelial cells (Figure 1, Inset 1) and the precultivation of pneumococci (Figure 1, Inset 2). The applied microfluidic system (Figure 1, Inset 3) is composed of a special channel slide that is connected via a Luer adapter to perfusion tubing with two medium reservoirs. The perfusion tubing set is placed on a fluidic unit that serves as a stand and employs the perfusion tubes as a valve system. For flow cultivation, the fluidic unit with the medium-filled perfusion set and the connected channel slide are placed in a CO2 incubator (Figure 1, Inset 3). The fluidic unit is connected to an air pressure pump via air tubing. The air in the tubing must pass through a drying bottle containing silica beads for the removal of moisture from the perfusion reservoirs before the air is repumped into the pump system (Figure 1, Inset 3). The air pressure pump is controlled by computer software (PumpControl v1.5.0) that enables the setting of a continuous, defined flow rate depending on the diameter of the channel slide, the length and diameter of the perfusion tubing set, and the viscosity of the medium used (Figure 1, Inset 3). The secretion of VWF by WPB exocytosis of confluently grown HUVEC is induced by histamine-stimulation8 applied into the medium circulation in the perfusion tubing using an injection port (Figure 1, Inset 4). At a minimum shear stress of 10 dyn/cm2, the released VWF proteins multimerize and form long protein strings reaching lengths of more than 100 µm (Figure 1, Inset 4,5,6 and Figure 2A). These protein strings are microscopically detected and visualized after the injection of fluorescein isothiocyanate (FITC)-conjugated VWF-specific antibodies into the medium. The circulating antibodies enable the immunofluorescence detection of the cell surface-bound VWF strings in real time (Figure 1, Inset 5)8.
The established microfluidic-based cell culture infection approach using primary endothelial cells mimics the situation of a locally inflamed vasculature upon infiltration of the blood circulation by bacterial pathobionts such as Streptococcus pneumoniae. Examples of the visualization and quantitative analysis of bacterial interaction with differentiated vascular cells under shear stress using microfluidic endothelial cell cultivation are shown (Figure 1, Insets 5,6). RFP-expressing pneumococci were injected into the flow and after 30 min of circulation, the first signs of bacterial attachment to VWF strings of histamine-stimulated HUVEC were microscopically detected (Figure 1, Insets 5,6 and Figure 2A,B white arrows)8. Thus, the use of RFP-expressing pneumococci enabled the quantification of bacterial attachment to the VWF strings on the endothelial cells without the requirement of bacterial antibody detection.
Histogram overlay plots of the fluorescence intensities were generated using the evaluation software provided by Leica (i.e., LasX) to visualize the colocalization of RFP-expressing pneumococci with the VWF strings detected with green fluorescent antibodies. This enabled a quantitative analysis of the colocalization probability at specific regions of interest (ROI) within the fluorescence image. Using histogram overlays of bacterial signals in combination with the fluorescence signals of the VWF, overlapping fluorescence peaks for both could be visualized and thereby confirmed pneumococcus attachment to the VWF strings. The bacterial attachment resists the continuously applied shear stress for a minimum of 25 min (Figure 2B)8.
In sum, the continuous flow cultivation of primary HUVEC enabled VWF secretion and the generation of long VWF protein strings that serve as adhesion sites for circulating bacteria8.
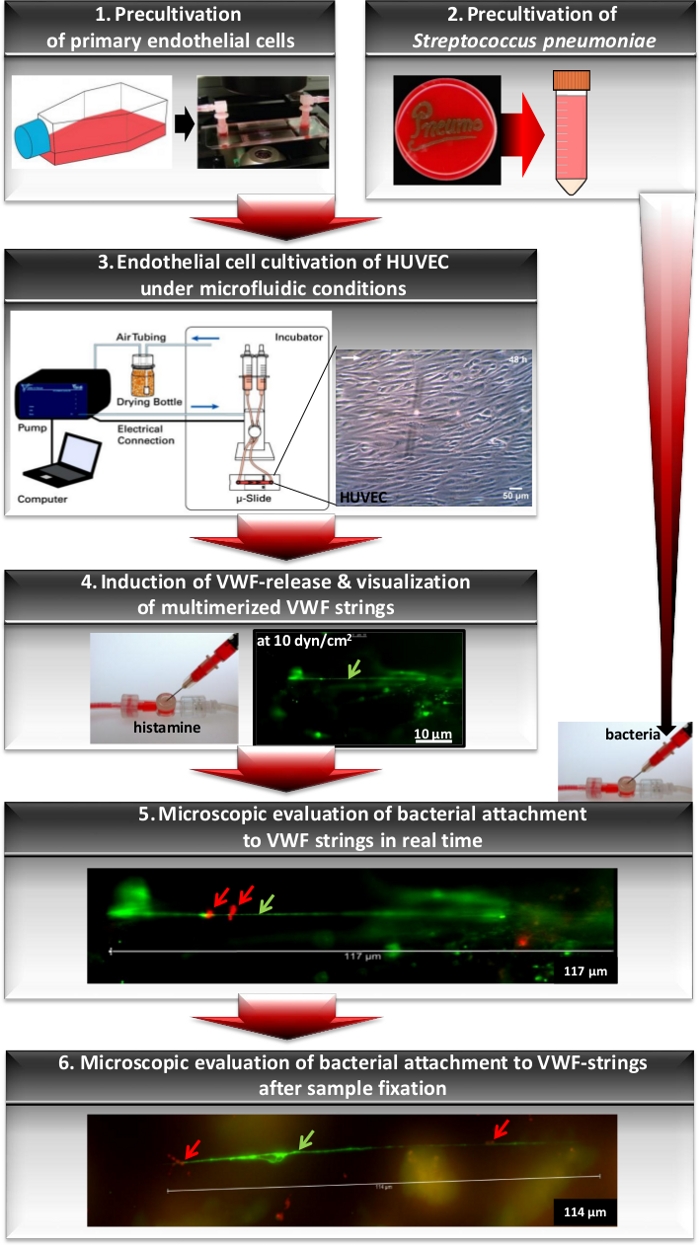
Figure 1: Workflow for analyses of bacterial attachment to VWF strings using microfluidic endothelial cell cultivation. Workflow of the principal experimental steps beginning with the precultivation of primary endothelial cells to subconfluency in cell culture flasks. Prior to cell cultivation in the flow, cells are seeded into a gelatin-coated channel slide (Inset 1). Bacteria are grown on agar plates followed by cultivation in a complex liquid medium to mid-log phase (Inset 2). For microfluidic cell culture, a channel slide with endothelial cells is connected to the perfusion tubes of a fluidic unit of the pump system and subjected to constant flow for cell differentiation (Inset 3). The generation of the VWF-strings (green arrow) was induced by histamine injection at a shear stress of 10 dyn/cm2 and microscopically monitored by immunofluorescence detection using FITC-labelled VWF-specific antibodies (Inset 4). After injection of RFP-expressing bacteria to the circulating medium, pneumococcus attachment (red arrow) to the VWF-strings was microscopically visualized in real time by fluorescence emission at 450 nm (Inset 5). After fixation of the cells using PFA, differential immunofluorescence staining provides the visualization of bacterial attachment at specific infection time points (Inset 6). The images of the pump system and the HUVEC cell layer described in steps 1, 3, and the image of the injection port (Inset 4) are included. The immunofluorescence images shown in Insets 4 and 5 have been modified and used with permission from Jagau et al.8. The lengths of the scale bars are indicated at the lower right.
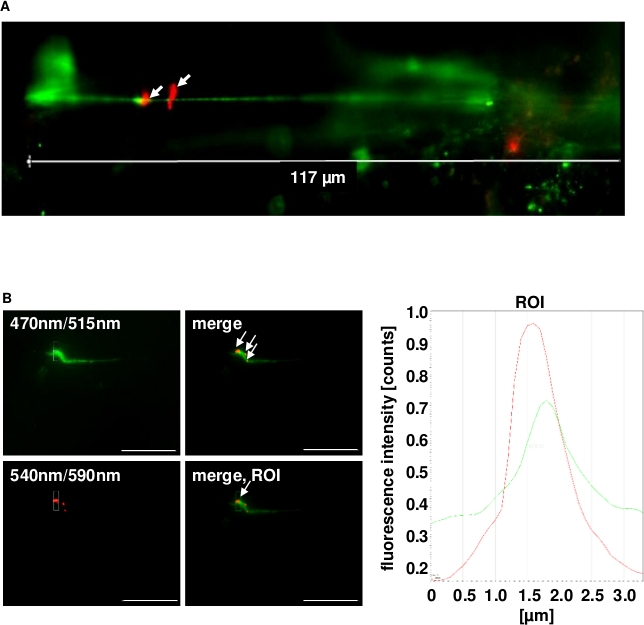
Figure 2: Representative immunofluorescence images of pneumococcus binding to the VWF strings generated in continuous flow on the surface of histamine-stimulated HUVEC. (A) The generation of the VWF strings was microscopically quantified after exposing confluently grown HUVEC to shear stress using a microfluidic pump system at 10 dyn/cm2. FITC-conjugated VWF-specific antibodies detected the VWF strings. White arrows point to RFP-expressing pneumococci with red fluorescence attached to long VWF strings. (B) Bacterial attachment to the green fluorescent VWF strings was microscopically observed for up to 2 h in constant flow (white arrows) and was confirmed by software-based evaluation of fluorescence intensities of a defined ROI. Real time images were taken with the fluorescence equipment of a confocal laser scanning microscope (SP8, Leica). Scale bars = 10 µm. This figure has been modified and used with permission from Jagau et al.8.
Supplemental Figure 1. Please click here to view a larger version of this figure.
Supplemental Figure 2. Please click here to view a larger version of this figure.
Supplemental Figure 3. Please click here to view a larger version of this figure.
Discussion
The simulation of bacterial interaction with mechanosensitive host proteins such as VWF requires a perfusable cell culture system that enables the generation of a defined, unidirectional, and continuous flow of liquids, thereby generating reliable shear stress. Several microfluidic pump systems have already been described. A comprehensive review from Bergmann et al. summarizes the key aspects of different two- and three-dimensional cell culture models17.
The microfluidic technology is a very young technique started in the early 1990s with the development of controllable, reproducible, and perfusable microenvironments in a micrometer and even in a nanometer scale18. The presented microfluidic approach can also be applied to study the broad range of bacterial adhesion mechanisms and involved proteins. It is best suitable for any interaction that involves mechanoresponsive adhesion components such as VWF, which in general are not approachable using standard cell culture techniques. For example, it is known that the conformation of several extracellular matrix proteins differs depending on their location. Within the blood system, glycoproteins like fibronectin circulate in a globular conformation, whereas in the extracellular matrix the proteins appear as multiconnected di- and multimerized scaffold19,20. Moreover, several distributors of microfluidic equipment offer standardized channel slides precoated with different types of collagen (e.g., subendothelial collagens or other plasma-derived proteins). These channel slides are suitable for visualization and quantitative analyses of bacterial adhesion to specific extracellular matrix proteins in different flow situations.
Different microfluidic systems can be categorized based on the different materials used for microdevice production. In this regard, the glass/silicone-based platforms differ from the polymer-based and the paper-based platforms21. Polymer-based channel slides are produced with an elastically supported surface (ESS), generally require a surface coating for cell attachment during flow experiments. As an alternative to a coating with adhesion-supporting components such as collagen, the surface of some commercially available channel slides is physically modified, which creates a hydrophilic and adhesive surface suitable for most cell types. Moreover, some channel slides are generated using substrates like polydimethylsiloxane (PDMS), which is oxygen-permeable and also enables the culture of blood vessel cells on the inner surface of microchannels in fluidic pump systems22,23.
The microfluidic system that was used in these studies served as an efficient and reliable system that was applied for the analysis the interaction of Staphylococcus aureus with multimerized VWF fibers after culture of human endothelial cells in flow24,25. This microfluidic system was a closed circular perfusion system that enabled the analysis of infection by pathogenic bacteria under biosafety 2 conditions. Moreover, the channel slides were suitable for microscopic monitoring during flow and are available with different precoatings (e.g., gelatin, poly-L-lysine, or collagen-IV) that ensure cell adhesion and a high degree of experimental reproducibility. This protocol uses a microfluidic system (see Table of Materials) for the establishment of a perfusable infection model for S. pneumoniae, thereby mimicking the flow situation within the human vascular system8.
The described general procedure of bacterial cell attachment in this microfluidic system can also be conducted with other types of pump systems generating a defined and continuous flow in a sterile environment. For microfluidic purposes, mainly four types of flow control systems are used: i) peristaltic pumps and recirculation pumps, which are used in this protocol, ii) syringe pumps, iii) pressure controllers, and iv) pressure controllers with flow switch matrices. Each kind of flow control system has advantages and drawbacks depending on the specific microfluidic application and the ability to carry out microscopic visualization in real time. In most applications requiring a continuous circulation of the samples, recirculation pumps are combined with software-based pressure controllers to ensure a defined flow situation. This is also optimized in the pump system demonstrated here. The syringe-based pump systems can be subdivided into “classic” syringe pumps, which generate flow oscillation, and “pulseless” microfluidic syringe pumps. These syringe pump-based systems are generally easy to use, but flow control in dead-end channels is challenging using syringe pumps. Moreover, flow changes inside the chip might take some time, and a flow meter is required to determine the flow rate. Even pulseless syringe pumps might generate periodic pulsation on the flow rate due to the step-by-step motor of the syringe pump. Another two syringe pump device is able to generate an “infuse and withdraw”-based fluidic movement that applies defined shear forces on cell surfaces and is suitable for microdialysis applications even in a PC-independent manner. This system must be combined with microtubing for connection of chambers or channel slides for cell cultivation followed by microscopic analyses.
The abovementioned pressure controllers are flow control systems that pressurize the tank containing the sample, which is smoothly injected in a microfluidic chamber or on a chip. The pressure controllers can establish a pulseless flow and also provide flow rates in combination with flow meters. A combination of pressure controllers with flow switch matrices enable a fast flow switch with no back flows. The recirculating system presented here enabled the generation of shear stress values typically reported for the human vascular system26. In vivo vascular wall shear stress has been estimated from wall shear rates as derived from non-invasively recorded velocity profiles, and whole blood viscosity in large arteries and plasma viscosity in arterioles26. Reneman and Hoeks recorded velocity profiles in large arteries by using a specially designed ultrasound system and in arterioles via optical techniques using fluorescent flow velocity tracers26. An average shear stress of 11-13 dyn/cm2 is reached in the carotid artery, as opposed to only 4-5 dyn/cm2 in the brachial artery. Peak values of up to 25-70 dyn/cm2 have been monitored for the carotid artery. The shear stress values of small and middle veins ranges between 0.1 and 0.5 dyn/cm2. In the microfluidic system described here, the applicable shear stress value depends on the selected perfusion tubing diameter, the height of the channel slide, and the viscosity of the fluidic medium used. The selected fluidic setting was composed of a slide with 0.4 cm in height (volume of 100 µl) in combination with a perfusion set of 50 cm in length and 1.6 mm in diameter and the medium viscosity was 0.0072 [dyn·s]/cm2. This setting is appropriate for a shear stress range between 3.5 and 31.2 dyn/cm2 at flow rates of 3.8−33.9 mL/min. In addition, the pressure pump software control can apply a pulsed medium flow that could mimic a pulsed arterial blood flow.
The successful, reliable, and reproducible use of this combined microfluidic infection method requires some precautions that must be kept in mind. During the infection process under microfluidic conditions, the cell layer might be exposed to cytotoxic or cytolytic bacterial compounds such as pneumolysin, which affect the eukaryotic cell viability and weaken the cell adherence to the slide surface. Therefore, keeping a constant flow is required throughout the whole course of the experiment and the integrity of the cell morphology must be frequently monitored. Moreover, the flow culture medium should provide all essential nutrients to the endothelial cells to ensure a tight surface attachment of the cells throughout the infection experiment. Nevertheless, it must be noted that medium supplements contain substances that might interfere or inhibit the interaction between the bacteria and specific host proteins. In studies of pneumococcus-VWF interaction for example, heparin must be depleted from the cell culture medium, because it inhibits the binding of pneumococci to VWF8.
Another critical step in flow culture of endothelial cells is the maintenance of tight cell adhesion, which depends on the overall cell vitality and on the level of differentiation. Shear flow-resistant cell adhesion of primary endothelial cells was only achieved when cells were strictly kept in subconfluency before exposure to shear stress. On the other hand, the production of WPB directly depends on the confluency of the endothelial cell layer promoting tight cell-cell-contacts13. The benefit of the microfluidic cell culture of primary endothelial cells covers the strongly induced cell proliferation that quickly leads to the formation of a confluent cell layer under flow conditions. The cells are tightly attached to each other, are collectively oriented in the direction of flow, and represent a highly differentiated phenotype that is required to produce secretory WPB. These WPB serve as storage vesicles for VWF, vasodilation activators, and cytokines that are exocytosed as protein bursts upon histamine stimulation or pneumolysin activity27,13. Therefore, the generation of a highly adhesive, confluent cell layer of differentiated primary endothelial cells in low proliferation passages is an indispensable prerequisite for efficient VWF release and the formation of VWF strings on the cell surface and the analyses of bacteria-VWF-interaction in flow conditions. Thus, it has to be noted that the differentiation of the endothelial cells took 48 h of flow cultivation at a minimum and required a constant shear flow without any variations in the pump pressure. Any variations might lead to a sudden medium blast, which would flush the cells out of the slide. Moreover, during microscopic visualization the microfluidic slide and the medium reservoirs of the pump system needed to be kept at a temperature of 37 °C as this represents the temperature optimum of the human cells.
Immortalized human cell lines are suitable for many scientific experiments and are often employed in cell culture infection studies. These cell lines share some general technical advantages, such as low or moderate culture requirements and unlimited proliferation, which enables passaging several hundred times without significant changes in morphology or receptor profiles. However, these cell lines represent a solitary monoculture and are subjected to artificial two-dimensional growth conditions.28 The missing physiological source tissue environment results in substantial changes of functional and morphological cell phenotype with every passage of the culture29. Prior to other bacterial adhesion experiments, the profile of surface-exposed cell-type specific marker proteins and receptors of human primary lung endothelial cells at different cell culture passages was determined. The flow cytometry analysis revealed a strongly reduced expression of specific surface proteins such as the platelet endothelial cell adhesion molecule 1 (PECAM1) within eight rounds of cell passaging. Moreover, the expressed integrin receptor profile was significantly changed in higher cell passages in favor of a cell type-unspecific integrin receptor pattern and a reduced cell type-specific integrin receptor pattern (Bergmann et al., unpublished data). These results are particularly relevant for analyses of pathogen-host interactions in infection biology studies. With the aim of sustaining the phenotypic cell characteristics and a high level of functional differentiation during microfluidic cell culture at the time of bacterial infection, primary endothelial cells from multiple donors were chosen in this case and used only up to five passages at maximum in order to keep the phenotypic characteristics as specific as possible8.
The combined visualization of bacteria and specific cell surface structures during flow requires an optimized fluorescence staining protocol. Using different combinations of directly labelled proteins, fluorescence protein-expressing bacteria, and fluorescence-conjugated antibodies, immunofluorescence staining procedures can be specifically adapted to the visualization targets. These procedures enable a defined and clear microscopic visualization and also a differentiation and quantification of bacterial adherence and internalization3,13,30,31. The immunofluorescence-based detection of internalized bacteria requires a cell permeabilization step such as a short Triton X-100 incubation, which might lead to cell detachment. Therefore, the detection of bacterial internalization processes occurring in a flow culture should be visualized after flow incubation using PFA-crosslinked samples. For real time visualization of bacteria in flow, the use of genetically modified bacteria expressing fluorescence proteins enables a quick and targeted microscopic detection. RFP-expressing pneumococcus strains that were generated using an efficient genetic construct designed by Kjos and Veening16,8 were used in this experimental study. For detection of VWF strings in flow, different VWF-specific fluorescein-labelled antibodies were tested and could be used. To obtain an optimal signal response at a minimized unspecific background, the adequate antibody concentration was consistently titrated for each applied antibody.
For microscopic live cell imaging, the fluidic unit and the channel slide are removed from the CO2 incubator and placed into a chamber prewarmed to 37 °C at the microscope. This chamber could not be adjusted to 5% CO2. Without a carbonate-buffered atmosphere, HUVEC morphology and bacterial fitness remained intact for up to 180 min, which is enough for analysis of VWF-mediated bacterial adherence. If longer infection times and microscopic monitoring outside of a CO2 incubator are required, a buffered cell culture medium should be used in order to compensate for pH shifts due to inadequate CO2 concentration. Alternatively, the whole system could be placed back into the CO2 incubator in between the steps of a time series of microscopic visualization.
In addition to the fluorescence detection in real time, the bacterial infection of the endothelium within the channel slide can be stopped and preserved by medium exchange with paraformaldehyde (PFA) as a fixation substance. After fixation in the flow, the optimized and stepwise immunofluorescence staining of the cell surface within the channel slide enables the generation of valuable microscopic snapshot visualizations and provides a versatile combined structure detection of specific cellular compounds involved in the interaction between bacteria and host cells. The scientific benefit of this combined method is that the PFA-treated channel slide can be stored and used for a post-flow immunofluorescence staining of the structures of interest. The PFA treatment inactivates the bacteria and thus arrests the expression of RFP-protein. Therefore, a pneumococcus-specific antiserum was generated in rabbit for bacterial immune detection and visualization was performed using a rabbit-specific AlexaFluor568-conjugated secondary antibody8. As already mentioned, the use of different antibody combinations requires an exact optimization of antibody amounts and the blocking buffer composition. Otherwise, unspecific background signals and cross-detection effects might lead to artificial staining results. An optimized immunofluorescence staining procedure can easily be used for the detection of many different cellular targets such as the actin cytoskeleton or endosomal markers13,31.
This procedure can be adapted to create a more complex tissue environment that mimics physiology from a histologic, physiological, and functional point of view. The presented infection analyses can be efficiently used to answer several scientific questions at the same time by using an in line-connection of several channel slides to one perfusion set. This extended set up would facilitate the analyses of different cell types, cell confluences, and different slide-coatings within the same flow setting in parallel and allow a direct comparison of bacterial infections of different cell types. Moreover, the serial in line connection and combined analyses of several channel slides also provides the possibility of time series experiments, which can be applied for gene expression profiling (e.g., the analysis of infection time-dependent virulence factor gene expression).
The infection analyses can also be extended to a constant laminar flow condition lasting several days or even weeks in order to analyze cellular response in conditions mimicking a long-term chronic infection phase. In addition to the presented single-cell type culture, some examples of heterotypic cell culture in microfluidic cell culture devices have already been reported32,33. This allows for high throughput pharmacological studies and might ultimately result in using microfluidic cell culture systems for regenerative purposes as well34.
In summary, the microfluidic system in combination with immune staining procedures served as a valuable model for the analysis of pathomechanisms between bacteria and host cells in an environment that simulates the conditions within the vascular system.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The project was funded by the DFG (BE 4570/4-1) to S.B.
Materials
| 1 mL Luer-syringe | Fisher Scientific | 10303002 | with 1 mL volume for gelatin injection using the luer-connection of the slides |
| 2 mL Luer-syringe | Sarstedt | 9077136 | For pieptting/injecting fluids into the luer connections of the channel chamber slides |
| Accutase | eBioscience now thermo fisher | 00-4555-56 | protease mix used for gentle detachment of endothelial cells |
| AlexaFluor350-conjugated Phalloidin | Abcam | ab176751 | no concentration available from the manufacturer, stock solution is sufficient for 300 tets, company recommends to use 100 µl of a 1:1000 dilution, blue fluorescence (DAPI-filter settings) |
| AlexaFluor488-conjugated goat-derived anti-mouse antibody | Thermo Fisher Sientific | A11001 | stock concentration: 2 mg/mL for immunostaining of human VWF in microfluidic slide after PFA fixation, green fluorescence |
| AlexaFluor568-conjugated goat-derived anti-rabbit-antibody | Thermo Fisher Scientific | A-11011 | stock concentration: 2 mg/mL for immunostaining of pneumococci in microfluidic slide after pFA fixation, red fluorescence |
| Bacto Todd-Hewitt-Broth | Becton Dickinson GmbH | BD 249210 | complex bacterial culture medium |
| Bovine Serum Albumin (BSA) | Sigma Aldrich | A2153-25G | solubilized, for preparation of blocking buffer |
| Cell culture flasks with filter | TPP | 90026 | subcultivation of HUVEC in non-coated cell culture flasks of 25 cm2 surface |
| Centrifuge Allegra X-12R | Beckman Coulter Life Sciences | 392304 | spinning down of bacteria (volumes of > 2mL) |
| Centrifuge Allegra X-30 | Beckman Coulter Life Sciences | B06314 | spinning down of eukaryotic cells |
| Centrifuge Z 216 MK | Hermle | 305.00 V05 – Z 216 M | spinning down of bacteria (volumes of less than 2 mL) |
| Chloramphenicol | Carl Roth GmbH + Co. KG, Karlsruhe | 3886.2 | used in a concentration of 0.2 /mL for cultivation of pneumococci transformed with genetic construct carrying red fluorescent protein and chloramphenicol resistance cassette |
| Clamp for perfusion tubing | ibidi | 10821 | for holding the liquid in the tube bevor connecting the slide to the pump system |
| CO2-Incubator | Fisher Scientific | MIDI 40 | incubator size is perfectly adapted to teh size of the fluidic unit with connected channel slide and was used for flow cultivation at 37°C and 5% CO2 |
| CO2-Incubator | Sanyo | MCO-18 AIC | for incubation of bacteria and cells in a defined atmosphere at 5% CO2 and 37°C |
| Colombia blood agar plates | Becton Dickinson GmbH | PA-254005.06 | agar-based complex culture medium for S. pneumoniae supplemented with 7% sheep blood |
| Computer | Dell | Latitude 3440 | Comuter with pressure pump software |
| Confocal Laser Scanning Microscope (CLSM) | Leica | DMi8 | An inverse microscope with a stage covered by a heatable chamber and with a fluorescence unit equipped with fluorescence filter, Xenon-light source (SP8, DMi8) and DFC 365 FX Kamera (1392 x 1040, 1.4 Megapixel) |
| Di Potassium hydrogen phosphate (KH2PO4) | Carl Roth GmbH + Co. KG, Karlsruhe | 3904.1 | used for PBS buffer |
| Drying material | Merck | 101969 | orange silica beads for drying used in a glass bottle with a tubing adaptor |
| ECGM supplement Mix | Promocell | C-39215 | supplement mix for ECGM -medium, required for precultivation of endothelial cells: 0.02 mL/mL Fetal calf serum, 0.004 mL/ mL endothelial cell growth supplement, 0.1 ng / mL epidermal growth factor, 1 ng / mL basic fibroblast growth factor, 90 µg / mL heparin, 1 µg / mL Hydrocortisone |
| ECGMS | Promocell | C-22010 | ECGM supplemented with 5 % [w/w] FCS and 1 mM MgSO4 to increase cell adhesion |
| Endothelial Cell growth medium (ECGM, ready to use) | Promocell | C-22010 | culture medium of HUVECs, already supplemented with all components of the supplement mix |
| Fetal Calf Serum (FCS) | biochrome now Merck | S 0415 | supplement for cell culture, used for infection analyses |
| FITC-conjugated goat anti-human VWF antibody | Abcam | ab8822 | stock concentration: 10 mg/mL, for immunodetection of globular and multimerized VWF in flow |
| Fluidic Unit | ibidi | 10903 | fluidic unit for flow cultivation |
| Gelatin (porcine) | Sigma Aldrich | G-1890-100g | for precoating of microslide channel surface |
| histamine dihydrochloride | Sigma Aldrich | H-7250-10MG | for induction of VWF secretion from endothelial Weibel Palade Bodies |
| Human Umbilical Vein Endothelial Cell (HUVEC) | Promocell | C-12203 Lot-Nr. 396Z042 | primary endothelial cells from pooled donor, stored crypcoserved in liquid nitrogen |
| Human VWF-specific antibody derived from mouse (monoclonal) | Santa Cruz | sc73268 | stock concentration: 200 µg/mL for immunostaining of VWF in microfluidic slide after PFA fixation |
| Injection Port | ibidi | 10820 | for injection of histamin or bacteria into the reservoir tubing during the flow circulation |
| Light microscope | Zeiss | Axiovert 35M | inverse light microscope for control of eukaryotic cell detachement and cell counting using a 40 x water objective allowing 400 x magnification |
| Luer-slides I0.4 (ibiTreat472microslides) | ibidi | 80176 | physically modified slides for fludic cultivation (μ–Slide I0.4Luer with a channel hight of 0.4 mm, a channel volume of 100 μl, a growth area of 2.5 cm and a coating area of 25.4 cm2) suitable for all kinds of flow assay, the physical treatment generates a hydrophilic and adhesive surface. |
| Magnesium sulfate (MgSO4, unhydrated) | Sigma Aldrich | M7506-500G | For preparation of ECGMS medium |
| Microfluidic Pump | ibidi | 10905 | air pressure pump |
| Neubauer cell counting chamber | Karl Hecht GmbH&Co KG | 40442002 | microscopic counting chamber for HUVECs |
| Paraformaldehyde 16% (PFA) | Electron Microscopy Sciences | 15710-S | for cross linking of samples |
| Perfusion Set | ibidi | 10964 | Perfusion Set Yellow/Green has a tubing diameter of 1.6 mm, a tube length of 50 cm, a total working volume of 13.6 mL, a dead tube volume of 2.8 mL and a reservoir size of 10 mL. combined with the µ-slide L0.4Luer, at 37°C and a viscosity of 0.0072 dyn x s/cm2 a flow rate range of 3.8mL/min up to 33.9 mL/min and shear stress between 3.5 dyn/cm2 and 31.2 dyn/cm2 can be reached. with 50 cm lenght for microfluidic |
| Phosphate-buffered saline (PBS) | the solution was prepared using the following chemicals: 0.2 g/L KCl, 1.44 g/L Na2HPO4, 0.24 g/L KH2PO4 , pH 7.4 | ||
| Plastic cuvettes | Sarstedt | 67,741 | (2 x optic) for OD measurement at 600 nm |
| Pneumococcus-specific antiserum | Pineda | raised in rabbit using heat-inactivated Streptococcus pneumoniae NCTC10319 and D39, IgG-purified using proteinA-sepharose column. | |
| Polystyrene or Styrofoam plate | this is a precaution step to avoid cold stress on the cells seeded in the channel slides. Any type of styrofoam such as packaging box-material can be used. The plate might by 0.5 cm thick and should have a size of 20 cm2. | ||
| Potassium chloride (KCl) | Carl Roth GmbH + Co. KG, Karlsruhe | 6781.1 | used for PBS buffer |
| Pump Control Software (PumpControl v1.5.4) | ibidi | v1.5.4 | Computer software for controlling the pressure pump, setting the flow conditions and start/end the flow |
| reaction tubes 1.5/ 2.0mL | Sarstedt | 72.706/ 72.695.500 | required for antibody dilutions |
| reaction tubes with 50 mL volume | Sarstedt | 6,25,48,004 | |
| RFP-expressing pneumococci | National Collection of Type Cultures, Public Health England | 10,319 | Streptococcus pneumoniae serotype 47 expressing RFP fused to ahistone-like protein integrated into the genome |
| serological pipets 5, 10 mL | Sarstedt | 86.1253.025/ 86.1254.025 | for pipeting larger volumes |
| Sodium Carbonate (Na2CO3, water free) | Sigma Aldrich | 451614-25G | for preparation of 100 mM Sodium Carbonate buffer |
| Sodium dihydrogen phosphate (NaH2PO4) | Carl Roth GmbH + Co. KG, Karlsruhe | P030.2 | used for PBS buffer |
| Spectral Photometer Libra S22 | Biochrom | 80-2115-20 | measurement of optical density (OD) of bacterial suspension at 600 nm |
| Sucrose | Sigma Aldrich | S0389-500G | for preparation of blocking buffer |
| Triton X-100 | Sigma Aldrich | T9284-500ML | Used in 0.1% end concentration diluted in dH20 for eukaryotic cell permeabilization after PFA fixation |
| Yeast extract | oxoid | LP0021 | bacteria are cultivated in THB supplement with 1% [w/w] yeast extract = complete bacterial cultivation medium THY |
Referencias
- Weiser, J. N., Ferreira, D. M., Paton, J. C. Streptococcus pneumoniae: transmission, colonization and invasion. Nature Reviews Microbiology. 16 (6), 355-367 (2018).
- Bergmann, S., Hammerschmidt, S. Versatility of pneumococcal surface proteins. Microbiology. 152, 295-303 (2006).
- Bergmann, S., et al. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. Journal of Cell Science. 122, 256-267 (2009).
- Bergmann, S., Schoenen, H., Hammerschmidt, S. The interaction between bacterial enolase and plasminogen promotes adherence of Streptococcus pneumoniae to epithelial and endothelial cells. International Journal of Medical Microbiology. 303, 452-462 (2013).
- Bergmann, S., Rohde, M., Chhatwal, G. S., Hammerschmidt, S. Alpha-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Molecular Microbiology. 40, 1273-1287 (2001).
- Bergmann, S., et al. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Molecular Microbiology. 49, 411-423 (2003).
- Bergmann, S., Rohde, M., Preissner, K. T., Hammerschmidt, S. The nine residue plasminogen-binding motif of the pneumococcal enolase is the major cofactor of plasmin-mediated degradation of extracellular matrix, dissolution of fibrin and transmigration. Thrombosis and Haemostasis. 94, 304-311 (2005).
- Jagau, H., et al. Von willebrand factor mediates pneumococcal aggregation and adhesion in flow. Frontiers in Microbiology. 10, 511 (2019).
- Tischer, A., et al. Enhanced local disorder in a clinically elusive von willebrand factor provokes high-affinity platelet clumping. Journal of Molecular Biology. 429, 2161-2177 (2017).
- Ruggeri, Z. M. Structure of von willebrand factor and its function in platelet adhesion and thrombus formation. Best Practice Research Clinical Haematology. 14, 257-279 (2001).
- Spiel, A. O., Gilbert, J. C., Jilma, B. Von willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation. 117, 1449-1459 (2008).
- Springer, T. A. Von Willebrand factor, Jedi knight of the bloodstream. Blood. 124, 1412-1425 (2014).
- Luttge, M., et al. Streptococcus pneumoniae induces exocytosis of weibel-palade bodies in pulmonary endothelial cells. Cellular Microbiology. 14, 210-225 (2012).
- Cornish, R. J. Flow in a Pipe of Rectangular Cross-Section. Proceedings of the Royal Society A. 786, 691-700 (1928).
- Michels, A., Swystun, L. L., Mewburn, J., Albánez, S., Lillicrap, D. Investigating von Willebrand Factor Pathophysiology Using a Flow Chamber Model of von Willebrand Factor-platelet String Formation. Journal of Visualized Experiments. 14 (126), (2017).
- Kjos, M., et al. fluorescent Streptococcus pneumoniae for live-cell imaging of host-pathogen interactions. Journal of Bacteriology. 197, 807-818 (2015).
- Bergmann, S., Steinert, M. From single cells to engineered and explanted tissues: New perspectives in bacterial infection biology. International Reviews of Cell and Molecular Biology. 319, 1-44 (2015).
- Harrison, D. J., et al. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science. 261 (5123), 895-897 (1993).
- Magnusson, M. K., Mosher, D. F. Fibronectin: Structure, assembly, and cardiovascular implications. Arteriosclerosis, Thrombosis, and Vascular Biology. 18, 1363-1370 (1998).
- Zerlauth, G., Wolf, G. Plasma fibronectin as a marker for cancer and other diseases. The American Journal of Medicine. 77 (4), 685-689 (1984).
- Li, X. J., Valadez, A. V., Zuo, P., Nie, Z. 2012. Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis. 4, 1509-1525 (2019).
- Fiddes, L. K., et al. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials. 31, 3459-3464 (2010).
- Schimek, K., et al. Integrating biological vasculature into a multi-organ-chip microsystem. Lab on a Chip. 13, 3588-3598 (2013).
- Pappelbaum, K. I., et al. Ultralarge von willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation. 128, 50-59 (2013).
- Schneider, S. W., et al. Shear-induced unfolding triggers adhesion of von willebrand factor fibers. Proceedings of the National Academy of Sciences of the United States of America. 104, 7899-7903 (2007).
- Reneman, R. S., Hoek, A. P. G. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Medical & Biological Engineering & Computing. 46 (5), 499-507 (2008).
- Valentijn, K. M., Sadler, J. E., Valentijn, J. A., Voorberg, J., Eikenboom, J. Functional architecture of Weibel- Palade bodies. Blood. 117, 5033-5043 (2011).
- Freshney, R. I. . Culture of animal cells: A manual of basic Technique, 5th edition. , (2005).
- Shaw, J. A., Shaw, A. J. . Epithelial cell culture- a practical approach. , 218 (1996).
- Elm, C., et al. Ectodomains 3 and 4 of human polymeric Immunoglobulin receptor (hpIgR) mediate invasion of Streptococcus pneumoniae into the epithelium. Journal of Biological Chemistry. 279 (8), 6296-6304 (2004).
- Nerlich, A., et al. Invasion of endothelial cells by tissue-invasive M3 type group A streptococci requires Src kinase and activation of Rac1 by a phosphatidylinositol 3-kinase-independent mechanism. Journal of Biological Chemistry. 284 (30), 20319-20328 (2009).
- Ho, C. T., et al. Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab on a Chip. 13, 3578-3587 (2013).
- Huh, D., et al. Reconstituting organ-level lung functions on a chip. Science. 328, 1662-1668 (2010).
- Harink, B., Le Gac, S., Truckenmuller, R., van Blitterswijk, C., Habibovic, P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab on a Chip. 13, 3512-3528 (2013).

