Methods for Imaging Intracellular pH of the Follicle Stem Cell Lineage in Live Drosophila Ovarian Tissue
Summary
We provide a protocol for imaging intracellular pH of an epithelial stem cell lineage in live Drosophila ovarian tissue. We describe methods to generate transgenic flies expressing a pH biosensor, mCherry::pHluorin, image the biosensor using quantitative fluorescence imaging, generate standard curves, and convert fluorescence intensity values to pH values.
Abstract
Changes in intracellular pH (pHi) play important roles in the regulation of many cellular functions, including metabolism, proliferation, and differentiation. Typically, pHi dynamics are determined in cultured cells, which are amenable to measuring and experimentally manipulating pHi. However, the recent development of new tools and methodologies has made it possible to study pHi dynamics within intact, live tissue. For Drosophila research, one important development was the generation of a transgenic line carrying a pHi biosensor, mCherry::pHluorin. Here, we describe a protocol that we routinely use for imaging live Drosophila ovarioles to measure pHi in the epithelial follicle stem cell (FSC) lineage in mCherry::pHluorin transgenic wild type lines; however, the methods described here can be easily adapted for other tissues, including the wing discs and eye epithelium. We describe techniques for expressing mCherry::pHluorin in the FSC lineage, maintaining ovarian tissue during live imaging, and acquiring and analyzing images to obtain pHi values.
Introduction
Recent studies revealed a role for changes in pHi during cellular differentiation and dysplasia in vivo1,2. These studies found that pHi is remarkably consistent in cells of the same type at the same stage of differentiation, but that it changes as cells transition from one stage to another. In some cases, blocking the changes in pHi partially disrupts differentiation, suggesting that the change in pHi is not just a consequence of changes in cell fate but instead helps to promote the change in cell fate, perhaps through effects on pH-sensitive regulatory proteins or chemical reactions required for differentiation. Future studies have the potential to reveal more insight into the many different roles of pHi dynamics in vivo. However, one of the challenges of studying pHi during differentiation in vivo is obtaining accurate measurements of pHi. Unlike other features of differentiation, such as changes in cellular morphology and gene expression, pHi is a labile chemical property of the cell that is not preserved in cells that have been fixed and permeabilized with standard methods. In addition, pHi may not be stable in cells that are stressed or dying as a result of experimental manipulation. Thus, it is important to keep cells alive and as healthy as possible when measuring pHi. Several vital dyes are available that work well for measuring the pHi of cells in culture3, but in many cases they are not suitable for in vivo studies because they do not penetrate the tissue deeply or evenly enough to provide accurate measurements.
To circumvent the problem of poor dye penetration, we and others have used a genetically encoded probe, mCherry::pHluorin4,5,6,7, that can be expressed specifically in the cell types of interest and imaged in live tissue. pHluorin is a variant of GFP with a higher pKa (~ 7.0 vs. ~ 4.0) that folds more readily at higher pH; so the total fluorescence intensity emitted from a population of pHluorin molecules in the cell increases with increasing pHi8. Importantly, fluorescence is linear within the normal cytosolic range of pHi values. In contrast, the fluorescence of mCherry (pKa ~ 4.5) is insensitive to pH changes within the cytosolic range. These two reporters are covalently linked together as a single chimeric protein, encoded by a single open reading frame, so they are always present in equal amounts. Therefore, the ratio of pHluorin to mCherry fluorescence intensity provides a measurement of pHi that is normalized to the probe concentration in each cell. The ratios can then be converted to estimates of pHi values using a standard curve that is generated by obtaining the pHluorin to mCherry ratios from tissues that have been equilibrated to known pH values.
Here, we describe the methods for using mCherry::pHluorin to measure the pHi of the epithelial FSC lineage in the Drosophila ovary. This well-characterized tissue has been used to model many different aspects of epithelial biology, such as stem cell self-renewal and differentiation9,10,11, collective cell migration12, and the development and maintenance of cell polarity13,14. The follicle epithelium is produced by two FSCs that reside at the anterior edge of the tissue in a structure called the germarium15,16. These cells divide regularly during adulthood to self-renew and produce progeny, called prefollicle cells (pFCs), that can either re-enter the niche and become an FSC or differentiate into one of three different follicle cell types: polar cells, stalk cells, or main body follicle cells. We showed previously that in wildtype tissue, the pHi increases steadily during the early stages of differentiation, from a pHi of approximately 6.8 in FSCs to 7.0 in pFCs, to 7.3 in follicle cells2. Blocking this increase by RNAi knockdown of a ubiquitously expressed sodium/proton exchanger, DNhe2, severely impairs pFC differentiation, whereas increasing pHi by overexpression of DNhe2 causes a mild excess differentiation phenotype. These findings demonstrate that pHi is stably maintained in the early FSC lineage and that it can be experimentally increased or decreased in vivo. The methods described here can be used to measure pHi in either wildtype tissue or various forms of mutant tissues, including RNAi knockdown or overexpression using a Gal4 of interest, and mitotic clones.
Protocol
NOTE: To measure pHi in the FSC lineage, we calculate the ratio of fluorescence intensities of pHluorin to mCherry in FSCs, pFCs, and follicle cells in physiological conditions, and convert the ratios into pHi values with standard calibration curves for each cell type7. First, live imaging experiments are performed to measure fluorescence intensities of pHluorin and mCherry in germaria dissected into a buffer containing NaHCO3, which mimics physiological conditions1,7. Next, standard curves are generated by measuring pHluorin and mCherry fluorescence intensities in a Na+-free, K+ buffer containing the ionophore nigericin adjusted to two different pH values, 6.5 and 7.5. In the presence of nigericin, pHi equilibrates with the pH of the buffer across the plasma membrane, causing pHi to match the extracellular pH. Lastly, the standard curves are used to convert pHluorin to mCherry ratios to estimated pHi values.
1. Pre-Trial: Preparation Before Measuring pHi In Vivo
NOTE: To measure pHi in vivo, the mCherry::pHluorin transgene must be expressed in the cell type of interest. Below are some common ways to generate transgenic pHluorin flies in the FSC lineage. Generating mCherry::pHluorin clones is particularly useful for identifying FSCs, which are located on the anterior edge of an FSC clone. Tissue specific mCherry::pHluorin expression is useful for measuring pHi across the entire tissue and is also more convenient when combining with expression of an RNAi or transgene.
- Generation of transgenic pHluorin flies
- mCherry::pHluorin-labeled FSC clones
- To make mCherry::pHluorin FSC clones, cross UAS-mCherry::pHluorin1 to an Act-Gal4 flipout stock or other stock with a Flp/FRT inducible Gal4.
- To make heatshock inducible clones, heatshock adult F1s for 2 days post eclosion in empty vials for 1 h in a 37 °C water bath, four times approximately every 12 h.
- To ensure normal growth and maximum rates of oogenesis during this period, maintain flies by providing fresh wet yeast (approximately equal parts dry baker's yeast and water) daily for at least 6 days after clone induction.
- Tissue specific mCherry::pHluorin expression
- Cross UAS-mCherry::pHluorin1 to a follicle cell specific driver, y1w*;P{GawB}10930/CyO (Bloomington ID: 7023).
- 3 days after flies start eclosing, collect and place them in vials containing wet yeast, for at least 24 h prior to dissection.
- mCherry::pHluorin-labeled FSC clones
- Making solutions
NOTE: It is important to prepare the following buffers on the day of the experiment because the pH and solute concentrations in the buffer can change over time.- Prepare bicarbonate, nigericin, and dissection buffers. Add the components in the order listed to avoid formation of precipitates (Refer to Table 1, Table 2, and Table 3).
2. Trial: Measuring pHi in the FSC Lineage
NOTE: The dissection, mounting, and live imaging steps for the bicarbonate buffer and the two nigericin buffer conditions may need to be performed twice: once to determine the microscope settings and a second time to collect experimental data. See the section 2.2 below for more information.
- Dissection and mounting
NOTE: For materials required to perform this step please refer to Figure 1 and Table of Materials. For 3D printed mounting chambers, 3D printer files are provided as Supplemental File 1 and Supplemental File 2.- Dissection:
- Prepare mounting chambers by applying a thin coating of vacuum grease to the flatter side of the 3D printed mounting chamber. Place that side on a 22 x 40 mM coverslip to seal the coverslip to the mounting chamber.
- Dissect ovaries from female adult flies in 500 µL dissection buffer (bicarbonate or nigericin buffer, Table 3)
- Anesthetize 3 – 4 flies using CO2 gas and transfer flies onto a fly pad perfused with CO2 gas.
- Pick up an anesthetized fly with forceps and place into the dissection media.
- Pinch the thorax of the fly with one forcep, while tugging on the tip of its abdomen until its ovaries are exposed.
- After detaching the ovaries from the other organs, tease apart the ovarioles using 221/2 syringe needles. Carefully separate the muscle sheath from the ovaries and separate individual ovarioles from the cluster of ovarioles. An effective technique to isolate the muscle sheath is to use one syringe needle to hold the cluster of ovarioles in place at its anterior end, and stroke downwards along the length of single ovarioles.
- Incubate the dissected ovaries in dissection media for exactly 10 min before proceeding to the mounting step so that there is enough time for the pHi of cells to fully equilibrate with the pH of the nigericin dissection media.
NOTE: Ensure that the muscle sheath is removed from the ovarioles. The muscle sheath may attenuate the fluorescence signal, which makes it difficult to identify individual cells using Concanavilin-A, and may cause ovarioles to move spontaneously during live imaging.
- Mounting
- Place a small drop of dissection media onto the glass coverslip inside the mounting chamber.
- Transfer separated ovarioles using forceps.
- Separate later stage egg chambers from germaria associated with earlier stage egg chambers, and try to ensure that the dissected germaria are placed at the center of the dissection media drop.
- Add two small drops of nail polish to opposite sides of a round 12-mm coverslip and let it air dry for about 10 s.
- Place the round coverslip on the drop of dissection media containing separated germaria such that that side with nail polish faces down and contacts the dissection media. Press down on the parts of the edge with nail polish to flatten and secure the germaria in position. We recommend using older nail polish because it smears less over the surface of the coverslip as it is being secured in place.
- Fill the mounting chamber with additional dissection media. Condition specific buffer that does not contain Concanavalin-A dye can be used to fill the chamber.
NOTE: The total time spent dissecting and mounting should not exceed 15 min. This is important to minimize the effects of tissue damage and cell death.
- Dissection:
- Live imaging:
NOTE: In this step, first collect images from the bicarbonate condition and the two nigericin conditions to determine the proper microscope settings; adjust the microscope settings as necessary, and then collect images for the experimental data. Use a confocal microscope capable of imaging in 3 channels: GFP (475 excitation/509 emission), mCherry (575 excitation/610 emission), and far-red (633 excitation/647 emission).
NOTE: Set Microscope Settings: The pixel intensities of the images collected will be quantified to determine the estimates of pHi, so it is important that the intensity values of the signal in each image set is neither too low nor saturated. The range of intensities that can be captured in an image is defined by the image bit depth. In many cases, 8-bit images are generated by default, but we recommend acquiring the data as 16-bit images, which have a broader dynamic range. It is important to ensure that the crosstalk between fluorescent channels is minimized, so the settings should be adjusted so that the emission spectrum collected from each fluorophore does not overlap. To test these settings, take a sample image using the excitation spectrum for GFP and collect in the emission spectrum for mCherry and vice versa. If the settings have been adjusted properly, there should be no signal in either case. Set the microscope settings (i.e., voltage settings on a laser scanning confocal, laser power and pinhole size) so that the pixel intensities of the signal in all three image sets are within the dynamic range of the image file. For all our experiments, we used a white light laser scanning confocal microscope with a 40x objective to acquire 16-bit images in 1,024 × 1,024 format, while we optimized voltage gain for every experiment as needed. For example, in one experiment, we set the voltage gain for GFP, mCherry, and far-red as 37.8%, 72.9%, and 260%, respectively.- Image ovarioles in nigericin dissection buffer at pH 6.5, and adjust settings such that pixel intensities of the pHluorin and mCherry images are low, but not below the limits of detection of the camera.
- Image another set of ovarioles in nigericin dissection buffer at pH 7.5 and adjust the settings such that the pixel intensities of the pHluorin and mCherry images are high, but not saturated.
- Image ovaries in bicarbonate dissection buffer and ensure that the pixel intensities of the pHluorin and mCherry images are not saturated with the chosen settings. If the pixels are saturated, adjust the settings as necessary.
NOTE: Microscope setting parameters may differ based on the exact microscope setup being used, and must be optimized for each experiment. After microscope settings have been set, do not change them for the remainder of the experiment. Settings should be consistent across control and experimental conditions. In our initial pHi experiments, we generated 2 and 3 point nigericin calibration curves and found no significant difference between calculated pHi using either method. However, in general, the addition of more points in the calibration curve would be expected to improve accuracy. Therefore, we recommend generating curves with different numbers of points to determine how many are needed for a given set of experimental conditions.
- Data collection:
- Perform dissection, mounting, and imaging of the samples in the bicarbonate and nigericin buffer conditions. After dissection and mounting, the maximum time spent imaging one set of samples should not exceed 45 min to ensure that the fluorescence intensity measurements are made in live, healthy tissue.
- For each condition, acquire images for at least 5 germaria.
3. Post-trial: Image Analysis
- Measure fluorescence intensities in FSCs, pFCs, and follicle cells
- Background subtraction:
- Open unprocessed images in FIJI with each channel in a separate window (Figure 4C).
- Use the rectangle tool to draw a region of interest (ROI) in the pHluorin channel window in a part of the image without signal.
- Optional step: Set the lower limit of the threshold so that pixels with intensity values below background are excluded and set the upper limit of the threshold to maximum. When the threshold is set in this way, the areas of the image without signal are mostly blue and the signal is clearly visible (Figure 4A).
- Measure the mean fluorescence intensity in the ROI. If the threshold was set in step 3, make sure to check the "Limit to threshold" box in the Set Measurements dialog box.
- Subtract the measured background intensity from each channel and slice of the image using the Subtract function, found in the Process → Math menu.
- Repeat steps 3.1.1.2-6 for the mCherry channel window except that, in step 3.1.1.2, instead of drawing a new rectangle with the rectangle tool, use the Restore selection function, found in the Edit→ Selection menu, to add a rectangle with the same dimensions and position on the image.
- Identify FSCs, pFCs, and follicle cells:
- Identify the FSCs, pFCs, and follicle cells by morphology and location using the Concanavalin-A staining to find cell boundaries (Figure 2).
- FSCs are thin triangular cells located on the edge of the germarium at the Region 2a/2b border. If mCherry::pHluorin is expressed in an FSC clone, FSC can also be identified as the anterior most cell of the clone.
- pFCs are irregular shaped cells in Region 2b, immediately adjacent to and downstream of FSCs.
- Follicle cells are square or columnar cells that surround the germ cell cyst in Region 3.
- Obtaining fluorescence intensity ratios
- Choose one or more slices in which the cell of interest has highest fluorescence intensity in the mCherry channel and make sure that the Concanavalin-A staining, seen in the far-red channel, is in focus within the selected slice.
- Draw ROI around each FSC and measure the mean fluorescence intensity of pHluorin and mCherry in all slices chosen for measurements.
- Divide the mean fluorescence intensity of the pHluorin channel by the mean fluorescence intensity of the mCherry channel to calculate a ratio of pHluorin to mCherry fluorescence.
- Background subtraction:
- Derive pHi values and generate pseudocolored images
- Derive pHi values
- In all cases, the linear regression curve should be generated using ovaries from flies of the same genotype for both experimental and control conditions. Generate a linear regression curve for each cell type using the data from two nigericin buffer conditions (Figure 3). In Excel, this can be done by plotting the data on a graph and adding a linear trendline. Alternately, see:
- Use the slope and the y-intercept of the linear regression curve calculated from the nigericin conditions and the equation of a line (y = mx + b) to convert the pHluorin to mCherry ratio values calculated from the samples in the bicarbonate condition to pH values. In this equation, substitute the slope form (the y-intercept) for b, put the ratio value in for x, and solve for y.
- Generate a pseudocolored image reflecting the ratio values in FIJI.
- Follow the procedure above for background subtraction (step 3.1.1 and Figure 4).
- Divide the pHluorin channel from the mCherry channel using the Image Calculator function, found in the Process menu. Be sure the "Create new window" and "32-bit (float) result" checkboxes are both checked. Change the lookup table (LUT), found under the Image menu, to Thermal or LUT of choice. See Figure 4 for other suitable options.
- In the adjust brightness and contrast dialog box (Image → Adjust → Brightness/Contrast), click the "Set" button. Set the Minimum displayed value to 0 and the Maximum displayed value to a ratio that best captures the data, usually 1.0-2.0 (Figure 5).
- Derive pHi values
Representative Results
Here we have described the process of measuring pHi in the follicle epithelium, which involves several steps. First, the ovaries are dissected from flies of the appropriate genotype using tools for dissecting and mounting (Figure 1). The ovarioles are then imaged using quantitative fluorescence microscopy and the images are analyzed to obtain measurements of pHi. For each image, the cell types of interest are identified as described in Section 3.1 (Figure 2). The ratio of the fluorescence intensities in the GFP and mCherry channels is converted to pHi values using a standard curve (Figure 3A) as described in Section 3.2. Using this method, we found that pHi increases with differentiation in the early FSC lineage, from 6.8 in FSCs, to 7.0 in prefollicle cells, to 7.3 in follicle cells (Figure 3B). The pHi values of each cell type can be represented graphically, as in Figure 3B, or as a pseudocolored micrograph that shows the differences in pHluorin to mCherry ratios (Figure 4 and Figure 5). In these images, differences in the pHluorin to mCherry ratios are displayed as differences in color, as defined by a LUT. It is important to select a LUT and maximum and minimum values for the range of colors to produce an image that is most representative of the data. Although, in general, the choice of LUT and range settings will not give the appearance of differences that are not present in the image, a less suited choice of LUTs may obscure pHi differences present in the micrograph (Figure 5).
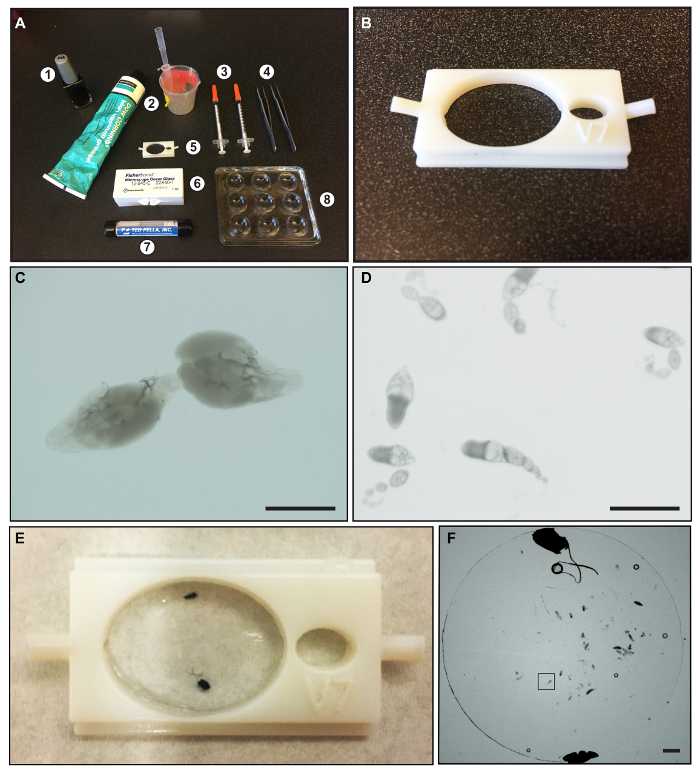
Figure 1: Materials for dissection and mounting Drosophila ovarioles. (A) An image showing: (1) nail polish; (2) vacuum grease and beaker and pipet tip used for applying grease; (3) 23-gauge syringe needles; (4) Dumont Inox forceps, Size 5; (5) 3D printed mounting chamber; (6) 22 X 40 mM glass coverslips; (7) Round Glass Coverslips, 12 mm diameter, 0.13-0.16 mm thickness; and (8) 9-well glass dissecting dish. (B) A close-up image of the 3D printed mounting chamber. (C) An image showing a dissected pair of wildtype ovaries. (D) An image showing well-separated ovarioles. (E) A picture of the 3D chamber with dissected ovaries mounted under a round glass coverslip. The black dots are drops of nail polish used to hold the round coverslip in place. (F) An image of dissected ovarioles underneath a round glass coverslip after mounting. The black box indicates a region of the image with a single dissected ovariole. Scale bars represent approximately 500 µm. Please click here to view a larger version of this figure.
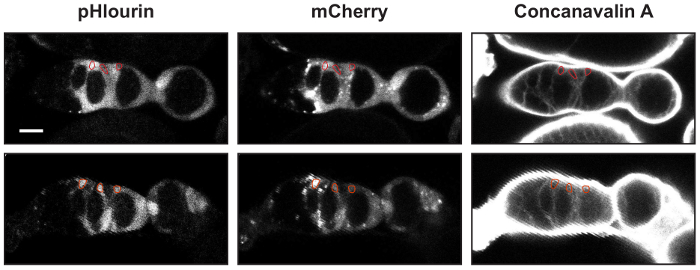
Figure 2: Identifying FSCs, pFCs, and follicle cells. Two examples of germaria with UAS-mCherry::pHluorin and 10930-Gal4 stained with Concanavilin-A. An ROI outlining an FSC, a pFC, and a follicle cell is shown in each image. The mean fluorescence intensities in the pHluorin and mCherry channels are used to calculate the pHluorin to mCherry ratios. Scale bars represent approximately 10 µm. Please click here to view a larger version of this figure.
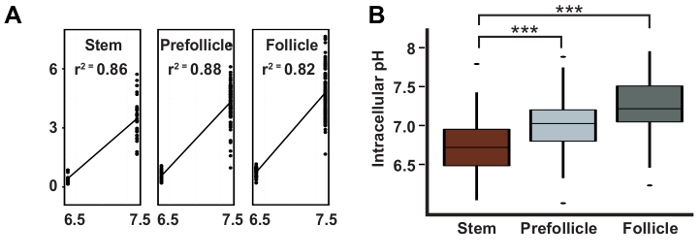
Figure 3: pHi increases during differentiation in the FSC lineage. Representative results taken from Ulmschneider et al.2 showing: (A) a typical linear regression curve used to calculate pH values from pHluorin to mCherry ratios; and (B) the calculated pHi values with 95% confidence intervals for FSCs, pFCs, and follicle cells. This figure has been adapted after permission, from Ulmschneider et al.2. Please click here to view a larger version of this figure.
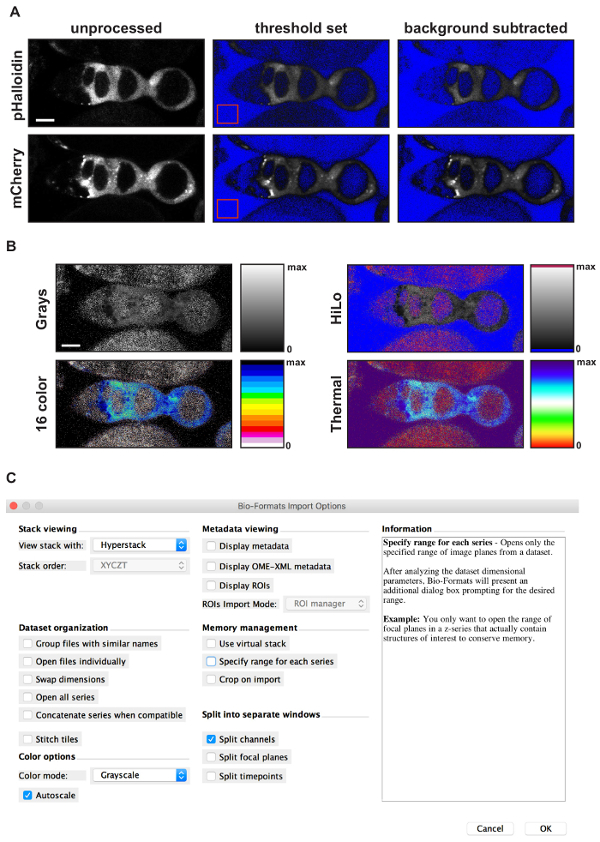
Figure 4: Using FIJI to generate a pseudocolored ratiometric image. (A) Images showing the results of each step in the background subtraction process. Starting with an unprocessed image (left panels), the first step is to set the threshold limits so that pixels with intensity values below background are excluded. The upper limit of the threshold is set to maximum. FIJI will color the excluded pixels blue, resulting in an image with a blue background and a clearly visible germarium (middle panels). Note, this step is optional. The second step is to draw an ROI in a part of the image without signal (red squares in middle panels), measure the mean fluorescence intensity of the ROI, and subtract that amount from the entire image, resulting in a background subtracted image (right panels). The third step is to use the Image calculation function to divide the image in the pHluorin channel by the image in the mCherry channel. The result will be an image displayed with the Greys lookup table and the image display values set to span the entire dynamic range provided by the bit depth of the image. The final step is to adjust the image display settings to a more appropriate range and select a lookup table. (B) Sample images showing the result of an image calculation with the minimum display value set to 0 and the maximum display value set to 2.5 using four different lookup tables. The rectangle next to each image shows the colors used across the dynamic range for each lookup table. Notice that for HiLo, 16 color, and Thermal, distinct colors are used for pixels with an intensity value of 0 or maximum (e.g., blue and red, respectively, in HiLo). This provides an easy visual reference of the limits of the dynamic range, allowing the viewer to see that the signal is within the dynamic range. (C) Screen shot showing the options selected when importing a file into ImageJ using the Bio-Formats Import Plug-In. Scale bars represent 10 µm. Please click here to view a larger version of this figure.
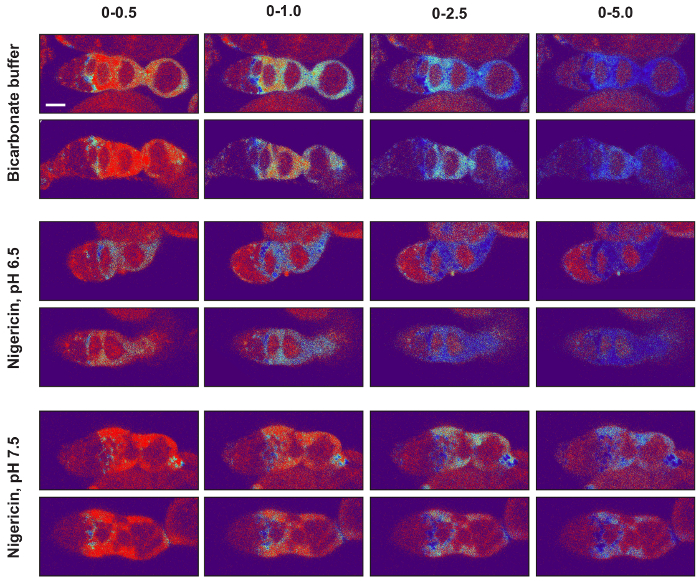
Figure 5: Setting the image display values of pseudocolored ratiometric images. The minimum and maximum image display values should be set so that the signal in images from the bicarbonate condition as well as from both nigericin conditions are within the dynamic range. To illustrate this point, two images from each of the three conditions are shown with four different image display settings (0-0.5, 0-1, 0-2.5, and 0-5) using the Thermal lookup table. Notice that, for all three experimental conditions, when the images are displayed with the maximum displayed value set to 0.5, much of the signal is at or near maximum on the colorimetric scale, and when it is set to 5.0, much of the signal is at or near minimum on the colorimetric scale. In both of these cases, the differences in the pHluorin to mCherry ratio across the tissue cannot be easily appreciated so these settings are not ideal. In contrast, maximum display values of 1.0 or 2.5 are much more appropriate. With these settings, the differences in ratios across the tissue can be easily appreciated, and the signals in the images from all three experimental conditions are displayed in colors that are within the dynamic range of the colorimetric scale. Scale bars represent approximately 10 µm. Please click here to view a larger version of this figure.
Discussion
Here, we describe a method for measuring the pHi of cells in the FSC lineage within wildtype tissue. This protocol has been developed and refined over the past five years, since we first began to study pHi in Drosophila ovarian tissue. During that time, the protocol has been used successfully by multiple investigators in our lab and on at least four different spinning disc and laser scanning microscopes. The reproducibility of our original observation, that pHi increases as cells in the FSC lineage differentiate from the stem cell to the pFC to the follicle cell state, across these multiple trials demonstrates both that this biological phenomenon is robust and that the methodology is reliable. However, in our experience, this is a challenging procedure to master. It requires close attention to detail at every step, and rapid, highly proficient execution of the dissection, mounting, and imaging steps. As stated in the protocol, the dissection and mounting procedure includes a 10 min incubation but should not exceed 15 min total and the imaging procedure must be completed in 45 min. Under ideal conditions, Drosophila ovarioles can be maintained in culture for up to 14 h17, but we found that the tissue begins to die much more rapidly in the nigericin buffers used to generate the standard curves. Our guidelines ensure that all data are collected while the tissue is still well within the window of time that it appears healthy and morphologically normal. This does not leave much time for each step, though, so it is important to plan ahead to make sure that everything is ready to proceed from one step to the next. In fact, it is much more efficient if two people work together on the dissection, mounting, and imaging procedures, with one person executing a step while the other person prepares for the next step.
Since pHi may be sensitive to the experimental manipulations required to image the tissue ex vivo, such as temperature and buffer composition, this method is best to identify relative changes in pHi. Given that the probe consists of two distinct fluorescent proteins, their fluorescence properties like quantum yield, life-time, and folding properties may be affected differently in different cell types. Therefore, it is essential to include samples treated with the nigericin buffer conditions and to use them to generate a new calibration curve for each cell type in every trial. In order to minimize additional sources for variability, keeping all conditions consistent during the course of imaging is emphasized. Samples in nigericin buffer conditions should be prepared and imaged on the same day as those in the bicarbonate buffer condition and the preparation and image acquisition of all samples should be kept as consistent as possible. This is important because the pHi measurements are based on a comparison between the image sets, so experimental differences that affect the detection of the mCherry and pHluorin in one or more of the image sets may decrease the accuracy of the pHi estimates. Factors such as changes in the voltage settings of the photomultiplier tubes (if using a laser scanning confocal) or exposure times (if using a spinning disc confocal) or a dirty lens that reduces the amount of light reaching the camera, will clearly affect the results. But there are a myriad of other more subtle factors that may vary from day-to-day and could also influence the results, such as if the flies are well-fed, the age of the flies, and how long the lasers have been on prior to imaging. Preparing and imaging all three conditions on the same day minimizes these differences and will thus produce the most consistent results. Notably, each time we switched to a new microscope or components of the microscope were upgraded, it was necessary to reoptimize the settings on the new equipment. This often resulted in a change in the average values of the individual measurements but, as long as new calibration curves were generated with each trial, the changes in equipment had a minimal impact on the pHi estimates. In addition, the relevant comparison is often between different cell types that are within the same tissue (e.g., the FSCs, pFCs and follicle cells) and thus are internally controlled for experimental variations.
Although this protocol has focused on measuring pHi of wildtype tissue, the methodology is compatible with standard Drosophila methods for manipulating gene expression, such as expression of RNAi or a transgene using UAS/Gal4, and mosaic analysis. Since mCherry::pHluorin is driven by a UAS promoter, it will always be co-expressed with the RNAi or transgene, and MARCM18 can be used to generate homozygous mutant clones with mCherry::pHluorin as the clonal marker. For the reasons described above, wildtype tissue should be analyzed as a control alongside every trial with a mutant genotype. Lastly, the general principles described here can be applied to the use of mCherry::pHluorin to measure pHi in other tissues as well. Indeed, this protocol was adapted from a protocol for using mCherry::pHluorin to measure pHi in the Drosophila eye7, and we have used similar methodology to image mCherry::pHluorin in the larval brain. Overall, the tools and methods described here provide new opportunities to investigate the many diverse functions of pHi in vivo within living, intact tissue.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Bryne Ulmschneider for contributions to the protocol and Diane Barber for suggestions on the manuscript. This work was funded by a National Institute of Health grant GM116384 to T.G. Nystul and D.L. Barber.
Materials
| Fly Stocks | |||
| UAS-mCherry::pHluorin[1] | |||
| y1 w*;P{GawB}10930/CyO | Bloomington Stock Center | 7023 | |
| Act-Gal4 flipout stock | Bloomington Stock Center | 4409 | |
| Name | Company | Catalog Number | Comments |
| Chemicals for Buffer preparation | |||
| NaCl | Sigma Aldrich | S5886 | |
| KCl | Sigma Aldrich | P-3911 | |
| glucose | Mallinckrodt | 4912 | |
| HEPES | Thermo Fisher Scientific | BP310 | |
| MgSO4 | Thermo Fisher Scientific | M63 | |
| CaCl2 | Sigma Aldrich | C-5080 | |
| HCO3 | Sigma Aldrich | S-5761 | |
| MgCl2 | Sigma Aldrich | M-9272 | |
| NMDG+ | Sigma Aldrich | M-2004 | |
| K2HPO4 | Mallinckrodt | 7088 | Use to Make KHPO4 pH 7.4 |
| KH2PO4 | Thermo Fisher Scientific | BP362 | Use to Make KHPO4 pH 7.4 |
| Concanavalin A, Alexa Fluor 647 Conjugate | Thermo Fisher Scientific | C21421 | 0.25 mg/ml dilution |
| Nigericin | Thermo Fisher Scientific | N1495 | |
| Name | Company | Catalog Number | Comments |
| Dissection and mounting tools | |||
| 2 Dumont Inox forceps (Size 5) | Thermo Fisher Scientific | NC9473431 | |
| 2 23-gauge syringe needles | Sigma Aldrich | Z192457 | |
| 9-well glass dissecting dish | Thermo Fisher Scientific | 13-748B | |
| Vacuum Grease | Dow Corning | 1018817 | |
| 22 X 40 mM glass coverslips | Thermo Fisher Scientific | 12545C | |
| Round Glass Coverslips, 12mm diameter, 0.13-0.16mm thickness | Ted Pella, Inc. | 26023 | |
| 3-D mounting chamber | custom manufactured | .stl and .ipt files for 3-D printer included as supplemental files | |
| Name | Company | Catalog Number | Comments |
| Other equipment | |||
| pH meter | Thermo Fisher Scientific | 13-620-183A | Model: Accumet AB15 |
| Dissection microscope | Olympus Corporation | 0H11436 | Model: SZ2-ST |
| Confocal Microscope | Leica Biosystems | SP5 or SP8 laser-scanning confocal microscope with a 40× objective with a numerical aperture of 1.3 |
Referencias
- Grillo-Hill, B. K., Choi, C., Jimenez-Vidal, M., Barber, D. L. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife. 03270, (2015).
- Ulmschneider, B., Grillo-Hill, B. K., Benitez, M., Azimova, D. R., Barber, D. L., Nystul, T. G. Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J. Cell Biol. 215 (3), 345-355 (2016).
- Han, J., Burgess, K. Fluorescent Indicators for Intracellular pH. Chemical reviews. 110 (5), 2709-2728 (2010).
- Koivusalo, M., Welch, C., et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188 (4), 547-563 (2010).
- Choi, C. -. H., Webb, B. A., Chimenti, M. S., Jacobson, M. P., Barber, D. L. pH sensing by FAK-His58 regulates focal adhesion remodeling. J. Cell Biology. 202 (6), 849-859 (2013).
- Rossano, A. J., Chouhan, A. K., Macleod, G. T. Genetically encoded pH-indicators reveal activity-dependent cytosolic acidification of Drosophila motor nerve termini in vivo. J. Phys. 591 (7), 1691-1706 (2013).
- Grillo-Hill, B. K., Webb, B. A., Barber, D. L. Ratiometric imaging of pH probes. Methods in Cell Biol. 123, 429-448 (2014).
- Miesenböck, G., De Angelis, D. A., Rothman, J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 394 (6689), 192-195 (1998).
- Kirilly, D., Xie, T. The Drosophila ovary: an active stem cell community. Cell research. 17 (1), 15-25 (2007).
- Sahai-Hernandez, P., Castanieto, A., Nystul, T. G. Drosophila models of epithelial stem cells and their niches. Wiley interdisciplinary reviews. Dev. Biol. 1 (3), 447-457 (2012).
- Losick, V. P., Morris, L. X., Fox, D. T., Spradling, A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell. 21 (1), 159-171 (2011).
- Pocha, S. M., Montell, D. J. Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: themes and variations. Ann Rev. of Gen. 48, 295-318 (2014).
- St Johnston, D., Ahringer, J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 141 (5), 757-774 (2010).
- Castanieto, A., Johnston, M. J., Nystul, T. G. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. eLife. 3, (2014).
- Margolis, J., Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 121 (11), 3797-3807 (1995).
- Nystul, T. G., Spradling, A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell stem cell. 1 (3), 277-285 (2007).
- Morris, L. X., Spradling, A. C. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 138 (11), 2207-2215 (2011).
- Lee, T., Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends in neuro. 24 (5), 251-254 (2001).

