シュレンク管を用いた Ti(III) メタロセンの合成
30,565 Views
•
•
Descripción
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Inorganic chemists often work with highly air- and water-sensitive compounds. The two most common and practical methods for air-free synthesis utilize either Schlenk lines or gloveboxes. This experiment will demonstrate how to perform simple manipulations on a Schlenk line with a focus on solvent preparation and transfer. Through the synthesis of a reactive Ti(III) metallocene complex, we will demonstrate a new, simple method to degas solvent as well as how to transfer solvent by cannula and by syringe on a Schlenk line.
The synthesis of a Ti(III) metallocene compound 3 is shown in Figure 1.1 Compound 3 is highly reactive with O2, (see oxidation of compound 3 to Ti(IV) metallocene 4 shown in Figure 1). Therefore, it is important to run the synthesis under anaerobic conditions. The synthesis of target compound 3 can be monitored visually and progresses through one additional color change before arriving at the desired product, which is blue in color. If during the experiment there is an observed color change from blue to yellow (or green = blue + yellow), this is an indication that O2 entered the flask and that undesired oxidation of compound 3 to the Ti(IV) analog (compound 4) has occurred.
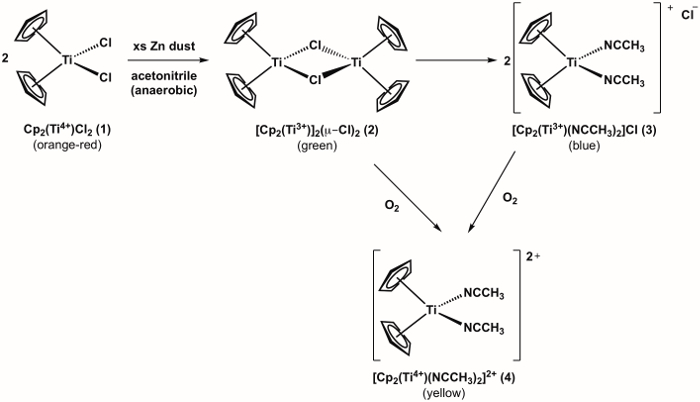
Figure 1. Synthesis of Ti(III) metallocene compound 3 and it's reaction with O2.
Principios
Schlenk line technique uses positive pressure of inert gases to keep air out of a system when handling air- and water-sensitive reagents. An introduction to Schlenk line technique can be found in the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series. In this module, two experimental techniques using the Schlenk line will be explored: solvent degassing and air-free solvent transfer.
Anaerobic synthesis requires removal of air that is dissolved in reaction solvents (i.e., degassing the solvent). The solubility of a gas in a liquid is dependent on the identity of the gas and the solvent, as well as the temperature of the system and the partial pressure of the gas above the liquid. Henry's law states that at a given temperature, the amount of gas dissolved in a specific volume of liquid is directly proportional to the partial pressure of that gas in the system. To degas a solvent, the air above the liquid is removed or replaced with an inert gas, such as N2 or Ar. By reducing or removing the pressure of air above the liquid, the amount of air dissolved in that liquid decreases. The process of degassing ultimately results in the removal of all of the air dissolved in the solvent.
There are several methods that can be used to degas solvent, including freeze-pump-thaw and bubbling inert gas through the solvent (purging). While the freeze-pump-thaw method is the more rigorous of the two methods for removing dissolved O2 (see the "Degassing Liquids" video in the Essentials of Organic Chemistry series), purging is useful when using smaller volumes of liquid and when the reactants and/or products are not water sensitive. Here we demonstrate how to degas solvent by purging. It is important to remember that degassing solvent does not remove water.
The most common methods to add solvent to a reaction using a Schlenk line include transfer by syringe or by cannula (a long double pointed needle, Figure 2). Syringes are used when a specific volume of liquid needs to be added to the reaction (i.e., adding a liquid reagent). Cannula transfers can be used to transfer an exact volume into a dropping funnel, or an approximate volume if transferring solvent to the reaction. Cannula transfer relies on a pressure difference between two flasks to transfer solvent from one vessel (donor flask) to another (receiving flask) (Figure 3), and the pressure differential can be achieved by either application of vacuum or pressure. Vacuum-based cannula transfer is conducted by putting the receiving flask under static or dynamic vacuum, while the donor flask is connected to positive N2 pressure. In pressure-based cannula transfer, the receiving flask is vented while positive N2 pressure is fed into the donor flask. In both cases, the lower pressure in the receiving flask results in solvent flowing through the cannula from the donor flask to the receiving flask. Here we demonstrate how to use the pressure method for cannula transfer.

Figure 2. Cannula.

Figure 3. Basics of cannula transfer. Schlenk flask A (the receiving flask, left) contains the solid reactants and Schlenk flask B (the donor flask, right) contains the degassed acetonitrile.
Procedimiento
1. Setup of the Schlenk Line
For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series. Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condenses and is explosive in the presence of organic solvents. If it is suspected that O2 has been condensed or a blue liquid is observed in the cold trap, leave the trap cold under dynamic vacuum. Do NOT remove the liquid N2 trap or turn off the vacuum pump. Over time the liquid O2 will sublime into the pump – it is only safe to remove the liquid N2 trap once all of the O2 has sublimed.
- Close the pressure release valve.
- Turn on the N2 gas and the vacuum pump.
- As the Schlenk line vacuum reaches its minimum pressure, prepare the cold trap with either liquid N2 or dry ice/acetone.
- Assemble the cold trap.
2. Preparation of the Solid Reactants
- Weigh 100 mg (0.40 mmol) of solid dicyclopentadienyltitanium(IV) dichloride (compound 1, Figure 1) and 78 mg (1.2 mmol) zinc dust into a Schlenk flask (Schlenk flask A).
- Fit Schlenk flask A with a greased glass stopper and attach the Schlenk flask side arm to the Schlenk line with Tygon tubing.
- Open the stopcock of the Schlenk line tube attached to Schlenk flask A to vacuum. Slowly open the stopcock on Schlenk flask A. Evacuate Schlenk flask A for 5 min.
- Repressurize Schlenk flask A with N2 by first closing the stopcock on the Schlenk flask. Slowly repressurize the Schlenk line tubing with N2 by turning the Schlenk line stopcock to N2. Make several (at least 5) quick 180 ° turns on the Schlenk flask stopcock, making sure the stopcock is closed after each turn. Slowly open the stopcock to finish filling Schlenk flask A with N2.
- Close the Schlenk flask A stopcock.
- Repeat steps 2.3-2.5 two more times. On the last cycle, leave the stopcock to the Schlenk flask A open.
3. Preparation of the Solvent
NOTE: Since the reaction is not water sensitive, glassware and solvents do not need to be dried. However, if the preparation is for use in the glovebox, all glassware and solvents should be appropriately dried.
- Measure 15 mL of acetonitrile and transfer the solvent to a new Schlenk flask (Schlenk flask B). Fit Schlenk flask B with a septum.
- Connect Schlenk flask B to the Schlenk line using Tygon tubing. Evacuate the tubing for 5 min and refill the tubing with N2 (the stopcock to the Schlenk flask should remain closed). Repeat the evacuation/refill cycles two more times. Leave the tubing under N2.
- Purge one of the unused Tygon tubes on the Schlenk line with N2, fitted with a long needle.
- Insert the needle into the septum of Schlenk flask B and lower the needle into the acetonitrile.
- Insert a second needle (not attached to the Schlenk line) into the septum of Schlenk flask B. This is the vent needle. Upon insertion of the vent needle, N2 should start bubbling through the acetonitrile.
- Allow the acetonitrile to degas for 15 min.
- Open the stopcock to Schlenk flask B.
- Remove the vent needle, followed by the needle connected to the Schlenk line. Close the stopcock on the Schlenk line that is connected to the long needle.
4. Addition of Solvent via Cannula (Figure 3)
- Make sure that the stopcocks to both of the Schlenk flasks (A & B) are open to N2.
- Replace the glass stopper on Schlenk flask A with a rubber septum.
- Insert one end of the cannula through the septum on Schlenk flask B (the donor flask). Do NOT put the needle into the acetonitrile.
- Ensure N2 is flowing through the cannula by putting the opposite end of the cannula close to the skin of the arm.
- Insert the other end of the cannula into Schlenk flask A (the receiving flask).
- Close the stopcock to Schlenk flask A.
- Lower the cannula in Schlenk flask B so that the tip reaches the bottom of the acetonitrile.
- Insert a vent needle in the septum of Schlenk flask A. Solvent should begin to flow. If no solvent is flowing, try increasing the N2 flow or raising the solvent flask above the height of the receiving flask.
- Transfer all 15 mL of the acetonitrile from Schlenk flask B to A. If only a portion of the solvent is desired, simply remove the cannula tip from the solvent in Schlenk flask B to stop the flow of liquid.
- Remove the vent needle from the septum and open the stopcock to Schlenk flask A.
- Remove the cannula from Schlenk flask A.
- Remove the cannula from Schlenk flask B.
5. Synthesis of Ti(III) Metallocene (Compound 3)
- Vigorously stir the solution for 15 min (or until the reaction mixture turns blue).
- If a green color persists, add more zinc dust (1-2 additional equivalents). To add more zinc dust to the system without introducing O2, make sure that the Schlenk flask stopcock is open to positive N2 pressure. Remove the rubber septum and add the solid to the flask. Re-attach the rubber septum. If the addition of excess zinc dust does not effect the desired color change to blue, O2 was likely introduced into the system.
6. Addition of Solvent via Syringe
- Degas 10 mL of acetonitrile as described in step 3 in Schlenk flask B.
- Make sure that both Schlenk flask A & B stopcocks are open to N2 and are fitted with rubber septa.
- Insert the syringe needle into either flask and pull N2 gas into the syringe.
- Remove the needle and eject the N2 into the hood.
- Repeat steps 6.3-6.4 two more times.
- Insert the syringe needle fitted to a 10 mL syringe into Schlenk flask B and pull up the desired volume of solvent (5 mL).
- Remove the needle from the solvent but leave the needle in the Schlenk flask. Bend the needle so that the syringe is pointing up (the needle should form an arch) and pull ~1 mL of N2 gas into the needle. There should be a gas "bubble" at the top of the syringe.
- While keeping the needle arched, remove the needle from Schlenk flask B. The syringe should still be pointed up with the bubble of N2 at the tip of the syringe where the needle is attached. The N2 bubble will prevent acetonitrile from leaking out of the syringe.
- With the needle still arched and the syringe pointing up, insert the needle into the septum of Schlenk flask A.
- Slowly add acetonitrile to Schlenk flask A. At this point, the position of the syringe is irrelevant.
- When solvent addition is complete, remove the syringe needle from Schlenk flask A.
Chemists frequently encounter air-sensitive chemical reagents and reactions, and thus have to apply special techniques when working with them.
The slightest trace of air in a chemical reaction would likely result in unwanted side products. To avoid this, first traces of oxygen are removed by purging equipment and reagents.
Then, in order to maintain an oxygen-free atmosphere, reagents are handled in a glovebox, or transferred from one closed system to another by cannulation using a Schlenk line.
This video will illustrate a procedure for purging oxygen from a reaction mixture and maintaining an air-free atmosphere in the synthesis of a Ti(III) metallocene. This will be followed by a few examples demonstrating the application of this technique.
Inorganic chemical reactions, such as the conversion of titanocene dichloride to its dimeric form and the final Ti(III) metallocene, are highly sensitive to oxygen, and therefore must be carried out in air-free conditions.
To start, in a fume hood equipped with a Schlenk line, also known as a double manifold, weigh Cp2(Ti4+)Cl2 and zinc dust into a 200 mL Schlenk flask equipped with a stir bar, labeled as "A". Seal the flask with a greased glass stopper and secure with a rubber band. Attach Tygon tubing from the Schlenk line to flask sidearm.
Open the stopcock to vacuum and evacuate for 5 min, then close the stopcock to the flask, switch to N2, and make at least five rapid 180 ° turns before slowly opening to fill the flask with N2.
In a separate Schlenk flask labeled "B", measure 15 mL of acetonitrile and seal with a rubber septum. Attach Tygon tubing from the Schlenk line to the flask sidearm, then evacuate the tubing for 5 min. Refill the tubing with N2.
Attach a long needle to a second Tygon tube on the Schlenk line, and purge with N2 for several minutes. Insert the purged needle into the Schlenk flask containing acetonitrile, followed by the venting needle. Bubble N2 into the solvent for 15 min, then open the flask stopcock to N2 and remove the needles.
With Schlenk flask A under N2, remove the glass stopper and replace it with a rubber septum. With the two Schlenk flasks open to N2, insert one end of the cannula into the donor flask, above the level of the solvent, and determine whether N2 is flowing through the other end. Then insert the other end of the cannula into the receiving flask containing the reagents, close the receiving flask's stopcock, and attach a venting needle.
Lower the cannula into the solvent, and allow all of the acetonitrile to drip or slowly flow along the sides of the receiving flask. Once the addition is complete, reopen the receiving flask stopcock to N2, and remove the cannula and venting needle.
After the solvent is added, vigorously stir the reaction mixture of acetonitrile, zinc dust, and Cp2(Ti4+)Cl2 until it turns blue, indicating formation of Ti(III) metallocene complex.
If the reaction mixture remains green after 15 min, keep the stopcock open to positive N2 pressure, remove the septum and add 1-2 equivalents of zinc dust. If the mixture is still green or has turned yellow, it is likely that oxygen has entered the system, which results in further oxidation to the Ti(IV) metallocene complex.
Now you know how to use a cannula transfer, but in case this is not possible, the solvent can be added via a syringe. First, make sure both the receiving and donor flasks are open to N2.
Insert the needle fitted to a 12 mL syringe into either flask and pull only N2 into it. Remove the needle and eject the N2 into the hood.
Once the needle and syringe are purged, insert the needle into the donor flask and pull up the desired volume of solvent. Then, raise the needle slightly, bend it to an arch and pull up 1 mL of N2. Keep the needle arched and syringe pointing up and remove it from the donor flask.
Insert the arched needle into the receiving flask. Slowly add the solvent, and remove the syringe needle from receiving flask when finished.
Now that we have discussed a procedure for an air-free synthesis, let's look at a few applications.
Cadmium selenide quantum dots are semiconductor nanocrystals composed of a cadmium selenide core and a ligand shell. These multicomponent structures are capable of manipulating electrons at the nanoscale.
The synthesis of these nanocrystals requires precise reaction conditions, especially an oxygen-free atmosphere.
Titanocene dichloride, the reagent used in this video, is an organotitanium compound commonly used in organic and organometallic synthesis. The compound itself is synthesized by reacting 2 equivalents of sodium cyclopentadiene (NaCp) with TiCl4 in anhydrous, oxygen-free THF. Titanocene dichloride is also used for the production of the Petasis reagent, which is a useful reagent applied in the conversion of esters to vinyl ethers.
Another titanocene dichloride reagent, called the Tebbe reagent, is applied to convert various carbonyl functional groups to alkenes, or also known as methylenation.
You've just watched JoVE's introduction to Synthesis of a Ti(III) metallocene using the Schlenk Line Technique. You should now understand how to perform degassing as well as cannula transfer, and some of its applications. Thanks for watching!
Resultados
Upon addition of the acetonitrile in step 4, the solution should change color from orange, to green, to blue (Figure 4). Failure to obtain the blue color indicates a leak in the system. Addition of acetonitrile by syringe in step 6 should result in no color change if anaerobic conditions are maintained. If oxygen is present, the solution will turn from blue, to green, to orange.



Figure 4. Three color stages during the synthesis of Ti(III) metallocene compound 3.
Applications and Summary
Here, we demonstrated standard Schlenk line technique to synthesize an air-sensitive Ti(III) metallocene complex. The solvent was degassed by bubbling N2 through the liquid in a Schlenk flask. We also demonstrated how to set up a reaction under anaerobic conditions on the Schlenk line and transfer solvent anaerobically by cannula transfer as well as by syringe.
Inorganic chemists use Schlenk line technique in the synthesis of air- and water-sensitive compounds. The solvent used in the synthesis of highly-reactive materials can be prepared using the Schlenk line. Air-sensitive reactions can also be set up and worked up using a Schlenk line. The Schlenk line technique is a powerful method for air-free manipulations used in synthesis, purification (i.e.,distillation, sublimation, and crystallization), catalysis, and gas reactions. In the next module, we will demonstrate how to use a glovebox for air-free synthesis. While some air-free manipulations are easier to perform in a glovebox, there are certain situations when one cannot use a glovebox and must rely on Schlenk line technique (such as heating a reaction). Some metallocene complexes (metal compounds featuring typically two cyclopentadienyl anions (Cp, C5H5–)) exhibit catalytic properties. For example, titanocene is a catalyst used in olefin metathesis.
The Ti(III) metallocene synthesized herein can be used on the Schlenk line or in the glove box as an atmospheric test. Oxidation of the Ti(III) metallocene by O2 on the Schlenk line or in the glove box would result in a color change and would provide a visual indication that the atmosphere contains O2.
Referencias
- Burgmayer, S. N. Use of a Titanium Metallocene as a Colorimetric Indicator for Learning Inert Atmosphere Techniques. J Chem Educ. 75, 460 (1998).
Transcripción
Chemists frequently encounter air-sensitive chemical reagents and reactions, and thus have to apply special techniques when working with them.
The slightest trace of air in a chemical reaction would likely result in unwanted side products. To avoid this, first traces of oxygen are removed by purging equipment and reagents.
Then, in order to maintain an oxygen-free atmosphere, reagents are handled in a glovebox, or transferred from one closed system to another by cannulation using a Schlenk line.
This video will illustrate a procedure for purging oxygen from a reaction mixture and maintaining an air-free atmosphere in the synthesis of a Ti(III) metallocene. This will be followed by a few examples demonstrating the application of this technique.
Inorganic chemical reactions, such as the conversion of titanocene dichloride to its dimeric form and the final Ti(III) metallocene, are highly sensitive to oxygen, and therefore must be carried out in air-free conditions.
To start, in a fume hood equipped with a Schlenk line, also known as a double manifold, weigh Cp2(Ti4+)Cl2 and zinc dust into a 200 mL Schlenk flask equipped with a stir bar, labeled as “A”. Seal the flask with a greased glass stopper and secure with a rubber band. Attach Tygon tubing from the Schlenk line to flask sidearm.
Open the stopcock to vacuum and evacuate for 5 min, then close the stopcock to the flask, switch to N2, and make at least five rapid 180 ° turns before slowly opening to fill the flask with N2.
In a separate Schlenk flask labeled “B”, measure 15 mL of acetonitrile and seal with a rubber septum. Attach Tygon tubing from the Schlenk line to the flask sidearm, then evacuate the tubing for 5 min. Refill the tubing with N2.
Attach a long needle to a second Tygon tube on the Schlenk line, and purge with N2 for several minutes. Insert the purged needle into the Schlenk flask containing acetonitrile, followed by the venting needle. Bubble N2 into the solvent for 15 min, then open the flask stopcock to N2 and remove the needles.
With Schlenk flask A under N2, remove the glass stopper and replace it with a rubber septum. With the two Schlenk flasks open to N2, insert one end of the cannula into the donor flask, above the level of the solvent, and determine whether N2 is flowing through the other end. Then insert the other end of the cannula into the receiving flask containing the reagents, close the receiving flask’s stopcock, and attach a venting needle.
Lower the cannula into the solvent, and allow all of the acetonitrile to drip or slowly flow along the sides of the receiving flask. Once the addition is complete, reopen the receiving flask stopcock to N2, and remove the cannula and venting needle.
After the solvent is added, vigorously stir the reaction mixture of acetonitrile, zinc dust, and Cp2(Ti4+)Cl2 until it turns blue, indicating formation of Ti(III) metallocene complex.
If the reaction mixture remains green after 15 min, keep the stopcock open to positive N2 pressure, remove the septum and add 1-2 equivalents of zinc dust. If the mixture is still green or has turned yellow, it is likely that oxygen has entered the system, which results in further oxidation to the Ti(IV) metallocene complex.
Now you know how to use a cannula transfer, but in case this is not possible, the solvent can be added via a syringe. First, make sure both the receiving and donor flasks are open to N2.
Insert the needle fitted to a 12 mL syringe into either flask and pull only N2 into it. Remove the needle and eject the N2 into the hood.
Once the needle and syringe are purged, insert the needle into the donor flask and pull up the desired volume of solvent. Then, raise the needle slightly, bend it to an arch and pull up 1 mL of N2. Keep the needle arched and syringe pointing up and remove it from the donor flask.
Insert the arched needle into the receiving flask. Slowly add the solvent, and remove the syringe needle from receiving flask when finished.
Now that we have discussed a procedure for an air-free synthesis, let’s look at a few applications.
Cadmium selenide quantum dots are semiconductor nanocrystals composed of a cadmium selenide core and a ligand shell. These multicomponent structures are capable of manipulating electrons at the nanoscale.
The synthesis of these nanocrystals requires precise reaction conditions, especially an oxygen-free atmosphere.
Titanocene dichloride, the reagent used in this video, is an organotitanium compound commonly used in organic and organometallic synthesis. The compound itself is synthesized by reacting 2 equivalents of sodium cyclopentadiene (NaCp) with TiCl4 in anhydrous, oxygen-free THF. Titanocene dichloride is also used for the production of the Petasis reagent, which is a useful reagent applied in the conversion of esters to vinyl ethers.
Another titanocene dichloride reagent, called the Tebbe reagent, is applied to convert various carbonyl functional groups to alkenes, or also known as methylenation.
You’ve just watched JoVE’s introduction to Synthesis of a Ti(III) metallocene using the Schlenk Line Technique. You should now understand how to perform degassing as well as cannula transfer, and some of its applications. Thanks for watching!
