Ideal Gas Law
139,435 Views
•
•
Derivation of the Ideal Gas Law
Gases are a fundamental state of matter. A gas is a collection of molecules that have a significant distance between their molecules. Due to this distance, colorless gases are invisible to the human eye and are studied using four measurable parameters: pressure (P), volume (V), number of moles (n), and temperature (T). The ideal gas law is a mathematical equation that relates all of these parameters. It is a combination of several different laws that describe the behavior of gases.
In 1662, Robert Boyle confirmed a prior discovery relating the pressure of a gas to its volume. Boyle’s law states that the pressure of a gas is inversely proportional to its volume if the temperature and number of moles of the gas are held constant.
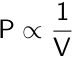
Boyle’s law can be extended to calculate the new pressure or volume of a gas if the initial pressure and volume are known.

In the 1780s, the unpublished work of French scientist Jacques Charles was credited by French scientist Joseph Louis Gay-Lussac for describing the direct relationship between the volume and temperature of a gas.

Charles’s law allows us to calculate the new volume or temperature of a gas if the initial volume and temperature are known, and the pressure and number of moles are constant.

Joseph Louis Gay-Lussac provided an extension to Charles’s law by relating pressure and temperature. Gay-Lussac’s law establishes that the pressure of an enclosed gas is directly proportional to its temperature.

Therefore, if a change is applied to a gas at a constant volume and number of moles, the new pressure or temperature can be calculated if the initial pressure and temperature are known.

Finally, in 1811, Amedeo Avogadro proposed the direct proportionality between the volume of a gas and the number of moles present.
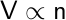
The law describes how equal volumes of two gases, with the same temperature and pressure, contain an equal number of molecules.
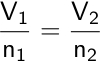
All of these relationships combine to form the ideal gas law, first proposed by Emile Clapeyron in 1834, as a way to combine these laws of physical chemistry. The ideal gas law accounts for pressure (P), volume (V), moles of gas (n), and temperature (T), with an added proportionality constant, the ideal gas constant (R). The universal gas constant, R, is equal to 8.314 J·K-1 mol-1.

Assumptions of the Ideal Gas Law
The ideal gas law assumes that gases behave ideally, meaning they adhere to the following characteristics: (1) the collisions occurring between molecules are elastic and their motion is frictionless, meaning that the molecules do not lose energy; (2) the total volume of the individual molecules is magnitudes smaller than the volume that the gas occupies; (3) there are no intermolecular forces acting between the molecules or their surroundings; (4) the molecules are constantly in motion, and the distance between two molecules is significantly larger than the size of an individual molecule. As a result of all these assumptions, an ideal gas would not form a liquid at room temperature.
However, as we know, many gases become liquids at room temperature and therefore deviate from ideal behavior. In 1873, Johannes D. Van der Waals modified the ideal gas law to account for the molecular size, intermolecular forces, and volume that define real gases.

In the Van der Waals equation, parameters a and b are constants that can be determined experimentally and differ from one gas to another. Parameter a will experience larger values for gases with strong intermolecular forces (i.e., water) and smaller values for gases that have weak intermolecular forces (i.e., inert gases). Parameter b represents the volume that 1 mole of gas molecules occupies; thus, when b decreases, the pressure increases as a result.
The Dumas Method
Invented by Jean Baptiste Andre Dumas, the Dumas method utilizes the ideal gas law to study gas samples. The ideal gas law includes Avogadro’s law, where the number of moles of two gas samples occupying the same volume is the same at a constant pressure and temperature. This relationship allows the Dumas method to calculate the molar mass of an unknown gas sample.
To accomplish this, a Dumas tube is used. A Dumas tube is an elongated glass bulb with a long capillary neck. Prior to the experiment, the volume and mass of the tube are measured. Then, a small amount of a volatile compound is placed in the Dumas tube. Volatile compounds have a high vapor pressure at room temperature and are vaporized at low temperatures. Thus, when the Dumas tube containing the volatile liquid is placed in boiling water, the liquid vaporizes and forces the air out of the tube, and the tube is solely filled with vapor. When the tube is removed from the water bath and left at room temperature, the vapor condenses back to a liquid. Since mass is conserved, the mass of the liquid in the tube is equal to the mass of the gas in the tube. Using the known mass and volume of the gas, along with the known water bath temperature and room pressure, the moles and therefore molecular weight of the gas can be calculated using the ideal gas law.
Here, three assumptions are made: (1) the vapor is acting ideally, (2) the volume of the tube does not vary between the room temperature and the working temperature, and (3) the gas and the water bath are at thermal equilibrium.
Referencias
- Kotz, J.C., Treichel Jr, P.M., Townsend, J.R. (2012) Chemistry and Chemical Reactivity. Belmont, CA: Brooks/Cole, Cengage Learning.
- Gay-Lussac, J.L. (1809). Memoir on the Combination of Gaseous Substances with Each Other. Mémoires de la Société d'Arcueil, Vol. 2, 207.
- Van der Waals, J.D. (1967). The equation of state for gases and liquids. Nobel Lectures, Physics. Elsevier: Amsterdam, pp. 254-265.
- Silderberg, M.S. (2009). Chemistry: The Molecular Nature of Matter and Change. Boston, MA: McGraw Hill.
